Abstract
Recently we discovered that intact kidneys release into the extracellular compartment 2',3'-cAMP (a positional isomer of 3',5'-cAMP with unknown pharmacology) and metabolize 2',3'-cAMP to 2'-AMP, 3'-AMP and adenosine. Because adenosine inhibits growth of vascular smooth muscle cells and mesangial cells, we tested the hypothesis that extracellular 2',3'-cAMP attenuates growth of preglomerular vascular smooth muscle and mesangial cells via production of adenosine. For comparison, all experiments were performed with both 2',3'-cAMP and 3',5'-cAMP.
Study 1
2',3'-cAMP, 3',5'-cAMP, 5'-AMP, 3'-AMP or 2'-AMP were incubated with cells and purines measured in the medium by mass spectrometry. Both preglomerular vascular smooth muscle and mesangial cells metabolized 3',5'-cAMP to 5'-AMP and adenosine, 5'-AMP to adenosine, 2',3'-cAMP to 2'-AMP, 3'-AMP and adenosine and 2'-AMP and 3'-AMP to adenosine. 3-Isobutyl-1-methylxanthine (phosphodiesterase inhibitor) and 1,3-dipropyl-8-psulfophenylxanthine (ecto-phosphodiesterase inhibitor) blocked conversion of 3',5'-cAMP to 5'-AMP and adenosine and α,β-methylene-adenosine-5'-diphosphate (CD73 inhibitor) blocked conversion of 5'-AMP to adenosine. These enzyme inhibitors had little effect on metabolism of 2',3'-cAMP, 2'-AMP or 3'-AMP.
Study 2
2',3'-cAMP and 3',5'-cAMP profoundly inhibited proliferation (thymidine incorporation and cell number) of both cell types with 2',3'-cAMP more potent than 3',5'-cAMP. Antagonism of A2B receptors (MRS-1724), but not A1 (1,3-dipropyl-8-cyclopentylxanthine), A2A (SCH-58261) or A3 (VUF-5574) receptors, attenuated the growth inhibitory effects of 2',3'-cAMP and 3',5'-cAMP.
Conclusions
Extracellular 2',3'-cAMP inhibits growth of preglomerular vascular smooth muscle and mesangial cells more profoundly than does 3',5'-cAMP. Although both cAMPs inhibit growth in part via conversion to adenosine followed by A2B-receptor activation, their metabolism is mediated by different enzymes.
Keywords: 2',3'-cAMP; 3',5'-cAMP; Adenosine; Adenosine Receptors; A2B Receptor; Vascular smooth muscle cells; glomerular mesangial cells
INTRODUCTION
Recently, we developed an assay employing high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure 3',5'-cAMP and other purines in the renal venous effluent from isolated, perfused kidneys1. While studying the release of 3',5'-cAMP from isolated, perfused rat kidneys, we observed a chromatographic peak that was due to an endogenous substance that was not 3',5'-cAMP but had the same parent ion as 3',5'-cAMP and fragmented to the same daughter ion as 3',5'-cAMP2. We identified the substance as a positional isomer of 3',5'-cAMP, namely 2',3'-cAMP2 and provided support for the notion that 2',3'-cAMP derives from mRNA breakdown triggered by energy depletion2. To our knowledge this was the first report of the endogenous generation of 2',3'-cAMP by any tissue, organ or cell.
The discovery that kidneys release 2',3'-cAMP into the extracellular compartment suggests the hypothesis that there may exist a novel mechanism for the extracellular production of adenosine: metabolism of extracellular 2',3'-cAMP to 2'-AMP and/or 3'-AMP followed by conversion of 2'-AMP and/or 3'-AMP to adenosine (i.e., the extracellular 2',3'-cAMP-adenosine pathway). Studies in isolated, perfused rat kidneys support the extracellular 2',3'-cAMP-adenosine pathway hypothesis3. In this regard, infusions of 2',3'-cAMP into the renal artery increase profoundly the levels of 2'-AMP, 3'-AMP and adenosine in the renal vein, and infusions of 2'-AMP and 3'-AMP increase the concentrations of adenosine in the renal vein3. Also, activation of mRNA breakdown by energy depletion increases renal venous levels of 2',3'-cAMP, 2'-AMP, 3'-AMP and adenosine3. Because renal venous measurements likely reflect vascular metabolism of the cAMPs, these findings indicate that the renal vasculature expresses the extracellular 2',3'-cAMP-adenosine pathway.
Preglomerular vascular smooth muscle cells (PGVSMCs) and glomerular mesangial cells (GMCs) are two important renovascular cell types that could contribute to the existence of the renovascular extracellular 2',3'-cAMP-adenosine pathway. Indeed, previous studies demonstrate the existence of an extracellular 3',5'-cAMP-adenosine pathway (metabolism of extracellular 3',5'-cAMP to 5'-AMP and conversion of 5'-AMP to adenosine) in both of these cell types4–6. Therefore, one goal of the present study was to investigate the renovascular extracellular 2',3'-cAMP-adenosine pathway by examining whether this pathway exists in PGVSMCs and GMCs and by determining, in side-by-side experiments, the quantitative importance of the extracellular 2',3'-cAMP-adenosine pathway compared with the extracellular 3',5'-cAMP-adenosine pathway in PGVSMCs and GMCs. A second goal of the present study was to ascertain whether the enzymes that mediate the extracellular 2',3'-cAMP-adenosine pathway are distinct from those that mediate the extracellular 3',5'-cAMP-adenosine pathway.
Adenosine is an important autacrine/paracrine factor that acts via A1, A2A, A2B and A3 receptors and has profound effects on blood vessels, particularly in the renal vasculature7–9. Studies show that adenosine powerfully inhibits the proliferation of vascular smooth muscle cells and mesangial cells10–15 and that the extracellular 3',5'-cAMP-adenosine pathway inhibits cell growth via generation of adenosine and stimulation of A2B receptors6, 16. Accordingly, a third goal was to determine whether extracellular 2',3'-cAMP inhibits proliferation of PGMVCs and GMCs, whether 2',3'-cAMP is more potent that 3',5'-cAMP in this regard, and whether the effects of 2',3'-cAMP are mediated via adenosine receptors.
METHODS
Animals
PGVSMCs and GMCs were harvested from adult male Wistar-Kyoto rats (Taconic Farms; Germantown, NY). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Drugs
2',3'-cAMP, 3',5'-cAMP, 5'-AMP, 3'-AMP, 2'-AMP, 3-isobutyl-1-methylxanthine (IBMX; broad spectrum phosphodiesterase inhibitor17), 1,3-dipropyl-8-psulfophenylxanthine (DPSPX; ecto-phophosphodiesterase inhibitor18–20), α,β-methylene-adenosine-5'-diphosphate (AMPCP; ecto-5'-nucleotidase (CD73) inhibitor21), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; selective A1 receptor antagonist22), 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH-58261; selective A2A receptor antagonist22), 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine (MRS-1754; selective A2B receptor antagonist22) and N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea (VUF-5574; selective A3 receptor antagonist23) were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Rat PGVSMCs and GMCs were harvested and cultured as described by us previously24, 25. All experiments were performed in cells in 3rd to 5th passage.
Metabolism Studies
Cells were washed with HEPES-buffered Hanks balanced salt solution and incubated with 0.5 ml of Dulbecco's phosphate-buffered saline in the presence and absence of various treatments. After 1 hour the medium was collected, heated for 3 minutes at 100°C to denature enzymes and then frozen at −80°C until assayed by LC-MS/MS.
Analytical Methods
Purines were quantified using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra, ThermoFisher Scientific, San Jose, CA) as previously described in detail3.
Cell Proliferation Studies
[3H]-thymidine incorporation (index of DNA synthesis) and cell number (cell proliferation) studies were conducted as previously described11.
Statistics
Data were analyzed by 1- or 2-factor analysis of variance (ANOVA), with post hoc comparisons using a Fisher's Least Significant Difference (LSD) test. The criterion of significance was p<0.05. All values in text and figures are means ± SEM.
RESULTS
Metabolism of 3',5'-cAMP, 2',3'-cAMP, 5'-AMP, 3'-AMP and 2'-AMP in PGVSMCs
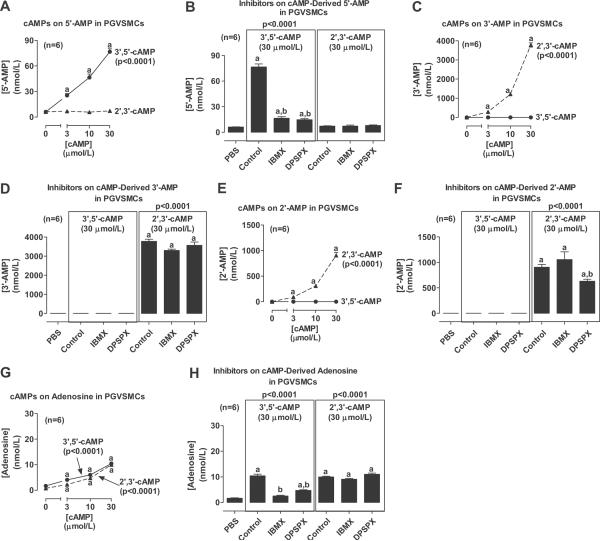
We first examined the conversion of 3',5'-cAMP versus 2',3'-cAMP to AMPs and adenosine in PGVSMCs. Confluent PGVSMCs in 24-well plates were incubated with 0, 3, 10 or 30 μmol/L of 3',5'-cAMP or 2',3'-cAMP. Also, in some culture wells, each cAMP (30 μmol/L) was incubated with either IBMX (1 mmol/L; a broad spectrum inhibitor of phosphodiesterase17) or DPSPX (1 mmol/L; an agent that does not penetrate cell membranes18 yet blocks ecto-phosphodiesterase19, 20). After 1 hour, the medium was collected and assayed for 5'-AMP, 3'-AMP, 2'-AMP or adenosine by LC-MS/MS. Incubation of PGVSMCs with 3',5'-cAMP, but not 2',3'-cAMP, concentration-dependently increased 5'-AMP levels (Fig. 1A). At 30 μmol/L, 3',5'-cAMP increased mean extracellular levels of 5'-AMP by approximately 12-fold (basal, 6.1 ± 0.3 nmol/L). This increase was nearly abolished by co-incubation with the inhibitors IBMX and DPSPX (Fig. 1B); however, neither IBMX nor DPSPX affected the lack of an increase in 5'-AMP levels in cells incubated with 2',3'-cAMP (Fig. 1B). In contrast, incubation of PGVSMCs with 3',5'-cAMP did not elevate 3'-AMP levels, yet incubation with 2',3'-cAMP concentration-dependently increased 3'-AMP levels (Fig. 1C). At 30 μmol/L, 2',3'-cAMP increased mean extracellular levels of 3'-AMP by approximately 19,000-fold (basal, 0.20 ± 0.08 nmol/L). Neither IBMX nor DPSPX affected the lack of an increase in 3'-AMP levels in cells incubated with 3',5'-cAMP (Fig. 1D), and also these inhibitors did not affect 2',3'-cAMP-induced increases in 3'-AMP levels (Fig. 1D). 3',5'cAMP also did not affect extracellular levels of 2'-AMP (Fig. 1E), whereas 2',3'-cAMP concentration-dependently increased 2'-AMP levels (Fig. 1E) approximately 25,000-fold at 30 μmol/L (basal, 0.04 ± 0.03 nmol/L). Neither IBMX nor DPSPX affected the lack of increase in 2'-AMP levels in 3',5'-cAMP-treated cells (Fig. 1F). Although IBMX did not affect 2',3'-cAMP metabolism to 2'-AMP, DPSPX did cause a slight reduction in the metabolism of 2',3'-cAMP to 2'-AMP (Fig. 1F). Both 3',5'-cAMP and 2',3'-cAMP concentration-dependently increased extracellular levels of adenosine (Fig. 1G) by approximately 6-fold (basal, 1.7 ± 0.1 nmol/L) and 13-fold (basal, 0.7 ± 0.1 nmol/L) at 30 μmol/L, respectively. IBMX and DPSPX attenuated the ability of 3',5'-cAMP to elevate adenosine levels (Fig. 1H), but in contrast had no effect on the metabolism of 2',3'-cAMP to adenosine (Fig. 1H).
Figure 1.
Line graphs show concentration-dependent effects of 3',5'-cAMP and 2',3'-cAMP on extracellular levels of 5'-AMP, 3'-AMP, 2'-AMP and adenosine in PGVSMCs. Bar graphs show the effects of 3',5'-cAMP and 2',3'-cAMP on extracellular levels of 5'-AMP, 3'-AMP, 2'-AMP and adenosine in the absence and presence of IBMX or DPSPX (1 mmol/L). Values represent means ± SEM for the indicated number of experiments (n). ap<0.05, compared with basal (0 or PBS) value; bp<0.05, compared with the corresponding cAMP in the absence of inhibitors. P-values in panels are from 1-factor analysis of variance.
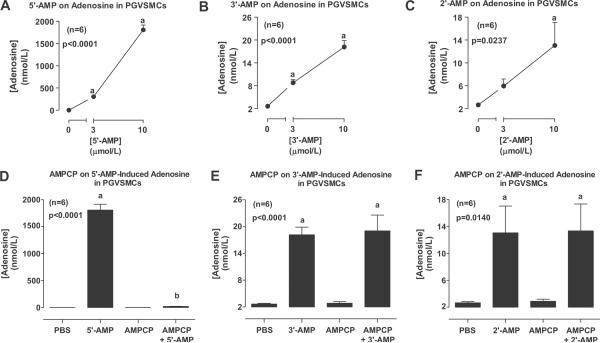
We next examined the conversion of the three different AMPs to adenosine in PGVSMCs. In these experiments, confluent PGVSMCs in 24-well plates were incubated with 0, 3 or 10 μmol/L of AMPs. Also, in some culture wells, each AMP (10 μmol/L) was incubated with AMPCP (0.1 mmol/L; an inhibitor of CD73, a well characterized ecto-5'-nucleotidase21). After 1 hour, the medium was collected and assayed for adenosine by LC-MS/MS. 5'-AMP (Fig. 2A), 3'-AMP (Fig. 2B) and 2'-AMP (Fig. 2C) all concentration-dependently increased extracellular levels of adenosine; however, 5'-AMP was more effective compared with 3'-AMP and 2'-AMP [680-fold, 5-fold and 5-fold (basal, 2.7 ± 0.2 nmol/L), respectively, at 10 μmol/L]. AMPCP abolished metabolism of 5'-AMP to adenosine (Fig. 2D), but did not affect the metabolism of 3'-AMP (Fig. 2E) or 2'-AMP (Fig. 2F) to adenosine.
Figure 2.
Line graphs show the concentration-dependent effects of 5'-AMP (A), 3'-AMP (B) and 2'-AMP (C) on extracellular levels of adenosine in PGVSMCs. Bar graphs show the effects of AMPCP (1 mmol/L) on extracellular adenosine levels derived from 5'-AMP (D), 3'-AMP (E) and 2'-AMP (F). Values represent means ± SEM for the indicated number of experiments (n). ap<0.05, compared with basal (0 or PBS) value; bp<0.05, compared with the corresponding AMP in the absence of inhibitor. P-values in panels are from 1-factor analysis of variance.
Metabolism of 3',5'-cAMP, 2',3'-cAMP, 5'-AMP, 3'-AMP and 2'-AMP in GMCs
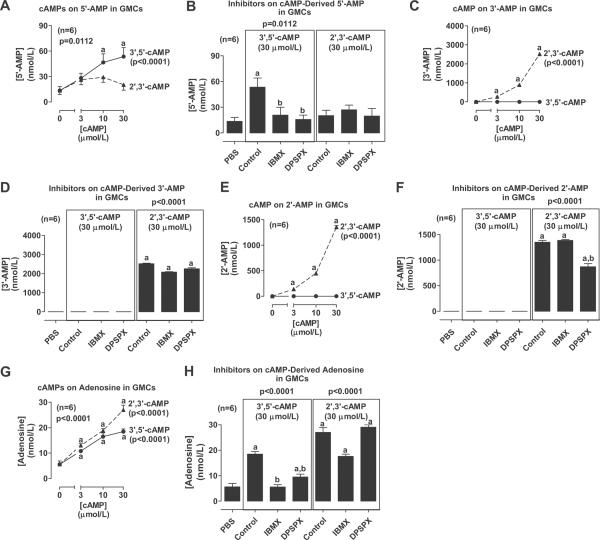
We conducted experiments with cAMPs in GMCs using the same protocol as with PGVSMCs. Incubation of GMCs with 3',5'-cAMP, but not 2',3'-cAMP, concentration-dependently increased 5'-AMP levels (Fig. 3A). At 30 μmol/L, 3',5'-cAMP increased mean extracellular levels of 5'-AMP by approximately 4-fold (basal, 13.4 ± 4.8 nmol/L). This increase was abolished by co-incubation with the inhibitors IBMX and DPSPX (Fig. 3B); however, neither IBMX nor DPSPX affected the lack of an increase in 5'-AMP levels in the cells incubated with 2',3'-cAMP (Fig. 3B). Incubation of GMCs with 3',5'-cAMP did not elevate 3'-AMP levels, yet incubation with 2',3'-cAMP concentration-dependently increased 3'-AMP levels (Fig. 3C). At 30 μmol/L, 2',3'-cAMP increased mean extracellular levels of 3'-AMP by approximately 5,800-fold (basal, 0.43 ± 0.14 nmol/L). Neither IBMX nor DPSPX affected the lack of increase in 3'-AMP levels in cells incubated with 3',5'-cAMP (Fig. 3D), and also these inhibitors did not affect metabolism of 2',3'-cAMP to 3'-AMP (Fig. 3D). 3',5'cAMP also did not affect extracellular levels of 2'-AMP (Fig. 3E), whereas 2',3'-cAMP concentration-dependently increased 2'-AMP levels (Fig. 3E) by approximately 64,000-fold at 30 μmol/L (basal, 0.02 ± 0.02 nmol/L). Neither IBMX nor DPSPX affected the absence of an increase in 2'-AMP levels in 3',5'-cAMP-treated cells (Fig. 3F). Although IBMX did not affect 2',3'-cAMP-induced increases in 2'-AMP levels, DPSPX did cause a slight reduction in the metabolism of 2',3'-cAMP to 2'-AMP (Fig. 3F). Both 3',5'-cAMP and 2',3'-cAMP concentration-dependently increased extracellular levels of adenosine (Fig. 3G) by approximately 3-fold and 5-fold at 30 μmol/L, respectively (basal, 5.6 ± 1.3 nmol/L). IBMX abolished and DPSPX nearly abolished the ability of 3',5'-cAMP to elevate adenosine levels (Fig. 3H), but in contrast had no significant effect on 2',3'-cAMP-induced increases in adenosine levels (Fig. 3H).
Figure 3.
Line graphs show concentration-dependent effects of 3',5'-cAMP and 2',3'-cAMP on extracellular levels of 5'-AMP, 3'-AMP, 2'-AMP and adenosine in GMCs. Bar graphs show the effects of 3',5'-cAMP and 2',3'-cAMP on extracellular levels of 5'-AMP, 3'-AMP, 2'-AMP and adenosine in the absence and presence of IBMX or DPSPX (1 mmol/L). Values represent means ± SEM for the indicated number of experiments (n). ap<0.05, compared with basal (0 or PBS) value; bp<0.05, compared with the corresponding cAMP in the absence of inhibitors. P-values in panels are from 1-factor analysis of variance.
5'-AMP (Fig. S1A), 3'-AMP (Fig. S1B) and 2'-AMP (Fig. S1C) all concentration-dependently increased extracellular levels of adenosine (please see http://hyper.ahajournals.org); however, 5'-AMP was again more effective in this regard compared with 3'-AMP and 2'-AMP [170-fold, 3-fold and 2-fold (basal, 8.2 ± 0.4 nmol/L), respectively, at 10 μmol/L]. AMPCP abolished 5'-AMP-induced increases in adenosine levels (Fig. S1D), but did not affect 3'-AMP-induced (Fig. S1E) or 2'-AMP-induced (Fig. S1F) increases in adenosine levels (please see http://hyper.ahajournals.org).
Growth Effects cAMPs in PGVSMCs
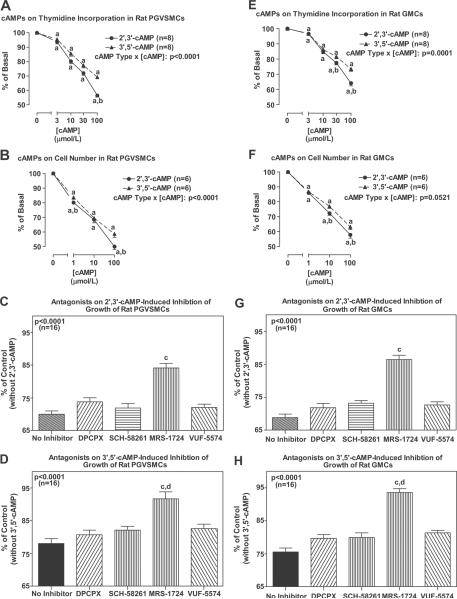
In PGVSMCs, both 2',3'-cAMP and 3',5'-cAMP significantly inhibited cell growth as assessed by either [3H]-thymidine incorporation or cell number (Fig. 4A and 4B). Inhibition of cell number was statistically significant for both cAMPs at the lowest concentration tested (1 μmol/L; 20% and 16% inhibition for 2',3'-cAMP and 3',5'-cAMP, respectively), and inhibition of cell number at the highest concentration tested (100 μmol/L) was 50% and 41% for 2',3'-cAMP and 3',5'-cAMP, respectively. For both the [3H]-thymidine incorporation experiments and cell number experiments, the inhibition by 2',3'-cAMP was statistically greater (p<0.0001 for both; interaction term in 2-factor ANOVA in which one factor was cAMP type and the other factor was concentration of cAMP) than inhibition induced by 3',5'-cAMP. DPCPX, SCH-58261 and VUF-5574 (each at 0.1 μmol/L) did not antagonize the inhibitory effects of 2',3'-cAMP or 3',5'-cAMP (each at 30 μmol/L) on [3H]-thymidine incorporation (Fig. 4C and 4D). In contrast, MRS-1724 (0.1 μmol/L) significantly (p<0.0001) attenuated the reduction in [3H]-thymidine incorporation induced by 2',3'-cAMP and 3',5'-cAMP (Fig. 4C and 4D). The attenuation by MRS-1724 of 3',5'-cAMP-induced inhibition was statistically greater than the attenuation of 2',3'-cAMP-induced inhibition.
Figure 4.
Line graphs show the concentration-dependent effects of exogenous 2',3'-cAMP and 3',5'-cAMP on [3H]-thymidine incorporation and cell number in PGVSMCs and GMCs. Bar graphs show the effects of DPCPX (A1 antagonist), SCH-58261 (A2A antagonist), MRS-1724 (A2B antagonist) and VUF-5574 (A3 antagonist) on inhibition of growth ([3H]-thymidine incorporation) by 2',3'-cAMP and 3',5'-cAMP in PGVSMCs and GMCs. aIndicates p<0.05 compared with basal (0) levels; bIndicates p<0.05 compared with 3',5'-cAMP at the same concentration; cIndicates p<0.05 compared with no inhibitor; dIndicates significantly (p<0.05) greater effect of MRS-1724 on 3',5'-cAMP compared with 2',3'-cAMP. P-values in figure are interaction term in 2-factor ANOVA.
Growth Effects of cAMPs in GMCs
In GMCs, both 2',3'-cAMP and 3',5'-cAMP significantly inhibited cell growth as assessed by either [3H]-thymidine incorporation or cell number (Fig. 4E and 4F). Inhibition of cell number was statistically significant for both cAMPs at the lowest concentration tested (1 μmol/L; 14% and 13% inhibition for 2',3'-cAMP and 3',5'-cAMP, respectively), and inhibition of cell number at the highest concentration tested (100 μmol/L) was 42% and 37% for 2',3'-cAMP and 3',5'-cAMP, respectively. For the [3H]-thymidine incorporation experiments, the inhibition by 2',3'-cAMP was statistically greater (p<0.0001; interaction term in 2-factor ANOVA in which one factor was cAMP type and the other factor was concentration of cAMP) than inhibition induced by 3',5'-cAMP. DPCPX, SCH-58261 and VUF-5574 (each at 0.1 μmol/L) did not antagonize the inhibitory effects of 2',3'-cAMP or 3',5'-cAMP (each at 30 μmol/L) on [3H]-thymidine incorporation (Fig. 4G and 4H). In contrast, MRS-1724 (0.1 μmol/L) significantly (p<0.0001) attenuated the reduction in [3H]-thymidine incorporation induced by 2',3'-cAMP and 3',5'-cAMP (Fig. 4G and 4H). The attenuation by MRS-1724 of 3',5'-cAMP-induced inhibition was statistically greater than the attenuation of 2',3'-cAMP-induced inhibition.
DISCUSSION
One goal of the present study was to determine whether PGVSMCs and GMCs express both the 2',3'-cAMP-adenosine and 3',5'-cAMP-adenosine pathways, and the results of the present study confirm this hypothesis. Another goal of this study was to investigate whether there is any overlap between the enzymes that mediate the extracellular 3',5'-cAMP-adenosine pathway and the enzymes that are responsible for the 2',3'-cAMP-adenosine pathway. The extracellular 3',5'-cAMP-adenosine pathway is mediated by an ecto-phosphodiesterase that is sensitive to inhibition by both IBMX and DPSPX and by CD73, an ecto-5'-nucleotidase that is sensitive to inhibition by AMPCP26. In both PGVSMCs and GMCs, IBMX inhibits the conversion of 3',5'-cAMP to 5'-AMP and to adenosine, but has little or no effect on the conversion of 2',3'-cAMP to 3'-AMP, 2'-AMP or adenosine. Thus the 2',3'-cAMP-phosphodiesterase that mediates the 2',3'-cAMP-adenosine pathway is distinct from the 3',5'-cAMP phosphodiesterase that mediates the 3',5'-cAMP-adenosine pathway. Because 3',5'-cAMP and 2',3'-cAMP are highly hydrophilic, these cAMPs would not be expected to penetrate cell membranes, and so the enzymes that metabolize these compounds must be ecto-enyzmes, i.e., ecto-2',3'-cAMP-phosphodiesterase and ecto-3',5'-cAMP-phosphodiesterase. In confirmation of this assertion, DPSPX, a methylxanthine that does not penetrate cell membranes, inhibits the ecto-3',5'-cAMP-phosphodiesterase in both PGVSMCs and GMCs. Consistent with the conclusion that ecto-3',5'-cAMP-phosphodiesterase and ecto-2',3'-cAMP-phosphodiesterase are separate enzymes, in PGVSMCs and GMCs, DPSPX has little or no effect on the conversion of 2',3'-cAMP to 3'-AMP. Importantly, in both PGVSMCs and GMCs, DPSPX somewhat reduces the conversion of 2',3'-cAMP to 2'-AMP. This suggests that there are two separate ecto-2',3'-cAMP-phosphodiesterases, one that metabolizes 2',3'-cAMP to 3'-AMP (i.e., ecto-2',3'-cAMP-2'-phosphodiesterase) and is resistant to inhibition by both IBMX and DPSPX, and a second that metabolizes 2',3'-cAMP to 2'-AMP (i.e., ecto-2',3'-cAMP-3'-phosphodiesterase) and is resistant to inhibition by IBMX, but is modestly inhibited by DPSPX. This finding may have important implications because if 3'-AMP and 2'-AMP have different biological effects apart from their conversion to adenosine, the relative expression of these two forms of ecto-2',3'-phosphodiesterase and their relative kinetic properties would determine whether extracellular 2',3'-cAMP is converted predominantly to 3'-AMP or to 2'-AMP.
Regarding the ecto-3',5'-cAMP-phosphodiesterase, to our knowledge whether this enzyme also metabolizes 3',5'-cAMP to 3'-AMP as well as 5'-AMP was until now unknown. The present study, however, clearly shows that ecto-3',5'-cAMP-phosphodiesterase is exclusively an ecto-3',5'-cAMP-3'-phosphodiesterase. Therefore, any 3'-AMP present in the extracellular compartment is most likely derived from ecto-2',3'-cAMP-2'-phosphodiesterase acting on extracellular 2',3'-cAMP.
The results of the present study show that in PGVSMCs and GMCs, AMPCP inhibits the conversion of extracellular 5'-AMP to extracellular adenosine, but has little or no effect on the metabolism of extracellular 3'-AMP or 2'-AMP to extracellular adenosine. We conclude, therefore, that the ecto-2'/3'-nucleotidase that mediates the conversion of 3'-AMP or 2'-AMP to adenosine is distinct from the ecto-5'-nucleotidase (CD73) that mediates the conversion of extracellular 5'-AMP to extracellular adenosine. It is conceivable that there exists distinct ecto-2'-nucleotidases and ecto-3'-nucleotidases and that there exists ecto-nucleotidases with activity against both 2'-AMP and 3'-AMP. However, the present results do not address this issue.
The fact that the extracellular 3',5'-cAMP pathway and the extracellular 2',3'-cAMP pathway are mediated by distinct ecto-enyzmes implies that small molecule inhibitors for each of the relevant ecto-enzymes could be developed to manipulate specifically the extracellular levels of 3',5'-cAMP, 2',3'-cAMP, 5'-AMP, 3'-AMP, 2'-AMP and adenosine. The utility of such inhibitors will become clearer as we learn more about the pharmacology of these purines and their biological roles in the extracellular compartment.
In PGVSMCs and GMCs, the increase in extracellular 3'-AMP and 2'-AMP induced by extracellular 2',3'-cAMP is much greater than the increase in extracellular 5'-AMP induced by 3',5'-AMP. In contrast, the increase in extracellular adenosine caused by extracellular 5'-AMP is much greater than the increase in adenosine caused by extracellular 3'-AMP and 2'-AMP. This implies that the rate-limiting step for the extracellular 3',5'-cAMP-adenosine pathway is the ecto-3',5'-cAMP-3'-phosphodiesterase, whereas the rate limiting step for the extracellular 2',3'-cAMP-adenosine pathway is the ecto-2'/3'-nucleotidase. Consequently, activation of the extracellular 2',3'-cAMP-adenosine pathway causes a greater increase in the intermediate AMPs, which could be of considerable importance if 3'-AMP and 2'-AMP have biological roles independent from adenosine formation.
The present study establishes for the first time that extracellular 2',3'-cAMP is an important modulator of cell growth, and is, in fact, more potent than extracellular 3',5'-cAMP in both PGVSMCs and GMCs. We postulate that the growth inhibitory effects of 2',3'-cAMP and 3',5'-cAMP are mediated in part by conversion of these cAMPs to AMPs with further processing of the AMPs to adenosine. This hypothesis is based on the facts that: 1) both PGVSMCs and GMCs metabolize extracellular 2',3'-cAMP and 3',5'-cAMP to adenosine; 2) adenosine inhibits growth of vascular smooth muscle cells, GMCs and cardiac fibroblasts10–15, 27–29; and 3) MRS-1724 attenuates the growth inhibitory effects of both 2',3'-cAMP and 3',5'-cAMP.
Because MRS-1724 is a selective A2B antagonist22, our results imply that adenosine produced by metabolism of the extracellular cAMPs inhibits growth of both cell types via A2B receptors. These results are consistent with our previous findings that A2B receptors mediate the growth inhibitory effects of adenosine and extracellular 3',5'-cAMP12, 13, 15, 16, 27–29. Importantly, the results of the present study show that neither DPCPX, SCH-58261 nor VUF-5574 attenuate the growth inhibitory effects of either 2',3'-cAMP or 3',5'-cAMP, findings that eliminate any involvement of A1, A2A or A3 receptors in the growth inhibitory effects of either 2',3'-cAMP or 3',5'-cAMP.
In PGVSMCs and GMCs, extracellular 2',3'-cAMP is more potent as a growth inhibitor compared with extracellular 3',5'-cAMP. The reason for this differential potency is unknown, but probably is due to direct pharmacological effects of 2',3'-cAMP (or 2'-AMP/3'-AMP) that are not expressed by 3',5'-cAMP (or 5'-AMP). The basis for this conclusion is that: 1) 2',3'-cAMP and 3',5'-cAMP appear to be equally effective in generating adenosine; and 2) MRS-1724 nearly fully prevents growth inhibition by 3',5'-cAMP, but only partially blocks growth inhibition by 2',3'-cAMP.
It is noteworthy that the growth inhibitory effects of extracellular 2',3'-cAMP are profound, generally inhibiting growth by approximately 50% at a concentration of 100 μmol/L. Importantly, even very low concentrations of 2',3'-cAMP can inhibit growth (levels less than 1 μmol/L). Calculations show that the degradation of mRNA (the source of 2',3'-cAMP) could readily achieve such concentrations of 2',3'-cAMP in the local cellular environment3.
Perspective
Neuroendocine activation or renal injury can induce pathological proliferation of PGVSMCs or GMCs leading to renal dysfunction, which in turn can contribute to the development and maintenance of hypertension, thus leading to further renal injury. As illustrated in Fig. S2 (please see http://hyper.ahajournals.org), both the 2',3'-cAMP-adenosine and 3',5'-cAMP-adenosine pathways may afford protection against these processes. The neuroendocrine system may activate the 3',5'-cAMP-adenosine pathway via stimulation of adenylyl cyclase to form 3',5'-cAMP and renal injury may activate the 2',3'-cAMP-adenosine pathway via breakdown of mRNA to release 2',3'-cAMP. These pathways, separately or in concert and possibly in combination with other mechanisms of adenosine production, would produce adenosine which via the A2B receptor would inhibit pathological remodeling of renovascular and glomerular structures. Thus, both endogenous 2',3'-cAMP and 3',5'-cAMP via adenosine may play an important role to protect against renal disease and hypertension, and drugs that modulate the extracellular 2',3'-adenosine or 3',5'-adenosine pathways may find utility in the prevention and treatment of renal diseases and hypertension.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING The work was supported by the National Institutes of Health [HL069846, DK068575, DK079307], the Swiss National Science Foundation [3200B0-106098/1, 320000-117998/1], and Oncosuisse [OCS-01551-08-2004], EMDO Stiftung.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther. 2008;325:920–926. doi: 10.1124/jpet.108.137752. [DOI] [PubMed] [Google Scholar]
- 2.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2',3'-cAMP release by the kidney. J Pharmacol Exp Ther. 2009;328:855–865. doi: 10.1124/jpet.108.146712. PMC2646794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson EK, Ren J, Mi Z. Extracellular 2',3'-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. PMC2785151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther. 1997;283:177–182. [PubMed] [Google Scholar]
- 5.Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther. 2000;295:23–28. [PubMed] [Google Scholar]
- 6.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Extracellular 3',5'-cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth. J Pharmacol Exp Ther. doi: 10.1124/jpet.110.166371. doi:10.1124/jpet.110.166371 http://jpet.aspetjournals.org/content/early/2010/03/01/jpet.110.166371.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal. 2003;285:F590–599. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 9.Inscho EW. Purinoceptor-mediated regulation of the renal microvasculature. J Auton Pharmacol. 1996;16:385–388. doi: 10.1111/j.1474-8673.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits PDGF-induced growth of human glomerular mesangial cells via A2B receptors. Hypertension. 2005;46:628–634. doi: 10.1161/01.HYP.0000178464.63393.88. [DOI] [PubMed] [Google Scholar]
- 11.Dubey RK, Gillespie D, Mi Z, Suzuki F, Jackson EK. Smooth muscle cell-derived adenosine inhibits cell growth. Hypertension. 1996;27:766–773. doi: 10.1161/01.hyp.27.3.766. [DOI] [PubMed] [Google Scholar]
- 12.Dubey RK, Gillespie D, Osaka K, Suzuki F, Jackson EK. Adenosine inhibits growth of rat aortic smooth muscle cells: possible role of A2b receptor. Hypertension. 1996;27:786–793. doi: 10.1161/01.hyp.27.3.786. [DOI] [PubMed] [Google Scholar]
- 13.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension. 1998;31:516–521. doi: 10.1161/01.hyp.31.1.516. [DOI] [PubMed] [Google Scholar]
- 14.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and total protein synthesis in vascular smooth muscle cells. Hypertension. 1999;33:190. doi: 10.1161/01.hyp.33.1.190. [DOI] [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Shue H, Jackson EK. A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension. 2000;35:267–272. doi: 10.1161/01.hyp.35.1.267. [DOI] [PubMed] [Google Scholar]
- 16.Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension. 1998;31:296–302. doi: 10.1161/01.hyp.31.1.296. [DOI] [PubMed] [Google Scholar]
- 17.Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 18.Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther. 1991;256:850–860. [PubMed] [Google Scholar]
- 19.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther. 1995;273:728–733. [PubMed] [Google Scholar]
- 20.Zacher LA, Carey GB. cAMP metabolism by swine adipocyte microsomal and plasma membranes. Comp Biochem Physiol B. 1999;124:61. doi: 10.1016/s0305-0491(99)00098-x. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptor ligands. In: Abbracchio MP, Williams M, editors. Purinergic and Pyrmidinergic Signalling I. Springer-Verlag; Berlin: 2001. pp. 129–175. [Google Scholar]
- 23.van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von Drabbe Kunzel J, de Groote M, Ijzerman AP. Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A3 receptor. J Med Chem. 2000;43:2227–2238. doi: 10.1021/jm000002u. [DOI] [PubMed] [Google Scholar]
- 24.Mokkapatti R, Vyas SJ, Romero GG, Mi Z, Inoue T, Dubey RK, Gillespie DG, Stout AK, Jackson EK. Modulation by angiotensin II of isoproterenol-induced cAMP production in preglomerular microvascular smooth muscle cells from normotensive and genetically hypertensive rats. J Pharmacol Exp Ther. 1998;287:223–231. [PubMed] [Google Scholar]
- 25.Inoue T, Mi Z, Gillespie DG, Jackson EK. Cyclooxygenase inhibition reveals synergistic action of vasoconstrictors on mesangial cell growth. Eur J Pharmacol. 1998;361:285–291. doi: 10.1016/s0014-2999(98)00720-1. [DOI] [PubMed] [Google Scholar]
- 26.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol. 2004;66:571–599. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- 27.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation. 1997;96:2656–2666. doi: 10.1161/01.cir.96.8.2656. [DOI] [PubMed] [Google Scholar]
- 28.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: potential role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.hyp.31.4.943. [DOI] [PubMed] [Google Scholar]
- 29.Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension. 2001;37:716–721. doi: 10.1161/01.hyp.37.2.716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.