Abstract
Solid phase oligosaccharide synthesis (SPOS) offers the promise to provide libraries of oligosaccharides for glycomics research. A major stumbling block to SPOS has been a lack of general methods to stereoselectively install 1,2-cis-glycosides, and intractable mixtures of compounds will be obtained if several of such glycosides need to be installed. We have prepared on-resin a biologically important glucoside containing multiple 1,2-cis-glycosidic linkages with complete anomeric control by using glycosyl donors having a participating (S)-(phenylthiomethyl)benzyl chiral auxiliary at C-2. A branching point could be installed by employing 9-fluorenylmethyloxycarbonyl (Fmoc) and allyloxycarbonyl (Alloc) as a versatile set of orthogonal protecting groups. The synthetic strategy made it possible for partial on-resin deprotection of the completed oligosaccharide thereby increasing the overall efficiency of the synthesis. The combination of classical and auxiliary mediated neighboring group participation for controlling anomeric selectivity is bringing the promise of routine automated solid supported oligosaccharides synthesis closer.
As many as 50% of human proteins are O- or N-glycosylated and the carbohydrate moieties of these glycoproteins have been implicated as essential mediators of cellular processes such as protein folding, regulation of cell signaling, fertilization, embryogenesis, neuronal development and hormone activities.1 However, carbohydrates are also important for pathogen recognition, modulation of innate immune responses, control of immune cell homeostasis, inflammation, and the development of autoimmune diseases and cancer.2–4 The ability of cells to generate information rich glycans has created a new field of research termed "glycomics", which seeks to identify and understand the processes involved in the formation of cell type and developmental stage specific oligosaccharide patterns.5–8 In this respect, collections of well-defined oligosaccharides are needed for the development of algorithms for the assignment of oligosaccharide MS spectra, for fabricating microarrays, for elucidating the biosynthetic pathways of glycoconjugate assembly, and as immunogens to produce monoclonal antibodies (MABs) for glycoprotein visualization and isolation by immunoprecipitation. In many cases, well-defined oligosaccharides can only be obtained by chemical or enzymatic approaches and although tremendous progress has been made, complex oligosaccharide synthesis is still very time consuming and it is not uncommon that the preparation of a single well-defined derivative can take as much as a year.9–13 Solid phase oligosaccharide synthesis (SPOS) offers the promise to increase the speed of oligosaccharide assembly, primarily by eliminating intermediate purification steps and by automation.14 However, SPOS requires that each glycosidic linkage is introduced with absolute stereoselectivity otherwise an intractable mixture of compounds will be obtained after several reaction cycles.
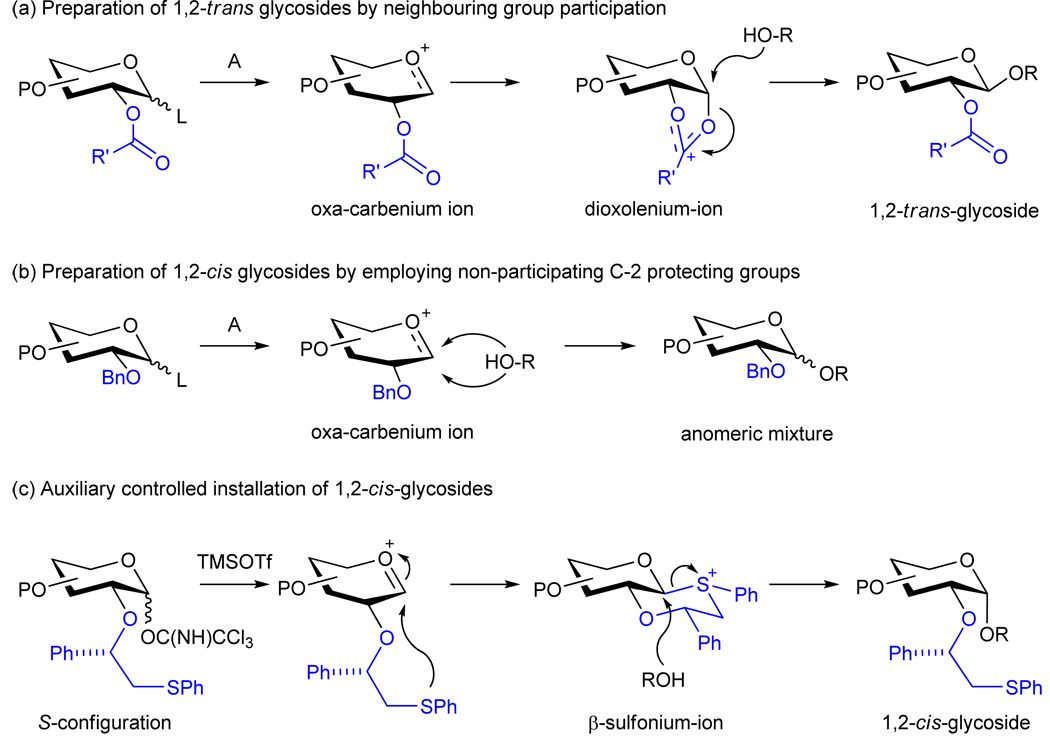
In this respect, 1,2-trans glycosides can be reliably introduced by exploiting neighboring group participation of a 2-O-acyl functionality (Figure 1a). The Seeberger group exploited this type of anomeric control for the automated solid phase synthesis of a phytoalexin eliciltor β-glycan using a modified peptide synthesizer.15 The introduction of 1,2-cis glycosidic linkages, such as α-glucosides and α-galactosides, requires glycosyl donors having a non-assisting functionality at C-2, and in general these glycosylations give mixtures of anomers (Figure 1b).16 Not surprisingly, only a few examples of SPOS of oligosaccharides containing 1,2-cis glycosides have been reported, which rely on tedious separation of the anomers by HPLC or the preparation of a 1,2-cis-linked disaccharide in solution, which after purification to remove the unwanted 1,2-trans-anomer, can be employed in solid phase synthesis.17–19 Thus, a major stumbling block in SPOS is the inability to reliably introduce 1,2-cis glycosides with complete stereoselectivity.
Figure 1.
Stereoselective introduction of glycosidic linkages. a, Preparation of 1,2-trans-glycosides by neighboring group participation of a C-2 ester. Activation of an anomeric-leaving group by a promoter results in its departure and the formation of an oxacarbenium ion. Subsequent, neighboring group participation by the 2-O-acyl protecting group will give a more stable five-membered dioxolenium-ion, which can only be formed as a 1,2-cis fused ring system. An alcohol can attack the anomeric center of the dioxolenium -ion from only one face providing a 1,2-trans-glycoside. b, The use of a non-participating protecting groups at C-2 of a glycosyl donor generally results in the formation of mixture of anomers although the axial glycoside often predominates. c, Chiral auxiliary controlled installation of 1,2-cis-glycosides. An intermediate β-sulfonium ion forces the incoming alcohol to attack the α-face resulting in the stereoselective introduction of a 1,2-cis-glycoside.
Herein, we report the solid phase synthesis of a well-defined biologically important branched 1,2-cis-linked penta-glucoside. Anomeric control was achieved by employing neighboring group participation by a (S)-(phenylthiomethyl)benzyl chiral auxiliary at C-2 of the glucosyl donors.20,21 In this approach, neighboring group participation by the C-2 auxiliary results in the formation of an anomeric sulfonium ion as a trans-decalin system because the alternative cis-decalin system will place the phenyl-substituent in an axial position inducing unfavorable steric interactions. Displacement of the equatorial anomeric sulfonium ion by a sugar alcohol will then lead to the formation of a 1,2-cis glycoside (Figure 1c). Furthermore, a branching unit could easily be installed by using the 9-fluorenylmethyloxycarbonyl (Fmoc) and allyloxycarbonyl (Alloc) as a versatile set of orthogonal protecting groups.22,23 Also, the synthetic strategy made it possible for partial on-resin deprotection of the completed oligosaccharide thereby increasing the overall efficiency of the synthesis.
Results and Discussion
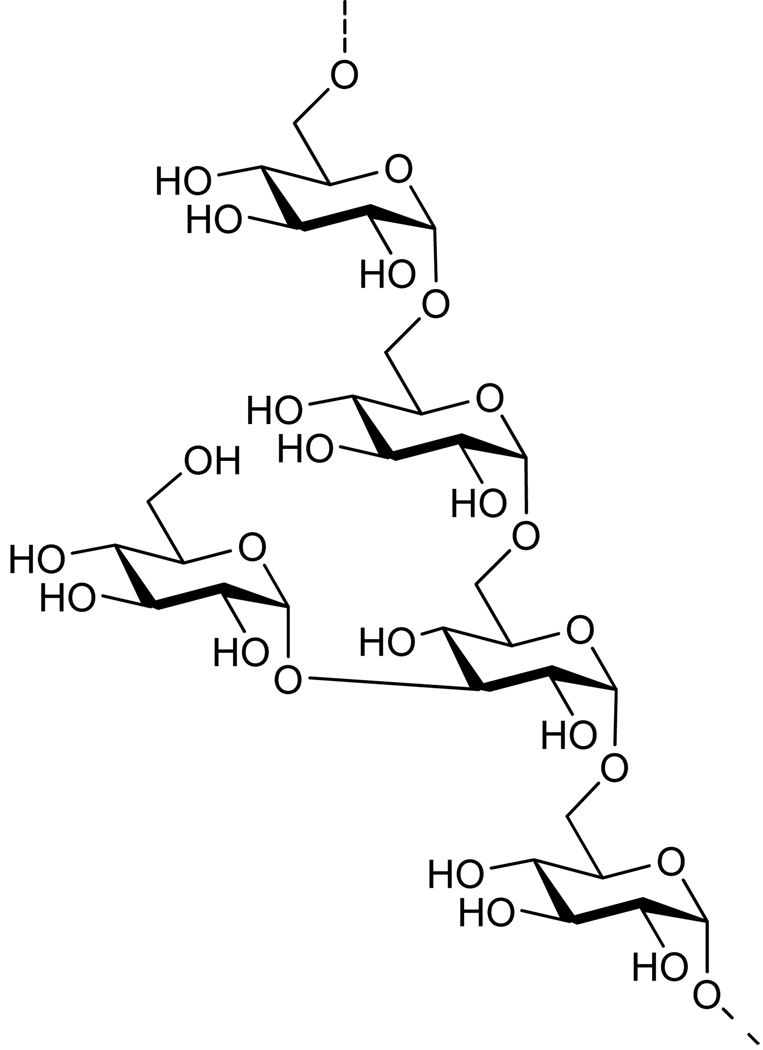
α-Glucans (Figure 2) are structural elements of an immuno-modulatory polysaccharide isolated from Aconitum carmichaeli that has the potential to be developed as an adjuvant.24 The polysaccharide is composed of an α(1,6)-linked glucosyl backbone branched with α(1,3)-linked glucoside moieties. α-Glucans have also been isolated from various microbial sources such as Pseudallescheria boydii, which produced a polysaccharide that was essential for conidial phagocytosis by macrophages and induction of innate immune responses in a TLR2 dependent manner.25 Furthermore, it has been found that the Streptococcus pneumoniae α-glucan metabolizing machinery is an important virulence factor.26 α-Glucans isolated from natural sources are heterogeneous in composition and well-defined derivatives are required to identify biologically active fragments.
Figure 2.
Structure of an α-glucan pentasaccharide repeating unit found in Aconitum carmichaeli. This adjuvant candidate is a significant synthetic challenge for SPOS due to the multiple α-1,6-linked glucosides in the backbone and the α-1,3-glucosidic branching point.
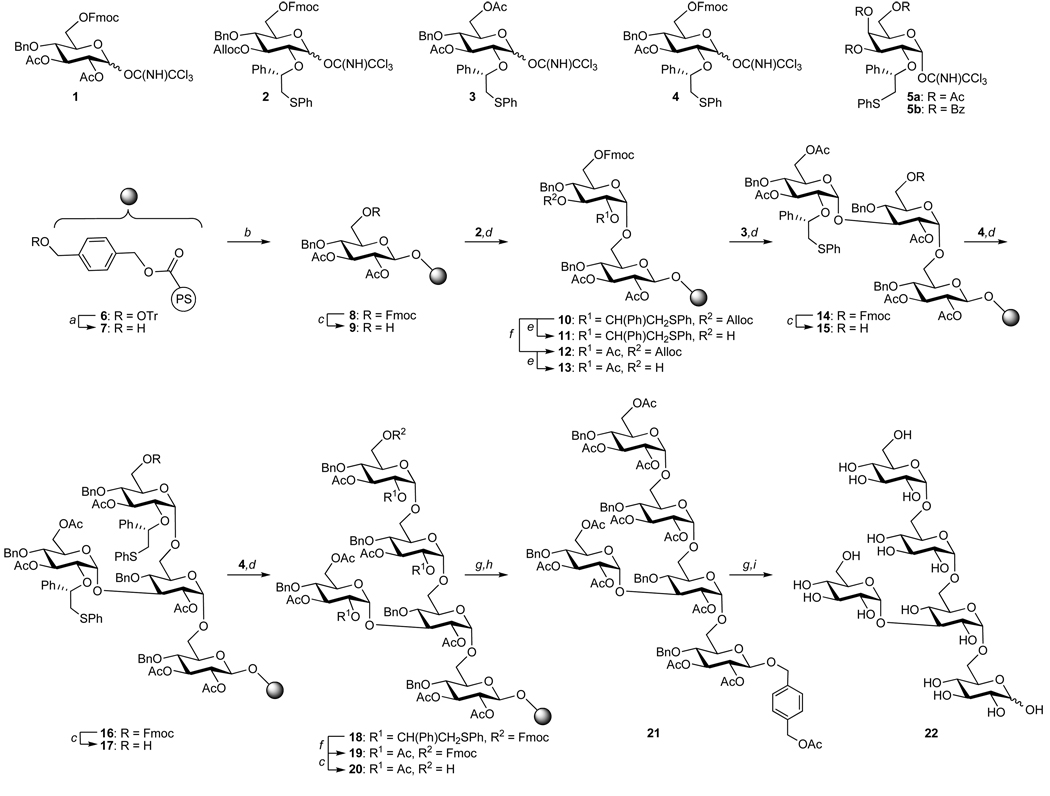
It was envisioned that pentasaccharide 22, which represents a significant synthetic challenge due to the presence of multiple α-glucosides at primary and secondary sugar alcohols and branched architecture, could be assembled from the four strategically selected glycosyl donors 1–4 and polystyrene modified resin 7 (Figure 3). In addition, galactosides 5a/b were expected to provide opportunities to prepare structural analogs. Glycosyl donors 2–5a/b are modified at C-2 with a (S)-(phenylthiomethyl)benzyl chiral auxiliary, and this functionality will ensure the stereoselective formation of α-glycosides.21 Furthermore, glycosyl donor 1, which is modified with an acetyl ester at C-2, will be coupled with the benzyl alcohol linker modified resin 7 to give resin bound β-linked glucoside 8. After completion of the synthesis, the resulting oligosaccharide can be cleaved from the resin by transesterification and the remaining anomeric 4-hydroxymethyl-benzyl ether can be removed during the final hydrogenation step providing the anomeric lactol. Thus, the anomeric identity of the glycosidic linkage will be lost and therefore it was installed as a straightforward β-glucoside. The temporary Fmoc carbonate of 2 and 4 can be cleaved under mild conditions using piperidine/DMF (1/9, v/v) and the use of this protecting group will make it possible to form the α(1,6)-linked backbone. Furthermore, monosaccharide building block 2 has a temporary Alloc carbonate at C-3, which can be removed with Pd(PPh3)4 in a mixture of THF and AcOH without affecting the Fmoc protecting group or the anomeric linker and allows for installment of the branching unit.
Figure 3.
The stereoselective solid supported synthesis of pentasaccharide 22 using monosaccharide building blocks 1–5 and linker modified resin 7. Reagents and conditions: a) TFA/CH2Cl2 (1/9, v/v), Et3SiH, 10 min, rt. b) 1, TMSOTf, CH2Cl2, MS4Å, 30 min, −40°C, double coupling. c) piperidine/DMF (1/9, v/v), 5 min, rt. d) 2, 3 or 4, TMSOTf, CH2Cl2, MS4Å, 15 min, −40°C then added to 9, 13, 15 or 17, DTBMP, CH2Cl2, MS4Å, 16 h −40°C → rt, double coupling. e) Pd(PPh3)4 (40 mol%), THF/AcOH (10/1, v/v), 16 h, rt. f) BF3˙Et2O, Ac2O/CH2Cl2 (1/2, v/v), 16 h, 0°C. g) NaOMe, MeOH/CH2Cl2 (1/1, v/v), 9 h for 21, 16 h for 22, rt. h) Ac2O/pyridine (1/3, v/v), 12 h, rt i) Pd(OH)2/C (20 wt%), H2, EtOH/H2O (1/1, v/v), 16 h, rt. MS = molecular sieves, PS = polystyrene, rt = room temperature, Tr = Trityl.
Thus, linker-modified resin 6 was prepared by ester formation between the carboxylic acids of carboxypolystyrene resin (Advanced ChemTech™, 2.0 mmol g−1) and (4-trityloxymethyl-phenyl)-methanol using N,N′-diisopropylcarbodiimide (DIC) and N,N-dimethyl-4-aminopyridine (DMAP). The remaining carboxylic acids were capped as methyl esters by subsequent addition of methanol. The trityl ether was removed using trifluoroacetic acid (TFA) in CH2Cl2 (1/9, v/v) and triethylsilane as the scavenger to afford 7 (loading 0.148 mmol g−1).27 It is important to note that different resin loadings could be achieved by employing different molar quantities of (4-trityloxymethyl-phenyl)-methanol. However, it was found that a loading of approximately 0.15 mmol g−1 resin gives optimal glycosylation results and further increases in loading led to decreases in coupling efficiencies.27,28
Next, the hydroxyl of resin 7 was coupled with glycosyl donor 1 (3.0 equivalents) in the presence of a catalytic amount of trimethylsilyl trifluoromethanesulfonate (TMSOTf) in CH2Cl2 at −40°C for 30 min to afford 8. The glycosylation was repeated to ensure completion of the reaction. Next, the Fmoc protecting group of 8 was removed by treatment with piperidine/DMF (1/9, v/v) to give resin bound acceptor 9, which was coupled with auxiliary containing glucosyl donor 2 to install the first 1,2-cis linkage. Thus, for this coupling, glucosyl donor 2 was preactivated in a separate flask with a stoichiometric amount of TMSOTf at −40°C to form an intermediate sulfonium ion. The solution containing the sulfonium ion was added via cannula to a cooled (−40°C) suspension of resin 9 and 2,6-di-tert-butyl-4-methylpyridine (DTBMP) in CH2Cl2. The reaction mixture was allowed to warm to rt over a period of 5 h and was shaken at ambient temperature for an additional 11 h. After this period of time, a small sample of resin (approximately 5.0 mg) was analyzed for product formation and the possible presence of starting material by treatment with methanolic sodium methoxide to cleave the ester linkage of the linker followed by analysis of the released product by TLC and MALDI-TOF. This study showed that mainly the disaccharide product had been formed but also the presence of a small amount of starting material (≤5%). Therefore, the glycosylation was repeated under identical conditions to ensure that a homogeneous product would be formed at the end of the synthetic sequence.
The Alloc function of 10 was easily removed by treatment with Pd(PPh3)4 in a mixture of THF and AcOH to give disaccharide acceptor 11. Interestingly, attachment of 10 to the insoluble polymeric support did not appear to influence the outcome of the deprotection reaction. A number of attempts were made to couple the resulting glycosyl acceptor with glycosyl donor 3. However, analysis of a small sample of resin indicated that no coupling had occurred. Subsequent model studies using solution phase chemistry indicated that the C-3′ hydroxyl of 11 was sterically shielded due to the neighboring (S)-(phenylthiomethyl)benzyl ether. Thus, the auxiliary of 10 was converted into acetyl ester 12 by the treatment with acetic anhydride in the presence of BF3˙OEt2. The Alloc of compound 12 could be removed under standard conditions to provide glycosyl acceptor 13, which was coupled with preactivated 3 to give smooth formation of resin bound trisaccharide 14. Thus, it was found that the auxiliary can be removed without affecting the Alloc and Fmoc protecting groups as well as the anomeric linker. The terminal Fmoc of 14 was cleaved by treatment with piperidine/DMF (1/9, v/v) and the resulting alcohol was glycosylated with preactivated 4 to afford tetrasaccharide 16. The reaction sequence of Fmoc removal and glycosylation was repeated to afford the fully protected pentasaccharide 18. Each glycosylation was performed twice with 2.0 equivalents of glycosyl donor to ensure complete conversion of the starting material. After each reaction step, a small sample of resin was treated with methanolic sodium methoxide and the resulting released material analyzed by TLC and MALDI-TOF MS. These studies showed clearly that each reaction step proceeded to completion with no or very little formation of side products. Furthermore, the pentaglucoside was also prepared in solution and careful examining of the products of each glycosylation confirmed complete α-anomeric selectivity (See supporting information).
Next, we explored whether pentasaccharide 18 could be partially deprotected when still attached to the resin. Thus, the auxiliaries of 18 were converted in the acetyl ester by treatment with acetic anhydride and BF3˙OEt2 in CH2Cl2 to give 19. Next, the Fmoc carbonate was removed with piperidine/DMF (1/9, v/v) to give 20, which was released from the polymeric support with concomitant acetyl ester removal using methanolic sodium methoxide in CH2Cl2. The crude pentasaccharide was re-acetylated and then purified by size exclusion chromatography (LH-20). HPLC analysis of the resulting product showed 21 as the major products and the presence of a small amount to monodebenzylated material (~10%), which was probably formed during removal of the auxiliaries. Importantly, no anomeric isomers of 21 were detected (see supporting information S34). Further purification by preparative HPLC gave pure 21 in an overall yield of 25%, which corresponds to a yield per step of 90% (thirteen on-resin steps).
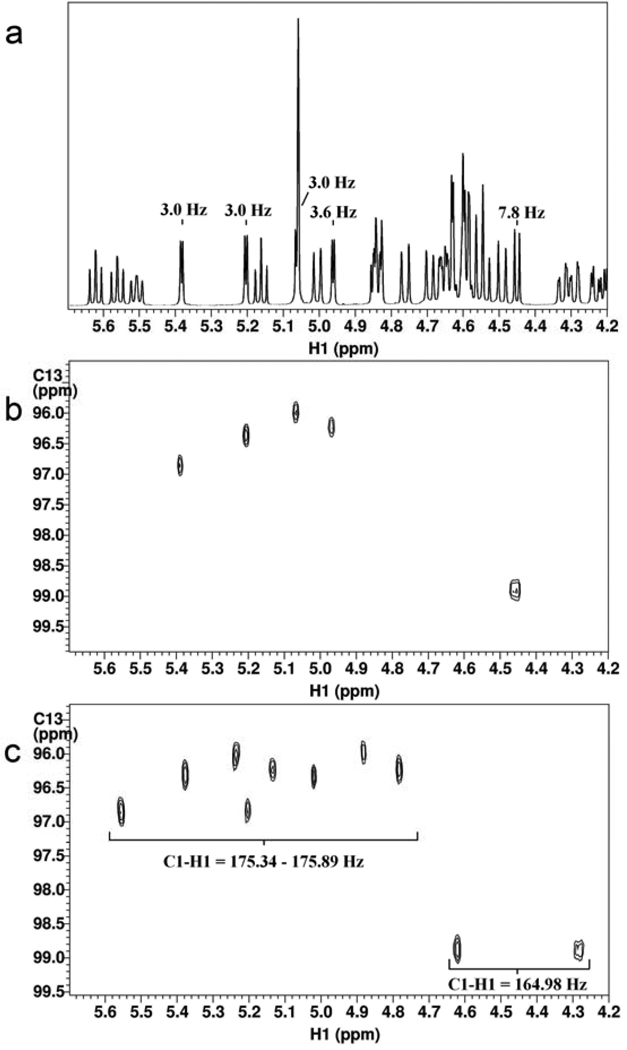
The identity and purity of 21 was confirmed by 1H NMR and coupled HSQC experiments (Figure 4). The homonuclear anomeric coupling constants as well as the heteronuclear one bond C1–H1 coupling constants unambiguously confirmed the presence of four 1,2-cis linkages and one 1,2-trans linkage. COSY, TOCSY and HSQC experiments were used for full spectral assignment (see supporting information S30, S31). Furthermore, an HMBC experiment confirmed the appropriate connectivity between the individual monosaccharides (see supporting information S32). Finally, 21 was converted into target compound 22 by removal of the acetyl esters using standard conditions followed by hydrogenation of the benzyl ethers using Pd(OH)2/C (20% wt) and H2 gas.
Figure 4.
NMR data of pentasaccharide 21: a) 1H NMR spectrum b) decoupled HSQC c) coupled HSQC. The homonuclear and heteronuclear coupling constants confirm the correct anomeric configuration of the product.
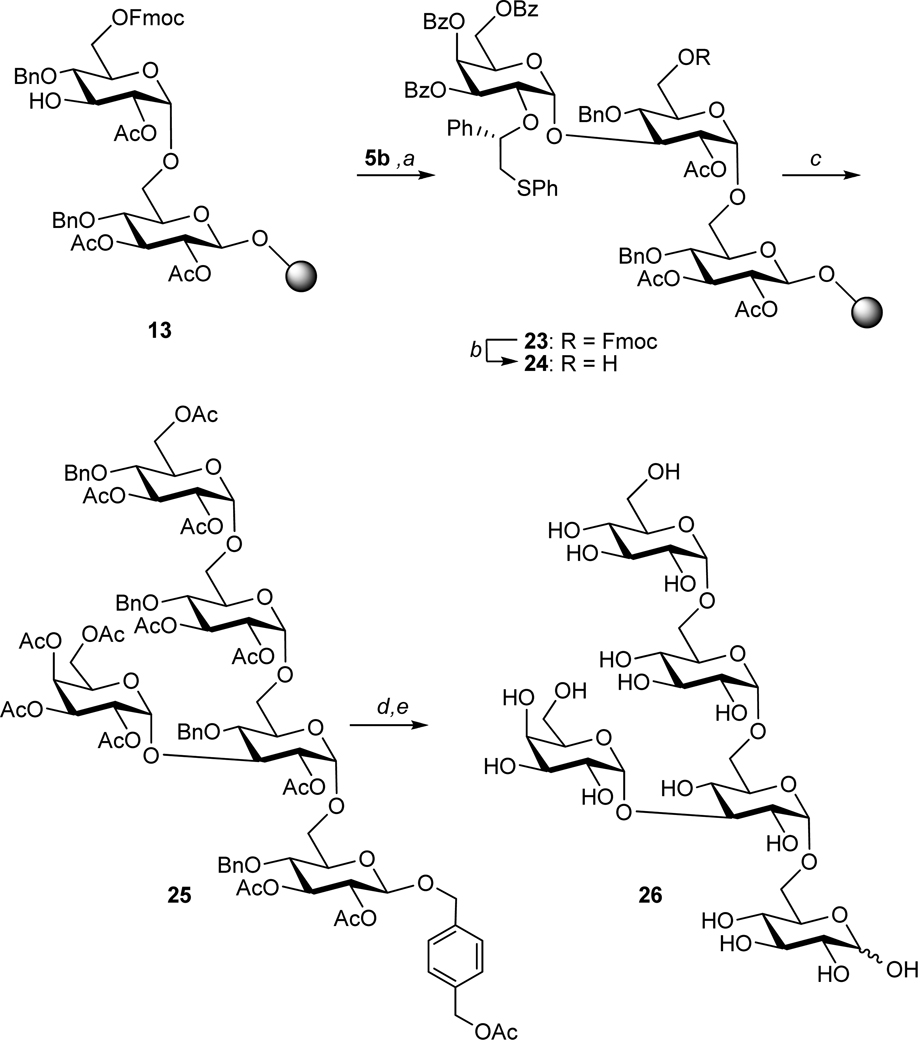
Next, attention was focused on the preparation of pentasaccharide 26 to demonstrate that the methodology can be extended to the stereoselective introduction of other types of monosaccharides and can be employed for the preparation of complex structural analogs (Figure 5). Compound 26 contains an α-galactoside at the C-3 branching position, which is challenging to introduce due the relative low reactivity of the corresponding glycosyl acceptor. Furthermore, branching points of α-glucans are expected to be critical for biological activity and hence compounds such as 26 provide an opportunity to explore the influence of subtle structural changes on biological activity.
Figure 5.
The stereoselective solid supported synthesis of galactoside containing analogue 26. The successful preparation of this derivative demonstrates that the methodology can be employed for a variety of glycosyl donors. Reagents and conditions: a) 5b, TfOH, CH2Cl2, MS4Å, 15 min, −40°C then added to 13, DTBMP, CH2Cl2, MS4Å, 16 h −40°C → rt, double coupling. b) piperidine/DMF, (1/9 v/v), 5 min, rt. c) same sequence and reagents as used for the conversion of 15 to 20. d) NaOMe, MeOH/CH2Cl2 (1/1, v/v), 8 h, rt. e) Pd(OH)2/C (20 wt%), H2, EtOH/H2O (1/1, v/v), 16 h, rt. MS = molecular sieves, rt = room temperature.
Solution phase studies showed that glycosylations with galactosyl donors 5a,b led to the exclusive formation of the corresponding α-galactosides. It was found that the sulfonium ion of the tri-O-benzoyl protected derivative 5b was stable at ambient temperature whereas acetylated derivative 5a required glycosylation at −20°C for optimal results. Thus, the more convenient to use galactosyl donor 5b was activated with a stochiometric amount of TfOH at −40°C to form the intermediate sulfonium ion, which was added to a cooled (−40°C) suspension of resin bound 13 and 2,6-di-tert-butyl-4-methylpyridine (DTBMP) in CH2Cl2 (Figure 5). The reaction mixture was allowed to warm to rt over a period of 5 h and was shaken at ambient temperature for an additional 11 h. A repetition of the glycosylation led to clean formation of trisaccharide 23 as judged by TLC and MS analysis of a small sample of oligosaccharide released from the resin by NaOMe treatment. Removal of the Fmoc protecting group of 23 followed by glucosylation with 4 and a repetition of the reaction sequence led to the formation of polymer bound pentasaccharide, which was partially deprotected and released from the resin employing standard procedures. Purification as before afforded anomerically pure 25 in an overall yield of 13%, which corresponds to a yield per step of 86% (thirteen on-resin steps). The same analytical procedures were used to confirm the purity and identity of 25 (see supporting information S36– S41). Finally, 25 was deprotected under the same conditions used before to afford 26.
In conclusion, glycosyl donors having a (S)-(phenylthiomethyl)benzyl chiral auxiliary at C-2 have been successfully employed for the solid supported synthesis of complex branched oligosaccharides. To the best of our knowledge, this is the first example of a stereoselective solid supported synthesis of an oligosaccharide having multiple 1,2-cis glycosidic linkages. A particular interesting feature was that a relatively small excess of glycosyl donor was required to drive the glycosylations to completion. Probably, the intermediate sulfonium ion is sufficiently stable to diffuse into the polymer support for glycosylation of the resin-bound sugar hydroxyls. Furthermore, it has been found that Fmoc and Alloc is an attractive set of orthogonal protecting groups for solid supported synthesis, which is compatible with the auxiliary based glycosylation methodology. Deprotection of the fully assembled oligosaccharide could partially be performed when still attached to the resin thereby further reducing the number of purification steps. The convenient protection of monosaccharides by a one-pot multi-step approach,29 combined with classical and auxiliary mediated neighboring group participation for controlling anomeric selectivity, is bringing the promise of routine automated solid supported oligosaccharides synthesis closer. Such an approach can deliver libraries of well-defined oligosaccharides needed for glycomics research.
Methods
General procedures
1H and 13C NMR spectra were recorded on a Varian inova-300 (300/75 MHz), a Varian inova-500 (500/125 MHz) and a Varian inova-600 (600/150 MHz) spectrometer equipped with sun workstations. Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane (TMS) as the internal standard. NMR data is presented as follows: Chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, dd = doublet of doublet, m = multiplet and/or multiple resonances), coupling constant in Hertz (Hz), integration. All NMR signals were assigned on the basis of 1H NMR, 13C NMR, COSY and HSQC experiments. HPLC analysis was performed using an Agilent Technologies 1200 series HPLC system with UV detection at 250 nm. Optical rotations were measured using a Jasco P-1020 polarimeter. Mass spectra were recorded on an Applied Biosystems 4700 MALDI-TOF proteomics analyzer. The matrix used was 2,5-dihydroxy-benzoicacid (DHB) and ultamark 1621 as the internal standard. Column chromatography was performed on silica gel G60 (Silicycle, 60–200 µm, 60 Å). TLC-analysis was conducted on Silicagel 60 F254 (EMD Chemicals inc.) with detection by UV-absorption (254nm) were applicable, and by spraying with 20% sulfuric acid in ethanol followed by charring at ~150°C or by spraying with a solution of (NH4)6Mo7O24˙H2O (25 g/L) in 10% sulfuric acid in ethanol followed by charring at ~150°C. CH2Cl2 was freshly distilled from calcium hydride under nitrogen prior to use. Molecular sieves (4Å) were flame activated under vacuum prior to use. Solid phase reactions were shaken using an IKA labortechnik KS 125 shaker. All reactions were carried out under an argon atmosphere.
General procedure for on-resin glycosylation with glycosyl donor 1
Glycosyl donor (0.3 mmol) was added to a suspension of the resin-bound glycosyl acceptor (0.670 g, 0.1 mmol, 0.148 mmol g−1) and activated 4Å molecular sieves in CH2Cl2 (6 mL). The mixture was shaken for 15 min at rt before being cooled to −40°C. TMSOTf (8.15 µl, 0.045 mmol) was added at this temperature and the mixture was shaken for 30 min. The resin was decanted into a filter to remove the molecular sieves, washed with CH2Cl2 (2 × 5 mL), MeOH (2 × 5 mL), CH2Cl2 (2 × 5 mL), and MeOH (2 × 5 mL) followed by drying under vacuum in a desiccator for 16 h. This procedure was repeated to complete one coupling cycle.
General procedure for on-resin glycosylation with (S)-(phenylthiomethyl)benzyl containing glycosyl donor 2, 3, 4 or 5b
Glycosyl donor (0.2 mmol) was dissolved in CH2Cl2 (2 mL) and activated 4Å molecular sieves were added. The mixture was stirred for 15 min at rt before being cooled to −40°C. TMSOTf (36.2 µl, 0.2 mmol for 2,3 and 4) or TfOH (17.6 µl, 0.2 mmol for 5b) was added at this temperature and the mixture was stirred at −40°C for 15 min. The mixture containing the activated donor was transferred via cannula to a cooled (−40°C) flask containing the resin-bound glycosyl acceptor (0.670 g, 0.1 mmol, 0.148 mmol g−1), DTBMP (82.4 mg, 0.4 mmol), activated 4Å molecular sieves and CH2Cl2 (6 mL). The mixture was shaken and allowed to slowly warm to rt during 5 h after which it was shaken for an additional 11 h at rt. The resin was decanted into a filter to remove the molecular sieves, washed with CH2Cl2 (2 × 5 mL), MeOH (2 × 5 mL), CH2Cl2 (2 × 5 mL), and MeOH (2 × 5 mL) followed by drying under vacuum in a desiccator for 16 h. This procedure was repeated to complete one coupling cycle.
General procedure for Fmoc cleavage
The resin (0.670 g, 0.1 mmol, 0.148 mmol g−1) was allowed to swell in DMF (7.0 mL) for 5 min. Piperidine (0.7 mL) was added and the mixture was shaken for 5 min at rt. The resin was filtered, washed with CH2Cl2 (2 × 5 mL), MeOH (2 × 5 mL), CH2Cl2 (2 × 5 mL), and MeOH (2 × 5 mL) followed by drying under vacuum in a desiccator for 16 h.
General procedure for (S)-(phenylthiomethyl)benzyl cleavage
The resin (0.670 g, 0.1 mmol, 0.148 mmol g−1) was allowed to swell in CH2Cl2 (5 mL) for 5 min at rt. Acetic anhydride (3 mL) was added and the mixture was cooled to 0°C. BF3˙Et2O (50.0 µl 0.4 mmol) was added and the mixture was shaken at 0°C for 16 h. The resin was filtered, washed with CH2Cl2 (2 × 5 mL), MeOH (2 × 5 mL), CH2Cl2 (2 × 5 mL), and MeOH (2 × 5 mL) followed by drying under vacuum in a desiccator for 16 h.
General procedure for Alloc cleavage
The resin (0.670 g, 0.1 mmol, 0.148 mmol g−1) was allowed to swell in a mixture of THF (7 mL) and acetic acid (0.7 mL) for 5 min at rt. The solution was purged with argon gas for 2 min followed by the addition of Pd(PPh3)4 (46.2 mg, 0.04 mmol). The mixture was shaken for 16 h at rt. The resin was filtered, washed with THF (2 × 5 mL), MeOH (2 × 5 mL), THF (2 × 5 mL), and MeOH (2 × 5 mL) followed by drying under vacuum in a desiccator for 16 h.
General procedure for product cleavage from the resin
The resin (0.670 g, 0.1 mmol, 0.148 mmol g−1) was allowed to swell in CH2Cl2 (5 mL) for 5 min at rt. MeOH (5 mL) and NaOMe (27.0 mg, 0.5 mmol) was added and the mixture was shaken for 3 h at rt. The resin was filtered and washed with a mixture of CH2Cl2/MeOH 1/1 (4 × 5 mL). This procedure was repeated three times to ensure complete product cleavage and the combined filtrates were neutralized using Dowex® 50W X8-200 H+ resin. The resin was removed by filtration and the filtrate was concentrated under reduced pressure.
Supplementary Material
Acknowledgement
This research was supported by the National Institute of General Medicine (NIGMS) of the National Institutes of Health (Grant Numbers 2R01GM065248 and 2R01GM061761).
Footnotes
Author contributions. T.J.B, J-H.K and G.J.B conceived and designed the experiments. T.J.B carried out the solid supported synthesis of 22 and 26 and analyzed the results. J-H.K carried out the solution phase synthesis of a pentaglucoside. J.P carried out the solution synthesis of a galactoside containing trisaccharide. T.J.B and G.J.B wrote the paper.
References
- 1.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 4.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 5.Raman R, et al. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 6.Pilobello KT, Mahal LK. Deciphering the glycocode: the complexity and analytical challenge of glycomics. Curr. Opin. Chem. Biol. 2007;11:300–305. doi: 10.1016/j.cbpa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Timmer MS, Stocker BL, Seeberger PH. Probing glycomics. Curr. Opin. Chem. Biol. 2007;11:59–65. doi: 10.1016/j.cbpa.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 8.Laurent N, Voglmeir J, Flitsch SL. Glycoarrays--tools for determining protein-carbohydrate interactions and glycoenzyme specificity. Chem. Commun. 2008:4400–4412. doi: 10.1039/b806983m. [DOI] [PubMed] [Google Scholar]
- 9.Codee JDC, et al. Thioglycosides in sequential glycosylation strategies. Chem. Soc. Rev. 2005;34:769–782. doi: 10.1039/b417138c. [DOI] [PubMed] [Google Scholar]
- 10.Buskas T, Ingale S, Boons GJ. Glycopeptides as versatile tools for glycobiology. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Ye XS, Zhang LH. Oligosaccharide assembly by one-pot multi-step strategy. Org. Biomol. Chem. 2007;5:2189–2200. doi: 10.1039/b704586g. [DOI] [PubMed] [Google Scholar]
- 12.Boltje TJ, Buskas T, Boons GJ. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 2009;1:611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu XM, Schmidt RR. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 2009;48:1900–1934. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- 14.Seeberger PH. Automated oligosaccharide synthesis. Chem. Soc. Rev. 2008;37:19–28. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 15.Plante OJ, Palmacci ER, Seeberger PH. Automated solid-phase synthesis of oligosaccharides. Science. 2001;291:1523. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 16.Demchenko AV. Stereoselective chemical 1,2-cis O-glycosylation: from 'sugar ray' to modern techniques of the 21st century. Synlett. 2003:1225–1240. [Google Scholar]
- 17.Jonke S, Liu KG, Schmidt RR. Solid-phase oligosaccharide synthesis of a small library of N-glycans. Chem.-Eur. J. 2006;12:1274–1290. doi: 10.1002/chem.200500707. [DOI] [PubMed] [Google Scholar]
- 18.Werz DB, Castagner B, Seeberger PH. Automated synthesis of the tumor-associated carbohydrate antigens Gb-3 and Globo-H: Incorporation of alpha-galactosidic linkages. J. Am. Chem. Soc. 2007;129:2770–2771. doi: 10.1021/ja069218x. [DOI] [PubMed] [Google Scholar]
- 19.Codee JD, Krock L, Castagner B, Seeberger PH. Automated solid-phase synthesis of protected oligosaccharides containing beta-mannosidic linkages. Chem.-Eur. J. 2008;14:3987–3994. doi: 10.1002/chem.200701864. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Yang H, Boons GJ. Stereoselective glycosylations using chiral auxiliaries. Angew. Chem. Int. Ed. 2005;44:947–949. doi: 10.1002/anie.200461745. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Yang H, Park J, Boons GJ. A general strategy for stereoselective glycosylations. J. Am. Chem. Soc. 2005;127:12090–12097. doi: 10.1021/ja052548h. [DOI] [PubMed] [Google Scholar]
- 22.Zhu T, Boons GJ. A new set of orthogonal-protecting groups for oligosaccharide synthesis on a polymeric support. Tetrahedron: Asymmetry. 2000;11:199–205. [Google Scholar]
- 23.Nicolaou KC, Winssinger N, Pastor J, DeRoose F. A general and highly efficient solid phase synthesis of oligosaccharides. Total synthesis of a heptasaccharide phytoalexin elicitor (HPE) J. Am. Chem. Soc. 1997;119:449–450. [Google Scholar]
- 24.Zhao C, Li M, Luo Y, Wu W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydr. Res. 2006;341:485–491. doi: 10.1016/j.carres.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Bittencourt VC, et al. An alpha-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J. Biol. Chem. 2006;281:22614–22623. doi: 10.1074/jbc.M511417200. [DOI] [PubMed] [Google Scholar]
- 26.van Bueren AL, et al. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat. Struct. Mol. Biol. 2007;14:76–84. doi: 10.1038/nsmb1187. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Schmidt RR. Solid-phase synthesis of complex oligosaccharides using a novel capping reagent. J. Org. Chem. 2004;69:1853–1857. doi: 10.1021/jo0354239. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Grathwohl M, Schmidt RR. Efficient solid-phase synthesis of a complex, branched N-glycan hexasaccharide: use of a novel linker and temporary-protecting-group pattern. Angew. Chem. Int. Ed. 2002;41:4489–4493. doi: 10.1002/1521-3773(20021202)41:23<4489::AID-ANIE4489>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, et al. Regioselective one-pot protection of carbohydrates. Nature. 2007;446:896–899. doi: 10.1038/nature05730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.