Abstract
Atypical adenomatous hyperplasia (AAH) is postulated to be the earliest morphologic precursor lesion in lung carcinogenesis. The epidermal growth factor receptor (EGFR), one of the members of the Erb-2 family of receptors, is commonly expressed in non-small cell lung carcinoma (NSCLC). A subset of the patients with NSCLC has molecular abnormalities in the EGFR gene, including missense mutations and deletions and/or abnormal gene copy numbers, and the relative importance of each of these for patient outcome is an area of great interest. Recent reports show that EGFR mutations are rare or absent in AAH and are rare in bronchioloalveolar carcinoma (BAC). However, the EGFR gene copy number status in AAH is unknown. In this study, we examined the EGFR gene copy number status in lung adenocarcinomas, synchronous AAH, and BAC in surgical pathology resection specimens. EGFR gene copy number was analyzed by chromogenic in situ hybridization (CISH) using formalin fixed paraffin embedded tissue sections and EGFR probes as recommended by the manufacturer. A known positive case of high-grade glioma was used as a positive control. The results indicate that four of eight adenocarcinomas (50%) had more than five EGFR signals per nucleus, suggesting a gain in copy number. Interestingly, in four of nine cases of AAH (44.4%) more than three EGFR signals per nucleus were noted, with scattered cells showing up to 6 signals per nucleus. In addition, in five of 12 cases of BAC (42%), more than three EGFR signals per nucleus were noted. In the remaining cases two to three intranuclear dot-like peroxidase positive signals were present consistent with non-amplification of the gene. Our study reveals an abnormal EGFR gene copy gain in several cases of AAH. In our cohort, the rate of EGFR gene copy abnormalities in AAH appears similar to BAC and lower than in lung adenocarcinomas. These findings suggest that although EGFR gene copy abnormalities may be an early event in lung carcinogenesis, they are associated with tumor progression to invasive cancer and highlight the complexity of tumor morphogenesis.
Keywords: EGFR, lung cancer, chromogenic in situ hybridization, copy number
Introduction
Dysregulation of epidermal growth factor receptor (EGFR) signaling has been implicated in the pathogenesis of numerous carcinomas, most notably non-small-cell lung carcinoma (NSCLC) [1]. Recognized mechanisms of EGFR gain of function in NSCLC include somatic activating mutations in the exons encoding the tyrosine kinase domain [2] and EGFR gene amplification [3]. A hypothesis of multistep tumorigenesis of lung adenocarcinoma starting with atypical adenomatous hyperplasia (AAH), progressing to bronchioalveolar carcinoma (BAC), and ultimately to invasive adenocarcinoma has been proposed and widely considered [4-6]. Although there have been intensive efforts to characterize the molecular abnormalities of BAC and invasive adenocarcinoma, the molecular alterations of precursor AAH are an area of more recent investigation [7-11]. Furthermore, the genetic alterations in the EGFR signaling pathway, which have been shown to characterize the late-stage of certain lung adenocarcinomas, are not clearly defined in AAH [12]. Studies have identified somatic mutations of the EGFR gene in lung adenocarcinoma [13, 14]. However, EGFR mutations are relatively uncommon in AAH and likewise rare in BAC [8]. Similarly, copy number changes of EGFR have also been identified in adenocarcinomas [15, 16] but to our knowledge, EGFR copy number changes in AAH have not been demonstrated in detail [17].
In this study, we therefore examined the extent of copy number variations in patients with AAH, BAC, and invasive lung adenocarcinoma by chromogenic in situ hybridization (CISH). CISH utilizes a peroxidase reaction to detect the locus of interest and can be performed in the clinical immunohistochemistry laboratory on formalin fixed paraffin embedded archival tissue and interpreted by standard light microscopy [15]. Recent studies from our group and others’ have shown that CISH is a useful assay for detecting EGFR gene amplification in patients with NSCLCand has an important role for identification of patients with a high number of EGFR copies [15, 18, 19]. Furthermore, the reliability of CISH as a technique for detecting gene amplification has been established for HER-2 in breast cancer specimens [20-22]. We therefore used CISH to investigate whether early morphologically detectable lesions such as AAH manifest copy number changes in the EGFR locus. The specimens used in this study were from patients with lung adenocarcinomas and synchronous AAH, in addition to patients who exclusively had pure BAC.
Materials and methods
We studied ten consecutive patients who had NSCLC with synchronous AAH (seven of the ten) in their surgical pathology lung specimens, and 12 patients with BAC, who were treated at Brigham and Women’s Hospital between 1999 and 2004. This study was approved by the local In-stitutional Review Board. Routine hematoxylin and eosinstained slides from formalin-fixed, paraffin-embedded tissue sections were reviewed and classified according to the WHO criteria [5, 23] and staged according to the American Joint Committee on Cancer [24]. Representative areas containing carcinoma and AAH were selected for CISH analyses on 5 mm tissue sections.
Chromogenic in situ hybridization
Paraffin-embedded tissue sections were de-paraffinized in two changes of xylene for 5 minutes each; xylene was removed in three washes of ethanol for three minutes each (100%, 100% and 95%) and the slides were washed in distilled running water for 5 minutes. The slides were placed in heated (>90°C) CISH Pretreatment Buffer (Invitrogen, Carlsbad, CA) and microwaved on high power for 30 minutes, then rinsed in distilled water for 5 minutes at room temperature. The tissue was digested for 10 minutes with pepsin digestion solution (Invitrogen, Carlsbad, CA) at room temperature, washed twice in distilled water for five minutes each, dehydrated in 90, 95, and 100% alcohol for 2 minutes each, then dried in a 37°C oven.
Five to seven µl of EGFR Amplification Probe (Invitrogen/Zymed, South San Francisco, CA) were applied to the designated area and a coverslip was applied and sealed with rubber cement. Slides were dried at 37°C, followed by probe denaturation at 95°C for five minutes and hybridization at 37°C overnight in a Ther-mobrite oven (MarketLab, Caledonia, MI). The slides were washed in 0.5% SSC for five minutes at room temperature, followed by 0.5% SSC for five minutes at 75°C, and water for five minutes at room temperature.
For immunodetection, slides were placed in 3% hydrogen peroxide in absolute methanol for ten minutes, and then washed in phosphate buffered saline with 0.025% Tween 20 (PBST) three times for 2 minutes each. Slides were incubated with nonspecific blocking solution (Zymed/ Invitrogen, South San Francisco, CA) for 10 minutes at room temperature, then incubated with mouse anti-digoxigenin antibody for 30 minutes at room temperature, washed in PBST twice for two minutes each, and incubated with horseradish peroxidase-conjugated goat anti-mouse anti-body for fifteen minutes, and finally washed again with PBST twice for two minutes each. Slides were then incubated with DAB Chromagen (Zymed/Invitrogen, South San Francisco, CA) for 30 minutes at room temperature, and washed in distilled water twice for two minutes each, and were counterstained with hematoxylin.
The CISH-prepared slides were examined at 400X magnification by bright field microscopy. Two pathologists (SS and LRC) examined the cases concomitantly and agreed on CISH scores; cases for which there was disagreement were further reviewed and a consensus was established. Both examiners were blinded to the FISH results. As in prior studies [15, 25], 200 tumor nuclei were examined in each case. In cases where the number of signals per tumor nucleus varied between tumor cells, the range of signals counted was recorded for each case. Further validation of this work comparing CISH to FISH was recently published [15].
Statistical analysis
A geometric average was calculated from the range of CISH scores to represent the point estimate for each case. CISH scores were correlated between groups using nonparametric analysis (Spearman correlation).
Results
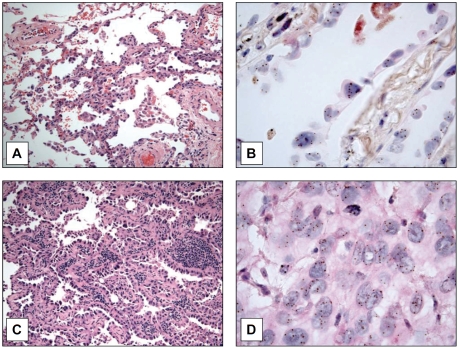
From the records of our institution, we identified seven patients with lung adenocarcinomas and synchronous AAH in the surgical pathology specimens, one patient with adenocarcinoma alone, two patients with AAH alone, and 12 patients with BAC. The clinical and pathologic features of the patient groups are summarized in Table 1. All surgical pathology specimens were reviewed morphologically and classified according to the WHO criteria [23], and each was subjected to analysis of EGFR gene copy number using CISH. We sought to examine whether gene amplification of EGFR was present in precursor lesions of lung adenocarcinomas. Using surgically resected specimens of lung adenocar-cinomas that demonstrated concurrent AAH, we used CISH to evaluate EGFR gene amplification. All specimens were from formalin fixed paraffin embedded tissue sections and EGFR probes were used as recommended by the manufacturer. Cases of BAC had features of involvement of alveolar walls by a proliferation of cuboidal cells with severe atypia, but without evidence of invasion. In contrast, the cases of AAH were localized proliferations of pneumocytes less than 0.5cm with marked hobnailing and mild to moderate atypia lining the alveolar walls and/or respiratory bronchioles. We were able to identify increased CISH signals representing amplification of the EGFR gene in cases of AAH (Figure 1A, B), suggesting the presence of increased EGFR gene copy number in this proposed earliest morphologic precursor of lung adenocarcinoma. We found up to six EGFR signals per nucleus (Figure 1B) in a case of AAH. In addition, we were able to detect increased copy numbers of EGFR by CISH in BAC. As previously reported, increased EGFR copy number was also identified in lung adenocarcinomas, with one example demonstrating up to ten EGFR signals per nucleus (Figure 1D).
Table 1.
EGFR copy number in patients with atypical adenomatous hyperplasia (AAH), bronchioloalveolar carcinoma (BAC) and adenocarcinoma of the lung*
| Case Nr | Age/Sex | Smoking Pack-yrs | Type of Lesion | EGFR Copy Number | Surgical Procedure |
|---|---|---|---|---|---|
| 1 | 50/F40 | AAH/ACA | 3/3 | RLL/RUL wedges | |
| 2 | 58/F | Unknown | AAH/ACA | 2/2 | RLL/RUL wedges |
| 3 | 56/F | Never | AAH/ACA | 5/7 | RUL wedge |
| 4 | 58/F | Unknown | AAH | 2 | Left lung, EPP |
| 5 | 72/F | 55 | ACA | 7 | RUL wedge |
| 6 | 62/F | 45 | AAH/ACA | 4/10 | LUL lobectomy |
| 7 | 75/F | Never | AAH/ACA | 2/2 | RML lobectomy |
| 8 | 69/F | 12 | AAH/ACA | 6/2 | LLL wedge |
| 9 | 76/M | Unknown | AAH | 2 | RUL lobectomy |
| 10 | 58/F | Unknown | AAH/ACA | 6/9 | LUL lobectomy |
| 11 | 58/F | 27 | BAC | 3 | RUL wedge |
| 12 | 59/F | Never | BAC | 3 | RLL wedge |
| 13 | 49/F | Unknown | BAC | 3 | RUL lobectomy |
| 14 | 66/F | 25 | BAC | 5 | RLL, wedge |
| 15 | 69/F | Unknown | BAC | 6 | RUL wedge |
| 16 | 80/M | 10 | BAC | 3 | RLL lobectomy |
| 17 | 71/M | Occasion | BAC | 6 | RML wedge |
| 18 | 66/F | Unknown | BAC | 5 | RUL lobectomy |
| 19 | 56/M | 20 | BAC | 5 | RML wedge |
| 20 | 75/F | Unknown | BAC | 3 | RLL wedge |
| 21 | 79/M | 40 | BAC | 3 | LUL wedge |
| 22 | 66/F | Unknown | BAC | 2 | LUL wedge |
ACA, adenocarcinoma; RLL, right lower lobe; RUL, right upper lobe; RML, right middle lobe; LLL, left lower lobe; LUL, left upper lobe.
Figure 1.
Surgical specimens from patients with atypical adenomatous hyperplasia (AAH), BAC (BAC) and adenocarcinoma of the lung. Panel A Atypical adenomatous hyperplasia (H&E 200x). Panel B Six EGFR signals per nucleus were noted in this case of AAH (H&E 1000x). Panel C Nonmucinous BAC. Uniform involvement of the alveolar walls by proliferation of cuboidal cells with severe atypia (H&E, 200x). Panel D Eleven EGFR signals per nucleus were noted in this case of adenocarcinoma (H&E 1000x).
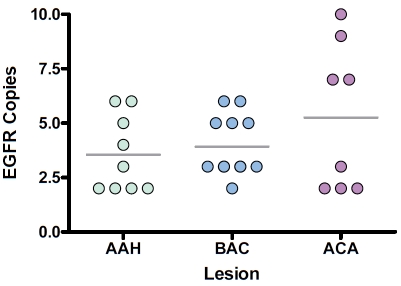
To determine whether there might be a correlation between early lesions of lung adenocarcinoma with EGFR gene amplification, we examined whether there was a progressive increase in EGFR gene expression as a function of proposed tumor development. We found an abnormal EGFR gene copy gain in several cases of AAH (mean 3.5 signals, 95% CI 2.2 to 4.9) and a progressive increase in the rate of EGFR gene copy abnormalities to invasive ACA (mean 5.3 signals, 95% CI 2.4 to 8.1). In four of 9 cases of AAH (44.4%) more than three EGFR signals per nucleus were noted (Figure 2).
Figure 2.
Scatter diagram illustrating the correlation between EGFR copy number and tumor progression, in AAH, BAC and lung adenocarcinoma. In four of 9 cases of AAH (44.4%) more than three EGFR signals per nucleus were noted. Horizontal bars represent the median value of EGFR copy number for each of the groups evaluated.
Discussion
In the current study, we used CISH to determine whether copy number changes to the EGFR locus might be an early event in lung tumorigenesis. We examined EGFR copy number changes in atypical adenomatous hyperplastic lesions, which are thought to represent the earliest morphologic entity that can be discriminated from either normal or reactive pulmonary epithelium. Although a previous report evaluated the prevalence of EGFR copy number abnormalities by CISH in AAH and lung adenocarcinoma [17], our findings are the first to demonstrate evidence for EGFR gene amplification in atypical adenomatous hyperplasia. The number of EGFR copies detected by CISH was higher in invasive adenocarcinoma than BAC and atypical adenomatous hyperplasia. Overall, we found that EGFR gene copy number in AAH is lower than in adenocarcinoma and very similar to that of BAC. Two other studies by Sakuma et al. found EGFR mutations by PCR-based methodology to be present in 32% of AAH cases, 88% of pure non-mucinous bronchioloalveolar carcinomas and 75% of invasive adenocarcinomas with a non-mucinous bronchioloalveolar component [26, 27]. Nevertheless further studies are needed to define this relationship. One limitation of our study was that most of the AAH were found concurrently in cases with invasive cancer. Ideally, examination of EGFR by CISH would occur in specimens devoid of an invasive lesion in order to definitively determine whether EGFR gene amplification is indeed an early event, or simply a correlate seen in cases of invasive adenocarcinoma with concurrent AAH. However, this would be difficult given that AAH lesions are small and are not usually or likely to be discovered by clinical or pathologic examination.
Activation of the EGFR signaling cascade has been shown to be due to a subset of mutations in the EGFR receptor kinase domain, which have been reported to occur with a frequency of 10% [1]. Such mutations appear to be more common in the adenocarcinoma histologic types and tend to be found in young, Asian women who have never smoked [28-30]. Notably, these patients appear to benefit substantially from inhibitors targeting EGFR. Other mechanisms of activation of the EGFR signaling cascade include gene amplification. Recent studies have highlighted the prognostic value of EGFR gene amplification in response to EGFR-inhibitor therapies [31-34]. Cappuzzo et al. showed that there was an increased responsiveness to kinase therapy in patients with lung adenocarcinomas that harbored increased EGFR gene copy number determined by FISH [31]. Similarly, Hirsch et al. demonstrated that increased EGFR gene copy number was associated with increased sensitivity to kinase inhibitors in patients with BAC's [34]. Adenocarcinomas with increased EGFR copy number were also found to correlate with EGFR overexpression in NSCLC and showed a trend towards poorer prognosis [16]. Importantly then, both mutations in EGFR and gene amplification are correlated with subsequent responsiveness to kinase inhibitor therapy. However, whether defects in kinase signaling are by themselves sufficient to induce malignancy is still unknown. Further research into the early mechanisms of lung tumorigenesis may reveal new strategies for the development of specific molecular therapeutics as well as provide for novel prognostic and diagnostic markers.
Our findings show that EGFR gene abnormalities are associated with tumor progression to invasive cancer and highlight the complexity of tumor morphogenesis.
Acknowledgments
This study was supported by Specialized Programs of Research Excellence (SPORE) grant in Lung Cancer CA090578 from the National Cancer Institute. Dr. Chirieac is supported by W81XWH06-1-0303 from the Department of Defense. We are indebted to Ms. Lillian Cruz for laboratory assistance and Ms. Brittany MacFarland for her editorial assistance in preparing the manuscript.
References
- 1.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-smallcell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Testa JR, Siegfried JM. Chromosome abnormalities in human non-small cell lung cancer. Cancer Res. 1992;52:2702s–2706s. [PubMed] [Google Scholar]
- 4.Westra WH. Early glandular neoplasia of the lung. Respir Res. 2000;1:163–169. doi: 10.1186/rr28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Kerr KM. Pulmonary preinvasive neoplasia. J Clin Pathol. 2001;54:257–271. doi: 10.1136/jcp.54.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiosea S, Jelezcova E, Chandran U, Luo J, Mantha G, Sobol RW, Dacic S. Overexpression of Dicer in precursor lesions of lung adenocarcinoma. Cancer Res. 2007;67:2345–2350. doi: 10.1158/0008-5472.CAN-06-3533. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Nomori H, Ohba Y, Shibata H, Mori T, Honda Y, Iyama K, Kobayashi T. Epidermal growth factor receptor mutations in multicentric lung adenocarcinomas and atypical adenomatous hyperplasias. J Thorac Oncol. 2008;3:467–471. doi: 10.1097/JTO.0b013e31816b4b14. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Orta L, Gil J, Li G, Hu A, Burstein DE. Immunohistochemical detection of XIAP and p63 in adenomatous hyperplasia, atypical adenoma-tous hyperplasia, bronchioloalveolar carcinoma and well-differentiated adenocarcinoma. Mod Pathol. 2008;21:553–558. doi: 10.1038/modpathol.2008.5. [DOI] [PubMed] [Google Scholar]
- 10.Licchesi JD, Westra WH, Hooker CM, Machida EO, Baylin SB, Herman JG. Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis. 2008;29:895–904. doi: 10.1093/carcin/bgn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartori G, Cavazza A, Bertolini F, Longo L, Marchioni A, Costantini M, Barbieri F, Migaldi M, Rossi G. A subset of lung adenocarcinomas and atypical adenomatous hyperplasia-associated foci are genotypically related: an EGFR, HER2, and K-ras mutational analysis. Am J Clin Pathol. 2008;129:202–210. doi: 10.1309/THU13F3JRJVWLM30. [DOI] [PubMed] [Google Scholar]
- 12.Licchesi JD, Westra WH, Hooker CM, Herman JG. Promoter hypermethylation of hallmark cancer genes in atypical adenomatous hyperplasia of the lung. Clin Cancer Res. 2008;14:2570–2578. doi: 10.1158/1078-0432.CCR-07-2033. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sholl LM, John Iafrate A, Chou YP, Wu MT, Goan YG, Su L, Huang YT, Christiani DC, Chirieac LR. Validation of chromogenic in situ hybridization for detection of EGFR copy number amplification in nonsmall cell lung carcinoma. Mod Pathol. 2007;20:1028–1035. doi: 10.1038/modpathol.3800946. [DOI] [PubMed] [Google Scholar]
- 16.Sholl LM, Yeap BY, Iafrate AJ, Holmes-Tisch AJ, Chou YP, Wu MT, Goan YG, Su L, Benedettini E, Yu J, Loda M, Janne PA, Christiani DC, Chirieac LR. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Res. 2009;69:8341–8348. doi: 10.1158/0008-5472.CAN-09-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awaya H, Takeshima Y, Furonaka O, Kohno N, Inai K. Gene amplification and protein expression of EGFR and HER2 by chromogenic in situ hybridisation and immunohistochemistry in atypical adenomatous hyperplasia and adeno-carcinoma of the lung. J Clin Pathol. 2005;58:1076–1080. doi: 10.1136/jcp.2004.025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallegos Ruiz MI, Floor K, Vos W, Grunberg K, Meijer GA, Rodriguez JA, Giaccone G. Epidermal growth factor receptor (EGFR) gene copy number detection in non-small-cell lung cancer; a comparison of fluorescence in situ hybridization and chromogenic in situ hybridization. Histopathology. 2007;51:631–637. doi: 10.1111/j.1365-2559.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SB, Lee HJ, Park JO, Choe G, Chung DH, Seo JW, Chung JH. Reliability of chromogenic in situ hybridization for epidermal growth factor receptor gene copy number detection in non-small-cell lung carcinomas: A comparison with fluorescence in situ hybridization study. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Gilcrease M, Sneige N. Reliability of chromogenic in situ hybridization for detecting HER-2 gene status in breast cancer: comparison with fluorescence in situ hybridization and assessment of interobserver reproducibility. Mod Pathol. 2005;18:1015–1021. doi: 10.1038/modpathol.3800432. [DOI] [PubMed] [Google Scholar]
- 21.Hanna WM, Kwok K. Chromogenic in-situ hybridization: a viable alternative to fluorescence in-situ hybridization in the HER2 testing algorithm. Mod Pathol. 2006;19:481–487. doi: 10.1038/modpathol.3800555. [DOI] [PubMed] [Google Scholar]
- 22.Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, Isola J. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/ neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000;157:1467–1472. doi: 10.1016/S0002-9440(10)64785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis WD BE, Muller-Hermelink HK, et al. World Health Organization Classification of Tumours. Lyon: IARC Press; 2004. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus, and Heart. [Google Scholar]
- 24.Greene FL PD, Fleming ID, Fritz A. AJCC Cancer Staging Manual (6th Edition) New York: Springer-Verlag; 2002. [Google Scholar]
- 25.Thorner PS, Ho M, Chilton-MacNeill S, Zielenska M. Use of chromogenic in situ hybridization to identify MYCN gene copy number in neuro-blastoma using routine tissue sections. Am J Surg Pathol. 2006;30:635–642. doi: 10.1097/01.pas.0000202163.82525.5c. [DOI] [PubMed] [Google Scholar]
- 26.Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Nakayama H, Kameda Y, Tsuchiya E, Miyagi Y. Epidermal growth factor receptor gene mutations in atypical adenomatous hyperplasias of the lung. Mod Pathol. 2007;20:967–973. doi: 10.1038/modpathol.3800929. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, Kameda Y, Tsuchiya E, Miyagi Y. Distinctive evaluation of nonmucinous and mucinous subtypes of bron-chioloalveolar carcinomas in EGFR and K-ras genemutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- 28.Huang SF, Liu HP, Li LH, Ku YC, Fu YN, Tsai HY, Chen YT, Lin YF, Chang WC, Kuo HP, Wu YC, Chen YR, Tsai SF. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10:8195–8203. doi: 10.1158/1078-0432.CCR-04-1245. [DOI] [PubMed] [Google Scholar]
- 29.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 30.Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, Ichimura K, Tsuda T, Yano M, Tsukuda K, Tabata M, Ueoka H, Tanimoto M, Date H, Gazdar AF, Shimizu N. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 31.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA, Jr., Varella-Garcia M. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 32.Cappuzzo F, Ligorio C, Janne PA, Toschi L, Rossi E, Trisolini R, Paioli D, Holmes AJ, Magrini E, Finocchiaro G, Bartolini S, Cancellieri A, Ciardiello F, Patelli M, Crino L, Varella-Garcia M. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol. 2007;25:2248–2255. doi: 10.1200/JCO.2006.09.4300. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch FR, Varella-Garcia M, Bunn PA, Jr., Franklin WA, Dziadziuszko R, Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T, von Pawel J, Watkins C, Flannery A, Ellison G, Donald E, Knight L, Parums D, Botwood N, Holloway B. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, Bunn PA, Jr, Franklin WA, Crowley J, Gandara DR. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23:6838–6845. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]