Abstract
We examined the hypothesis that persistent pulmonary hypertension of the newborn (PPHN) associated with ibuprofen is due to alterations in biochemical and molecular regulators of oxidative stress and NO signaling. Newborn rats breathing 50% O2 or room air from the first day of life (P1), received early IP injections of: 1) indomethacin (0.2 mg/kg) on P1 and 0.1 mg/kg on P2 and P3; 2) ibuprofen (10 mg/kg) on P1 and 5 mg/kg on P2 and P3; or 3) saline on P1, P2 and P3, then euthanized on P4; or late treatment on P4, P5 and P6, then euthanized on P7. Lung biomarkers for oxidative stress (8- epi-PGF2a), DNA damage (8-hydroxy-2’-deoxyguanosine) and pulmonary hypertension (ET-1, big ET-1, and total NO) were assessed. Despite timing of the dose and oxygen exposure, both drugs resulted in increased alveolar size. Both drugs had no beneficial effects on oxidative stress. Indomethacin treatment in O2 resulted in higher pulmonary levels of 8-epi-PGF2α which was associated with downregulation of most antioxidant genes with early treatment and overexpression of GPX5 and 6 with late treatment. Early and late ibuprofen treatment suppressed hyperoxia-induced NOx production and downregulated iNOS. Postponing treatment had no significant beneficial effects on biomolecular regulators of oxidative stress and NO signaling. The effect of ibuprofen on pulmonary NOx may explain in part, the transient PPHN seen in ibuprofen-treated preterm infants.
Keywords: Antioxidants, DNA damage, endothelins, nitric oxide, oxidative stress
Introduction
Indomethacin is a non-selective inhibitor of prostaglandin (PG) synthesis used prenatally for tocolysis and postnatally for the closure of a symptomatic patent ductus arteriosus (PDA). Its use is associated with significant renal, gastro-intestinal and central nervous system effects [1-3]. Ibuprofen, another non-selective inhibitor of PG synthesis, is as effective as indomethacin for closure of a symptomatic PDA with fewer side effects [2,4,5]. However, postnatal ibuprofen, but not indomethacin, has been shown to cause persistent pulmonary hypertension of the newborn (PPHN) [6-10] which resolved with inhaled nitric oxide (iNO). The mechanism(s) responsible for this difference between the two drugs have not been investigated and may be due to differential effects on factors that regulate pulmonary vasomotor tone.
Pulmonary vascular circulation increases approximately 10-fold after birth secondary to lung inflation and dilatation of the pulmonary vascular bed. Control of this transition involves a dynamic interaction between pulmonary vasodilators and vasoconstrictors, such as PGs, endothelin (ET)-1, and NO. Failure of the pulmonary vessels to transition, due to disturbances in the harmonious interplay between pulmonary vasodilators and vasoconstrictors results in PPHN [11]. Administration of PG synthetase inhibitors particularly during a critical time of lung development produces changes in pulmonary vascular smooth muscle architecture and tone [12]. This effect may be mediated in part by suppression of dilator PGs which modulate the intrinsic constrictor activity of ET-1 [13]. ET-1 is a potent vasoconstrictor that plays a significant role in the pathophysiology of neonatal PPHN. Over-expression of its receptors, particularly ETA, was noted in the thickened pulmonary arteries of neonates who had PPHN with or without diaphragmatic hernia [14,15]. Studies have reported an association between elevated endogenous plasma ET-1 during the first few days of life and the incidence of PPHN [16-18].
Exposure to hyperoxia predisposes the preterm neonate to generation of reactive oxygen species (ROS) and oxidative injury due to immature antioxidant defense mechanisms. 8-epi-PGF2α (or 8-isoprostane) is a free radical lipid peroxidation product of arachidonic acid via a non-enzymatic pathway [19-23], and a reliable and proven biomarker for oxidative stress [19,24]. Elevated plasma 8-isoprostane has been shown to be associated with BPD and PVL in preterm infants [25]. Therefore, considering the involvement of NO and ET-1 in the development of PPHN and the role of 8-isoprostane in oxidative injury, we proposed the hypothesis that the reported effects of ibuprofen on the immature lungs are due to alterations in biochemical and molecular regulators of oxidative stress and NO signaling. Our hypothesis was tested with the following objectives: 1) to compare the effects of ibuprofen and indomethacin on biomolecular regulators of oxidative stress and NO signaling; 2) to determine whether the effects are different when exposed to normoxia or hyperoxia; and 3) to establish whether postponing treatment is more beneficial than early postnatal treatment.
Material and methods
All experiments were approved by Memorial Health Services Institutional Animal Care and Use Committee, Long Beach, CA. Animals were cared for according to the guidelines outlined by the Guide for the Care and Use of Laboratory Animals, and euthanasia was carried out according to the 2007 American Veterinary Medical Association Guidelines on Euthanasia.
Experimental design
Certified infection-free, timed-pregnant Sprague Dawley rats carrying fetuses of known gestational age (18 days) were purchased from Charles River Laboratories (Wilmington, MA). The pregnant rats were placed in USDA-compliant cages and remained undisturbed until delivery of their pups (22 days gestation). The animals were housed in an animal facility with a 12-hour-day/12-hour-night cycle and provided standard laboratory diet and water ad libitum. On the day of birth, newborn rat pups delivering on the same day were weighed, measured for linear growth (crown to rump length), pooled and randomly assigned to expanded litters of 17 pups/litter. One dam remained with the same litter for the entire study. On the day of birth, litters were randomly assigned to either room air (RA) or 50% O2 (a mid range of hyperoxia). For early treatment, litters received intraperitoneal (IP) injections of: 1) indomethacin (0.2 mg/kg IP) on day 1 (P1), followed by 0.1 mg/kg on P2 and P3; 2) ibuprofen (10 mg/kg) on P1 followed by 5 mg/kg on P2 and P3; or 3) equivalent volume saline IP on P1, P2, & P3, prior to euthanasia on P4. For late treatment, litters were randomized to IP injections of: 1) indomethacin (0.2 mg/kg IP) on P4, followed by 0.1 mg/kg on P5 and P6; 2) ibuprofen (10 mg/kg) on P4 followed by 5 mg/kg on P5 and P6; or 3) equivalent volume saline IP on P4, P5, & P6, prior to euthanasia on P7. Litters assigned to hyperoxia were placed in the oxygen chamber from the first day of life until euthanasia at either P4 or P7. Indomethacin IV (Merck & Co., Inc., West Point, PA) was diluted in 0.9% saline prior to injection, and ibuprofen lysine IV solution was provided by Ovation Pharmaceuticals (Deerfield, IL) and diluted in 0.9% saline prior to injection. The doses of indomethacin and ibuprofen were based on doses currently administered to preterm neonates for symptomatic PDA. Immediately following euthanasia, the whole lungs were removed and the wet weight was determined. Tissue samples were excised from the left lower lobe, rinsed in ice-cold phosphate buffered saline (PBS) to remove blood elements and frozen immediately at -80°C for RNA isolation. Other samples were placed in 2 mL sterile normal saline and homogenized for enzyme immunoassay of 8-epi-PGF2a, 8-OHdG, ET-1, big ET-1, NOx), or placed in 10% phos-phate-buffered formalin for histopathology.
Hyperoxia
On the day of birth, the litters were placed with the dams in specialized oxygen chambers (BioSpherix, New York) attached to an oxycycler. The chambers are optimized for gas efficiency and provide adequate ventilation for the animals in a controlled atmosphere with minimal gas usage. From outside the chamber, the oxycycler senses oxygen inside the chamber and infuses nitrogen to reduce oxygen and oxygen to raise it to maintain an environment of 50% hyperoxia throughout the experimental time. Oxygen content inside the chamber was continuously monitored and recorded on a Dell Computer. Carbon dioxide in the chamber was monitored and removed from the atmosphere by placing soda lime within the chamber. The animals remained undisturbed except during the bedding change, and food and water refill which occurred every 3 days, and lasted no more than 3 minutes.
Measurement of 8-iso-PGF2α
8-iso-PGF2α levels in lung tissue homogenates were determined using the Direct 8-iso-PGF2α EIA kit for use with serum, plasma, and tissue from Assay Designs, Inc (Ann Arbor, MI). Samples were first hydrolyzed with NaOH and neutralized with HCl, prior to centrifugation for 5 minutes at 14,000 rpm in a microcentrifuge and the clear supernatant was used for the assay, according to the manufacturer's protocol. The standard curve ranged from 0 to 100,000 pg/mL. The sensitivity of the assay was 40 pg/mL and the inter- and intra-assay variations for each assay were <10%.
Measurement of 8-hydroxy-2’deoxyguanosine
8-hydroxy-2’deoxyguanosine levels in lung tissue homogenates were determined using the DNA Damage ELISA kit for the detection and quantitation of 8-OHdG, from Assay Designs, Inc. Following dilution with sample diluent samples were assayed according to the manufacturer's protocol. The standard curve ranged from 0 to 60 ng/ mL. The sensitivity of the assay was 0.59 ng/mL and the inter- and intraassay variations for each assay were <10%.
Measurement of endothelins
ET-1 and Big ET-1 in lung homogenates were measured using TiterZyme enzyme immunoassay kits obtained from Assay Designs, Inc. according to the manufacturer's recommendations. For big ET-1 and ET-1, the standard curves ranged from 0.78 pg/mL to 100 pg/mL. The sensitivity of the assay big ET-1 assay was 0.72 pg/mL and the sensitivity for ET-1 was 0.14. The inter- and intra-assay variations for each assay were <10%.
Measurement of NOx
Total NOx levels in lung homogenates were examined using commercially available Total NO assay kits purchased from Assay Designs, Inc., according to the manufacturer's protocol. Briefly, samples were diluted in reaction buffer and nitrate reductase was added to the sample to convert nitrate to nitrite. Nitrite was determined using the Griess Reagent. The standard curve ranged from 0 to 100 mmole/L and the inter- and intra-assay variations were <10%.
Total cellular protein assay
On the day of the assay, the lung homogenates were assayed for total cellular protein using the dye-binding Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard. The standard curve was linear from 0.05 to 1.45 mg/ml of protein.
Histology
At the time of euthanasia, total lung wet weight was determined and tissue from the left lobe as fixed with 10% phosphate-buffered formalin for at least 24 hours. After fixation, the samples were cut into 5 pieces of equal thickness and taken to Memorial Medical Center Pathology laboratory for processing and staining according to standard laboratory techniques for a total of 5 blocks per group. Digital images (20X magnification) were taken using Spot software version 4.6 (Diagnostic Instruments, Sterling Heights, MI), in combination with a high resolution Spot RT3 camera (Diagnostic Instruments), Olympus BH-2 microscope (Center Valley, PA) and Dell Optiplex GX280 computer (Dell Computer Corporation, Dallas, TX).
Real-time polymerase chain reaction
At euthanasia, tissue from the left lobe was frozen immediately for RNA isolation. Total RNA was extracted using RNA Pro solution (MP Bio, Solon, Ohio) and allowed to digest for 5 minutes at room temperature. The samples were transferred to microtubes containing ceramic beads and placed in a FastPrep-24 instrument (MP Bio) for 40 seconds. The samples were centrifuged at 13,000 rpm at 4°C for 5 minutes, and the supernatant transferred to a clean eppendorf tube. After addition of chloroform, the samples were vortexed for 30 seconds and centrifuged at 13,000 rpm at 4°C for 5 minutes. The upper aqueous phase was transferred to a clean eppendorf tube containing 0.5 mL of ice-cold 100% ethanol. The samples were placed in a -20°C freezer for 60 minutes to precipitate the RNA. Following precipitation, the samples were centrifuged at 13,000 rpm at 4°C for 20 minutes and the resulting pellet was washed in 75% ethanol/water and redissolved in 100 μL nuclease-free water. The amount of RNA was quantified at 260 nm using a Beckman spectrophotometer and diluted to 1 mg/mL. Cleanup of the RNA was performed using RNEasy mini cleanup kits (Qiagen) followed by on-column treatment with DNase I (Qiagen). Reverse transcriptase was performed using RT2 Profiler Rat NO Signaling PCR Array System (a pathway focused gene expression profiler) from SABiosciences, Frederick, MD. The real-time PCR arrays were carried out in duplicate on 96-well plates precoated with 84 genes regulating NO signaling.
Statistical analysis
Unpaired t-tests or Mann Whitney U tests were used to determine differences between RA and hyperoxia groups, or early versus late effects, following Levene's test for equality of variances. One -way analysis of variance was used to determine differences among the treatment groups for normally-distributed data, and Kruskal-Wallis test was used for non-normally-distributed data following Bartlett's test for equality of variances. Post hoc analysis was performed using the Tukey and Student-Newman-Keuls test for significance, as appropriate. Significance was set at p<0.05 and data are reported as mean ± SEM. All analyses were two-tailed and performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL) and GraphPad Prism software version 5.02 (GraphPad Inc., San Diego, CA).
Results
Effects on growth
Percentage change in body weight (calculated as: total body weight in grams, at euthanasia - total body weight at birth/total body weight at birth x 100) were calculated to determine effects of the drug on growth (data not shown). For the early treatment group, percentage change in body weight was not different between the RA and hyperoxia groups treated with saline and ibuprofen, but was significantly elevated with indomethacin treatment in hyperoxia (48.3±3.5%) versus RA (31.7±5.5%, p<0.05), as well as saline (36.7±2.8%, p<0.05) and ibuprofen (33.6±3.4%, p<0.01) treatment in hyperoxia. In the late treatment groups, hyperoxia resulted in decreased weight gain with saline treatment (71.7±3.5%, p<0.001) compared to RA (103.1±4.4%). This effect appeared to be reversed in the indomethacin-treated group exposed to hyperoxia (110.7±9.0%, p<0.01). Treatment with ibuprofen suppressed weight gain in RA (73.5±7.6%, p<0.001) compared to saline in RA. A similar but diminished effect was noted with indomethacin treatment in RA (82.5±5.1%, p<0.05) compared to saline in RA and indomethacin in hyperoxia (p<0.01). Lung to body weight ratios were comparable among the early and late treatment groups, and were not affected by hyperoxia.
Effect on oxidative stress
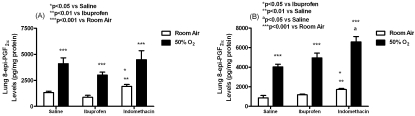
As expected, lung tissue 8-iso-PGF2α levels (pg/ mg protein) were similarly elevated in all groups exposed to hyperoxia with early (saline:4096.2± 566.1; indomethacin :4485.7±846.0; ibuprofen:3012.2±279.1; p<0.001) and late (saline:4022.5±288.1; indomethacin :6592.2± 535.9; ibuprofen:4957.8±488.5, p<0.001) treatment compared to the early (saline:1329.7 ±125.1; indomethacin:1921.6±183.5; ibuprofen:870.3±197.4;) and late (saline:859.4± 250.5; indomethacin :1720.8±101.1; ibuprofen:1175.9±64.3) RA groups (Figure 1). 8-iso-PGF2α levels were significantly higher with early indomethacin treatment in RA than saline (p<0.05) and ibuprofen (p<0.01) in RA. A similar response was noted with late indomethacin treatment in RA compared to saline (p<0.01) and ibuprofen (p<0.05) in RA, as well as late indomethacin treatment in hyperoxia versus saline treatment in hyperoxia (p<0.05).
Figure 1.
Effects of early (A) and late (B) saline, ibuprofen lysine and indomethacin, administered IP in room air (opened bar) or 50% O2 (solid bar) on lung 8-iso-PGF2α levels in neonatal rats. Data are mean±SEM (n=17 pups).
Effect on DNA damage
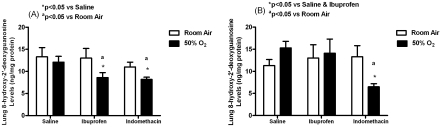
Early treatment with both indomethacin (8.2± 0.5, p<0.05) and ibuprofen (8.6±1.1, p<0.05) reduced lung 8-OHdG levels (ng/mg protein) in hyperoxia compared to saline-treated littermates in hyperoxia (12.1±1.3). The effect was also significantly different from indomethacin in RA (11.0±1.1) or ibuprofen in RA (13.0±2.2). For late treatment, only indomethacin effectively decreased lung 8-OHdG levels in hyperoxia (6.5±0.7, p<0.05) compared to indomethacin RA (13.3±2.5), saline hyperoxia (15.3±1.5), and ibuprofen hyperoxia (14.1±3.2) as demonstrated in Figure 2.
Figure 2.
Effects of early (A) and late (B) saline, ibuprofen lysine and indomethacin, administered IP in room air (opened bar) or 50% O2 (solid bar) on lung 8-hydroxy-2’-deoxyguanosine levels in neonatal rats. Data are mean±SEM (n=17 pups).
Effect on nitric oxide metabolites (NOx)
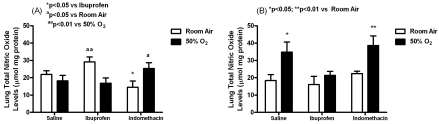
Early treatment with ibuprofen elevated NOx levels (mmol/mg protein) in RA (29.1±2.9) compared to ibuprofen treatment in hyperoxia (16.8±3.1, p<0.01) and indomethacin treatment in RA (14.5±3.6, p<0.05). Similar elevations were noted with indomethacin treatment in hyperoxia (25.3±3.4, p<0.05) compared to indomethacin treatment in RA. For late treatment, lung NOx levels were significantly elevated in the saline hyperoxia group (34.8±5.8, p<0.05) compared to saline RA (18.4±3.4) and in the indomethacin hyperoxia (38.6±5.6, p<0.01) compared to indomethacin RA (22.4±1.4) groups. No elevations were noted with ibuprofen (Figure 3).
Figure 3.
Effects of early (A) and late (B) saline, ibuprofen lysine and indomethacin, administered IP in room air (opened bar) or 50% O2 (solid bar) on lung NOx levels in neonatal rats. Data are mean±SEM (n=17 pups).
Effect on big ET-1
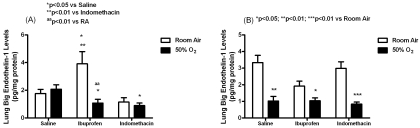
Figure 4 shows that in the early treatment groups, ibuprofen increased big ET-1 levels (pg/ mg protein) in RA (3.91±0.88) compared to saline RA (1.77±0.3, p<0.05) and indomethacin in RA (1.15±0.31, p<0.01). In contrast, ibuprofen treatment in hyperoxia decreased big ET-1 levels (1.07±0.28) compared to saline in hyperoxia (2.08±0.31, p<0.05) and ibuprofen in RA (p<0.01). A similar reduction in big ET-1 levels was noted with indomethacin treatment in hyperoxia (0.9±0.18, p<0.05) compared to saline treatment in hyperoxia (2.08±0.32). Lung big ET -1 levels were decreased in all late treatment groups in hyperoxia (saline:1.02±0.28, p<0.01; indomethacin :0.83±0.13, p<0.001; ibuprofen:1.04±0.17, p<0.05;) compared to RA (saline:3.33±0.44; indomethacin :3.0±0.39; ibuprofen:1.92±0.3;).
Figure 4.
Effects of early (A) and late (B) saline, ibuprofen lysine and indomethacin, administered IP in room air (opened bar) or 50% O2 (solid bar) on lung big ET-1 levels in neonatal rats. Data are mean±SEM (n=17 pups).
Effect on ET-1
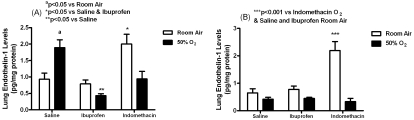
Treatment with early indomethacin resulted in higher lung ET-1 levels (pg/mg protein) in RA (2.0±0.3, p<0.05) compared to saline in RA (0.93±0.19) and ibuprofen in RA (0.79±0.12) as shown in Figure 5. Treatment with saline in hyperoxia resulted in higher ET-1 levels (1.9±0.24, p<0.05) compared to saline in RA (0.93±0.19), indomethacin in hyperoxia (0.94±0.23, p<0.05), and ibuprofen in hyperoxia (0.43±0.06, p<0.01). In the late treatment groups, indomethacin treatment in RA caused similar elevations in ET-1 levels (2.2±0.33, p<0.001) compared to indomethacin in hyperoxia (0.33±0.12), saline in RA (0.65±0.15) and ibuprofen in RA (0.78±0.12).
Figure 5.
Effects of early (A) and late (B) saline, ibuprofen lysine and indomethacin, administered IP in room air (opened bar) or 50% O2 (solid bar) on lung ET-1 levels in neonatal rats. Data are mean±SEM (n=17 pups).
Histology
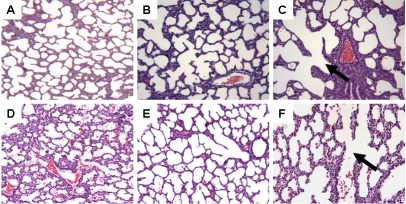
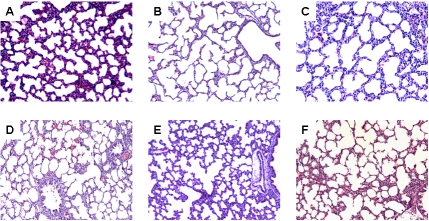
Histological analysis showed that early treatment with ibuprofen and to a greater extent, indomethacin resulted in increased alveolar size and reduction in the appearance of secondary crests (Figure 6). These characteristics were sustained with late treatment (Figure 7).
Figure 6.
Hematoxylineosin staining of left lung sections from neonatal rats administered early saline (A, D), ibuprofen lysine (B,E) and indomethacin (C,F), administered IP in room air (top three panels) or 50% O2 (bottom three panels). Images are 20× magnification.
Figure 7.
Hematoxylineosin staining of left lung sections from neonatal rats administered late saline (A, D), ibuprofen lysine (B,E) and indomethacin (C,F), administered IP in room air (top three panels) or 50% O2 (bottom three panels). Images are 20× magnification.
Profile of genes regulating NO signaling and oxidative stress
Following early treatment, hyperoxia alone resulted in downregulation of a majority of the genes of interest that are involved in regulation of NO signaling except nNOS, and oxidative stress. Of note, indomethacin treatment in RA resulted in upregulation of all the GPX and SOD genes (Table 1). Following late treatment, a reversal of gene expression was noted in the group exposed to hyperoxia. Most notably were the 6-fold downregulation with saline treatment, and the 3-fold increased expression with indomethacin treatment of nNOS compared to RA controls. Increased expression of most genes involved in regulation of oxidative stress was observed with late treatment. However, a 32-fold decrease in GPX2 was noted in the saline-treated hyperoxia group, as well as a 106-fold downregulation in GPX7 in the ibuprofen-treated hyperoxia group and an 8-fold, and a 60-fold upregulation in GPX5 and 6, respectively, in the indomethacin-treated hyperoxia group (Table 2).
Table 1.
Real-time polymerase chain reaction profile of genes involved in nitric oxide signaling and oxidative stress following early ibuprofen or indomethacin treatment in room air or 50% oxygen
| Gene | Sal-O2-E | Ibu-RA-E | Ibu-O2-E | Indo-RA-E | Indo-O2-E |
|---|---|---|---|---|---|
| Genes involved in Regulation of NO signaling pathway | |||||
| CALM1 | -1.2 | -1.1 | -1.2 | -1.6 | 1.0 |
| CAV-1 | -2.0 | -1.9 | -2.9 | 1.1 | -1.3 |
| eNOS | -2.0 | -2.2 | 1.0 | -1.4 | -1.5 |
| iNOS | -1.6 | 1.0 | -1.2 | -1.2 | 1.1 |
| nNOS | 1.1 | 1.5 | 3.9 | 2.7 | -1.2 |
| Genes involved in Regulation of Oxidative Stress | |||||
| CAT | 1.3 | 1.1 | 1.3 | -2.5 | 1.1 |
| GPX1 | -1.5 | -1.4 | -2.8 | 1.3 | -1.9 |
| GPX2 | -1.1 | 1.0 | -1.2 | 1.1 | 1.2 |
| GPX3 | 1.2 | 1.1 | -2.3 | 2.2 | -1.3 |
| GPX4 | 1.1 | 1.1 | 1.1 | 3.7 | -1.1 |
| GPX5 | -2.8 | 1.5 | 3.9 | 2.7 | -1.9 |
| GPX6 | -2.8 | 1.5 | 3.9 | 2.7 | -1.9 |
| GPX7 | -1.6 | -1.3 | -1.7 | 1.4 | -1.5 |
| SOD1 | 1.1 | 1.2 | 1.5 | 1.6 | 1.4 |
| SOD2 | -1.1 | -1.3 | -1.3 | 1.1 | -1.2 |
The data are presented as fold change from Sal-RA-E. All data were corrected using 5 different housekeeping genes. Genes are selected from a profile of 84 genes. In alphabetical order the genes of interest are: CALM1, calmodulin 1; CAT, catalase; CAV-1, caveolin- 1; eNOS, endothelial nitric oxide synthase; GPX1-7, glutathione peroxidase 1-7; iNOS, inducible nitric oxide synthase; nNOS, neuronal nitric oxide synthase; SOD1,2, superoxide dismutase 1,2.
Table 2.
Real-time polymerase chain reaction profile of genes involved in nitric oxide signaling and oxidative stress following late ibuprofen and indomethacin treatment in room air or 50% oxygen
| Gene | Sal-O2-L | Ibu-RA-L | Ibu-O2-L | Indo-RA-L | Indo-O2-L |
|---|---|---|---|---|---|
| CALM1 | -1.2 | -1.1 | -1.4 | -1.3 | -1.6 |
| CAV-1 | 1.8 | 1.4 | -1.3 | 1.1 | -1.3 |
| eNOS | 1.4 | -1.3 | -1.0 | -1.1 | -1.9 |
| iNOS | -1.2 | -1.2 | -1.8 | 1.5 | -1.6 |
| nNOS | -6.0 | -1.2 | -1.3 | -1.8 | 3.0 |
| Genes involved in Oxidative Stress | |||||
| CAT | -1.1 | -1.4 | -1.5 | -1.2 | -1.0 |
| GPX1 | 2.3 | 2.3 | 1.5 | 1.7 | -1.1 |
| GPX2 | -31.6 | 1.2 | 1.3 | 1.5 | 1.2 |
| GPX3 | 2.0 | 2.0 | -1.2 | 1.1 | -1.7 |
| GPX4 | 2.2 | 1.5 | 1.5 | 1.3 | 1.4 |
| GPX5 | 1.1 | -1.8 | 2.3 | -1.0 | 7.7 |
| GPX6 | 1.1 | -1.8 | 2.3 | -1.0 | 59.5 |
| GPX7 | 1.3 | 1.1 | -106.3 | -1.1 | -1.4 |
| SOD1 | 1.3 | -1.1 | 1.1 | -1.1 | -1.1 |
| SOD2 | 1.6 | 1.2 | 1.2 | 1.3 | -1.0 |
>The data are presented as fold expression difference compared to Sal-RA-E. All data were corrected using 5 different housekeeping genes. Genes are selected from a profile of 84 genes. Dowregulation is represented by parentheses and upregulation is represented by no parentheses. Genes of interest are as presented in Table 1.
Discussion
To examine our hypothesis that the reported effects of ibuprofen on the immature lungs are due to alterations in biochemical and molecular regulators of oxidative stress and NO signaling, we raised three questions: 1) do the effects of ibuprofen and indomethacin on biomolecular regulators of oxidative stress and NO signaling differ despite their similar action as non-selective COX inhibitors? 2) are the effects of ibuprofen and indomethacin different when administered in normoxia and hyperoxia (50% O2)?; and 3) does postponing treatment of ibuprofen and indomethacin to a later maturational age result in more beneficial effects? To address these questions, we first measured pulmonary levels of 8-epi-PGF2α which is a free radical lipid peroxidation product of arachidonic acid via a non-enzymatic pathway [19-23], and a reliable and proven biomarker for oxidative stress [19,24]. We also measured 8-OHdG, a reliable biomarker for oxidative DNA damage [26-30]. As expected, 8-epi-PGF2α was higher in the lungs of hyperoxic rats, reflecting oxidative stress, and treatment with both drugs did not significantly ameliorate this response, confirming a non-enzymatic ROS pathway. However, treatment with early ibuprofen tended to lower oxidative stress in contrast to early and late indomethacin. This may suggest that a portion of the isoprostane may be COX-derived. Surprisingly, we found that the levels of 8-epi-PGF2α were increasingly higher with indomethacin treatment and was most evident with late treatment. Whether this is due to a shift in arachidonic acid (AA) to the non-enzymatic ROS pathway due to inhibition of COX, remains to be determined.
Hyperoxia results in the production of ROS such as superoxide anion and hydroxyl radical. ROS causes oxidation of protein, DNA and lipids and initiate inflammatory responses in the lungs. Regulators of ROS include catalase (CAT), glutathione peroxidase (GPX) and superoxide dismutase (SOD). In the lungs, GPX content is approximately 140 times higher than the circulation [31], suggesting a key role in antioxidant defense of the lungs. SODs are the only enzymatic system-decomposing superoxide radicals and are important for oxidative stress [31]. In our study, early treatment with indomethacin in RA resulted in a 2.5-fold decrease in CAT, while GPX1-7 and SOD2 were either upregulated or remained unchanged from controls, as well as a robust upregulation in GPX5 and 6 with late treatment in 50% O2. This may suggest a compensatory response to oxidant/antioxidant imbalance. We also found that GPX2 was the only isoform of pulmonary GPX that responded after 6 consecutive days of hyperoxia with a marked (32-fold) reduction in gene expression. Increasing evidence suggest a role for GPX2 in protecting the lungs against hyperoxic injury [32]. This finding may suggest inability of the immature lungs to mount a defense against increasing levels of ROS. GPX7 was markedly downregulated (106-fold) with late ibuprofen treatment in hyperoxia. Our understanding of the mechanism for decreased GPX7 with ibuprofen is limited and requires further study. Interestingly, GPX5 (8-fold) and to a greater extent, GPX6 (60-fold) was abundantly expressed with late indomethacin in hyperoxia. This may be a compensatory effect resulting from the increasing levels of 8-epi-PGF2α in that group. GPX6 has been shown to be upregulated 30-fold in response to sodium nitroprusside (a nitric oxide donor) in legumes [33]. Alternatively, high GPX6 expression in response to late indomethacin treatment in hyperoxia may be due higher ROS and higher NO production in that group. Our data support a role for GPX6 in redox sensing and regulation in the hyperoxic lungs.
Hyperoxic DNA damage results from the interaction of ROS with DNA, lipids and proteins, cellular targets for oxidative damage. 8-OHdG is a modified nucleoside base which is a by-product of DNA damage. It is widely used as a bio-marker for oxidative DNA damage because of its ability to cause guanine to thymine transversions in DNA, its mutagenicity, and it is perceived by the cell as a threatening lesion that has to be removed rapidly [28]. We were surprised to find a lack of increase in 8-OHdG in the hyperoxia-exposed, early saline-treated lungs, and only a trend for higher levels in the late saline-treated lungs, despite clear evidence of oxidative stress. This may be due to insufficient exposure time to cause oxidative DNA damage. Interestingly, both ibuprofen and indomethacin suppressed pulmonary 8-OHdG during early treatment in hyperoxia, but had no effect in normoxia. This may suggest an involvement of PGs in oxidative stress. However, since only indomethacin maintained the suppressive effect during late treatment, we suggest that indomethacin may be more effective and longer-lasting than ibuprofen. Postponing treatment of ibuprofen and indomethacin had no beneficial effects on markers for oxidative DNA damage.
NO is produced by nitric oxide synthase (NOS) and plays a major role in lung development [34], maintenance of alveolar epithelial cell phenotype [35] and vasomotor tone [36]. All three NOS isoforms (eNOS, iNOS and nNOS) are present in the lungs and their expression and NO production are increased with lung maturation. We found that pulmonary NOx was increased with early and late indomethacin treatment in hyperoxia, early ibuprofen in RA, and late saline in hyperoxia. However, we did not find any appreciable effects with ibuprofen treatment in hyperoxia. NO is also a free radical that is upregulated in hyperoxia and results in vasodilation [37], the lack of response noted with ibuprofen treatment may explain at least in part, the transient PPHN noted in some infants exposed to ibuprofen for symptomatic PDA, an effect that was resolved with inhaled NO [6,7,9]. The difference in response to hyperoxia-induced NO upregulation between the two NSAIDs may be due to differences in their pharmacologic characteristics and selectivity for COX-2 which interacts with NO synthase [39]. Gene profile of NO signaling genes during early treatment showed that while some genes are upregulated and some were downregulated, increased NOx in the ibuprofen RA group was associated with upregulation of iNOS and nNOS and increased NOx in the indomethacin O2 group was associated with iNOS. This suggests that iNOS is most likely the source of pulmonary NO in this setting. Conversely, NOx elevation with late saline in hyperoxia was associated with eNOS upregulation while NOx elevation in the late indomethacin hyperoxia group was associated with nNOS upregulation. Together these findings suggest that hyperoxia-induced NO is generated from eNOS, whereas NO generated from ibuprofen and indomethacin, whether in RA or hyperoxia is predominantly from iNOS.
ETs are potent vasoactive mediators that are produced by the vascular endothelium. NO interacts with ET-1 by either blocking the conversion of big ET-1 to ET-1, or diffusing to the smooth muscle cell and activating cGMP to produce vasodilation [39-41]. Our data showed that both early and late indomethacin and ibuprofen suppressed pulmonary big ET-1 levels in hyperoxia, suggesting either increased conversion to ET-1 or decreased production. However, since ET-1 was also decreased in the hyperoxia-exposed groups it is unlikely that these effects are due to increased conversion. Although ibuprofen blunted the hyperoxia-induced NO response, we did not observe a compensatory increase in ET-1. In fact, early treatment with ibuprofen suppressed pulmonary ET-1 levels. This may be due to an indirect effect through suppression of PGs, since an interaction between ET-1 and COX -2 has been demonstrated [42]. Other studies have shown that ET-1 is a potent agonist for AA release and PG synthesis [43]. Our findings that ibuprofen regulates ET-1 levels corroborate those previous reports.
The most interesting finding was the effect of indomethacin and ibuprofen on the pulmonary architecture which was consistent despite the timing of treatment or the atmospheric oxygen environment. The rats were exposed to treatment at a critical time when the alveoli are being formed by septation of the large saccules, which is approximately the 3rd to 4th postnatal day and completed by the 2nd postnatal week [44]. Inhibition of septation produce larger and fewer alveoli, an effect that appears to be permanent, persisting until adulthood [45]. Our findings are consistent with and confirm an earlier study which showed that indomethacin treatment resulted in increased alveolar size, suggesting a role for PGs in alveolar formation [46]. Our findings are also consistent with a previous report of increased alveolar surface area with ibuprofen treatment in ventilated premature baboons who had improved pulmonary mechanics [34], further implicating a key role for PGs in alveolar development.
In conclusion, we found that ibuprofen and indo-methacin treatment resulted in similar lung architecture when administered in RA or hyperoxia suggesting an involvement of PGs in lung development. Both drugs had no beneficial effects on oxidative stress. Indomethacin treatment resulted in higher pulmonary levels of 8-epi-PGF2α which was associated with overexpression of GPX6 with late treatment suggesting a role in redox sensing and regulation in the hyperoxic lungs. Postponing treatment had no significant beneficial effects on biomolecular regulators of oxidative stress and NO signaling. We therefore speculate that suppression of hyperoxia-induced NOx production with early and late ibuprofen treatment may explain in part, the transient PPHN seen in ibuprofen-treated preterm infants.
Acknowledgments
The authors thank the technicians in the Pathology Department of Long Beach Memorial Medical Center for their participation in processing and staining the lung tissue samples.
This work was made possible through grants from the Memorial Medical Center Foundation, Long Beach, CA; Ovation Pharmaceuticals, Deer-field, IL; and Ikaria, Clinton NJ.
References
- 1.Srinivasjois RM, Nathan EA, Doherty DA, Patole SK. Renal impairment associated with indo-methacin treatment for patent ductus arteriosus in extremely preterm neonatesis postnatal age at start of treatment important? J Matern Fetal Neonatal Med. 2006;19:793–799. doi: 10.1080/14767050600922610. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RL, Parker GC, Van Overmeire B, Aranda JV. A meta-analysis of ibuprofen versus indo-methacin for closure of patent ductus arteriosus. Eur J Pediatr. 2005;164:135–140. doi: 10.1007/s00431-004-1596-5. [DOI] [PubMed] [Google Scholar]
- 3.Zanardo V, Vedovato S, Lago P, Piva D, Faggian D, Chiozza L. Effects of ibuprofen and indo-methacin on urinary antidiuretic hormone excretion in preterm infants treated for patent ductus arteriosus. Fetal Diagn Ther. 2005;20:534–539. doi: 10.1159/000088046. [DOI] [PubMed] [Google Scholar]
- 4.Aranda JV, Thomas R. Systematic review: intravenous Ibuprofen in preterm newborns. Semin Perinatol. 2006;30:114–120. doi: 10.1053/j.semperi.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 6.Bellini C, Campone F, Serra G. Pulmonary hypertension following L-lysine ibuprofen in preterm infant with patent ductus arteriosus. CMAJ. 2006;174:1843–1844. doi: 10.1503/cmaj.051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gournay V, Savagner C, Thiriez G, Kuster A, Rozé JC. Pulmonary hypertension after ibuprofen pro-phylaxis in very preterm infants. Lancet. 2002;359:1486–1488. doi: 10.1016/S0140-6736(02)08424-6. [DOI] [PubMed] [Google Scholar]
- 8.Levin DL, Mills LJ, Weinberg AG. Hemodynamic, pulmonary vascular, and myocardial abnormalities secondary to pharmacologic constriction of the fetal ductus arteriosus. A possible mechanism for persistent pulmonary hypertension and transient tricuspid insufficiency in the newborn infants. Circulation. 1979;60:360–364. doi: 10.1161/01.cir.60.2.360. [DOI] [PubMed] [Google Scholar]
- 9.Mosca F, Bray M, Stucchi I, Fumagalli M. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet. 2002;360:1023–1024. doi: 10.1016/S0140-6736(02)11109-3. [DOI] [PubMed] [Google Scholar]
- 10.Tarcan A, Gurakan B, Yildirim S, Ozkiraz S, Bilezikci B. Persistent pulmonary hypertension in a premature newborn after 16 hours of ante-natal indomethacin exposure. J Perinat Med. 2004;32:98–9. doi: 10.1515/JPM.2004.019. [DOI] [PubMed] [Google Scholar]
- 11.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- 12.Levin DL, Fixler DE, Morriss FC, Tyson J. Morphologic analysis of the pulmonary vascular bed in infants exposed in utero to prostaglandind synthetase inhibitors. J Pediatr. 1978;92:478–483. doi: 10.1016/s0022-3476(78)80453-3. [DOI] [PubMed] [Google Scholar]
- 13.de Vroomen M, Lopez-Cardoso RH, Steendijk P, Frolich M, Baan J, van Bel F. Endothelin-1 plasma concentration increases in the early phase of pulmonary hypertension development during respiratory distress syndrome: a study in newborn lambs. Early Hum Dev. 2001;63:9–21. doi: 10.1016/s0378-3782(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 14.de Lagausie P, de Buys-Roessingh A, Ferkdadji L, Saada J, Aisenfisz S, Martinez-Vinson C, Fund X, Cayuela JM, Peuchmaur M, Mercier JC, Berrebi D. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. J Pathol. 2005;205:112–118. doi: 10.1002/path.1677. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald PD, Paton RD, Logan RW, Skeoch CH, Davis CF. Endothelin-1 levels in infants with pulmonary hypertension receiving extracorporeal membrane oxygenation. J Perinatal Med. 1999;27:216–220. doi: 10.1515/JPM.1999.030. [DOI] [PubMed] [Google Scholar]
- 16.Endo A, Ayusawa M, Minato M, Takada M, Takahashi S, Harada K. Endogenous nitric oxide and endothelin-1 in persistent pulmonary hypertension of the newborn. Eur J Pediatr. 2001;160:217–222. doi: 10.1007/pl00008431. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Kazzi NJ, Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol. 1996;13:335–341. doi: 10.1055/s-2007-994352. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive Endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1994;124:489–490. doi: 10.1016/s0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- 19.Awad JA, Roberts LJ, Burk RF, Morrow JD. Iso-prostanes- prostaglandin-like compounds formed in vivo independently of cyclooxygenase. NSAIDs Eicosanoids, and the Gastroenteric Tract. 1996;25:409–427. doi: 10.1016/s0889-8553(05)70255-7. [DOI] [PubMed] [Google Scholar]
- 20.Jankov RP, Luo X, Cabacungan J, Belcastro R, Frndova H, Lye SJ, Tanswell AK. Endothelin-1 and O2-mediated pulmonary hypertension in neonatal rats: a role for products of lipid peroxidation. Pediatr Res. 2000;48:289–298. doi: 10.1203/00006450-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella BT, O’Mahony DJ, Fitzgerald GA. The human thromboxane A2 receptor a isoform (TPa) functionally couples to the G proteins G9 and G11 in vivo and is activated by the isoprostane 8 -epi prostaglandin F2a. J of Pharmacol Exper Ther. 1997;281:957–964. [PubMed] [Google Scholar]
- 22.Kromer BM, Tippins JR. The vasoconstrictor effect of 8-epi prostaglandin F2a in the hypoxic rat heart. Br J Pharmacol. 1999;126:1171–1174. doi: 10.1038/sj.bjp.0702433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med. 1998;158:1524–1527. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- 24.Roberts LJ, Morrow JD. The generation and actions of isoprostanes. Biochem Biophys Acta. 1997;1345:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 25.Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R. Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr Res. 2004;56:88–93. doi: 10.1203/01.PDR.0000130478.05324.9D. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht C, Knaapen AM, Becker A, Höhr D, Haberzettl P, van Schooten FJ, Borm PJ, Schins RP. The crucial role of particle surface reactivity in respirable quartz-induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res. 2005;6(1):129. doi: 10.1186/1465-9921-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–356. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr. 2000;72:1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Cho IH, Kim HS, Jung JE, Kim JE, Lee KH, Park T, Yang YM, Seong SY, Ye SK, Chung MH. Anti-inflammatory effects of 8-hydroxydeoxyguanosine in LPS-induced microglia activation: suppression of STAT3-mediated inter-cellular adhesion molecule-1 expression. Exp Mol Med. 2006;38:417–427. doi: 10.1038/emm.2006.49. [DOI] [PubMed] [Google Scholar]
- 30.Witherell HL, Hiatt RA, Repogle M, Parsonnet J. Helicobacter pylori and urinary excretion of 8-hydroxy-2-deoxyguanosine, and oxidative DNA adduct. Cancer Epidem, Biomarkers & Prev. 1998;7:91–96. [PubMed] [Google Scholar]
- 31.Kinnula VL, Crapo JD. Superoxide diusmutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 32.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Keeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 33.Ramos J, Matamoros MA, Naya L, James EK, Rouhier N, Sato S, Tabata S, Becana M. The glutathione peroxidase gene family of Lotus japonicus: characterization of genomic clones, expression analyses and immunolocalization in legumes. New Phytol. 2009;181(1):103–114. doi: 10.1111/j.1469-8137.2008.02629.x. [DOI] [PubMed] [Google Scholar]
- 34.McCurnin DC, Pierce RA, Willis BC, Chang LY, Yoder BA, Yuhanna IS, Ballard PL, Clyman RI, Waleh N, Maniscalco W, Crapo JD, Grubb PH, Shaul PW. Postnatal estradiiol up-regulates lung nitric oxide synthases and improves lung function in bronchopulmonary dysplase. Am J Respir Crit Care Med. 2009;179:492–500. doi: 10.1164/rccm.200805-794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vyas-Read S, Shaul PW, Yuhanna IS, Willis BC. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L212–L221. doi: 10.1152/ajplung.00475.2006. [DOI] [PubMed] [Google Scholar]
- 36.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cel mol Physiol. 2005;288:L450–L459. doi: 10.1152/ajplung.00347.2004. [DOI] [PubMed] [Google Scholar]
- 37.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 38.Gomez NN, Davicino RC, Biaggio VS, Bianco GA, Alvarez SM, Fischer P, Masnatta L, Rabinovich GA, Gimenez MS. Overexpression of inducible nitric oxide synthase and cyclooxygenase-2 in rat zincdeficient lung: Involvement of a NF-kappaB dependent pathway. Nitric Oxide. 2006;14:30–38. doi: 10.1016/j.niox.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Ovadia B, Bekker JM, Fitzgerald RK, Kon A, Thelitz S, Johengen MJ, Hendricks-Munoz K, Gerrets R, Black SM, Fineman JR. Nitric oxide-endothelin-1 interactions after acute ductal constriction in fetal lambs. Am J Physiol Heart Circ Physiol. 2002;282:862–871. doi: 10.1152/ajpheart.00417.2001. [DOI] [PubMed] [Google Scholar]
- 40.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Human Reprod 1998. 4:3–24. doi: 10.1093/humupd/4.1.3. Update. [DOI] [PubMed] [Google Scholar]
- 41.Salvemini D, et al. Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallat A, Gallois C, Tao J, Habib A, Maclouf J, Mavier P, Préaux AM, Lotersztajn S. Platelet-derived growth factor-BB and thrombin generate positive and negative signals for human hepatic stellate cell proliferation. Role of a pros-taglandin/cyclic AMP pathway and cross-talk with endothelin receptors. J Biol Chem. 1998;273:27300–27305. doi: 10.1074/jbc.273.42.27300. [DOI] [PubMed] [Google Scholar]
- 43.Yousufzai SY, Abdel-latif AA. Endothelin-1 stimulates the release of arachidonic acid and pros-taglandins in cultrured human ciliary muscle cells: activation of phospholipase A2. Exp Eye Res. 1997;65:73–81. doi: 10.1006/exer.1997.0305. [DOI] [PubMed] [Google Scholar]
- 44.Burri PH. The postnatal growth of the rat lung III. Morphology. Anat Rec. 1974;180:77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- 45.Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest. 1985;76:1297–1305. doi: 10.1172/JCI112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagai A, Katayama M, Thurlbeck WM, Matsui R, Yasui S, Konno K. Effect of indomethacin on lung development in postnatal rats: possible role of prostaglandin in alveolar formation. Am J Phys. 1995;268:L56–L62. doi: 10.1152/ajplung.1995.268.1.L56. [DOI] [PubMed] [Google Scholar]