Abstract
Carcinoids are slow growing neuroendocrine tumors that often cause debilitating symptoms due to excessive secretion of hormones such as serotonin. Surgery is the only potentially curative treatment, but many patients have unresectable metastatic disease. Lithium is a non- competitive inhibitor of GSK-3 with an established safety profile. The objective of this study was to investigate the effects of lithium on carcinoid cell growth in vitro. Lithium treatment caused a dose-dependent reduction in carcinoid cancer cell (BON and H727) growth. Western blot analysis revealed increased expression of cleaved poly (ADP-ribose) polymerase (PARP), indicating the induction of apoptosis. Lithium treatment also suppressed cellular levels of serotonin and chromogranin A. In summary, lithium inactivates GSK-3, induces apoptosis, and suppresses carcinoid cancer cell growth in vitro. The drug has been used clinically since the 19th century to treat a variety of diseases including bipolar disorder, and its safety profile is well documented. Therefore, based on these findings, we have undertaken a clinical trial of lithium chloride in the treatment of patients with unresectable carcinoid cancer.
Keywords: Carcinoid tumors, neuroendocrine tumors, H727 cells, BON cells, lithium chloride, chromogranin A, Achaete-Scute Complex-Like 1, serotonin
Introduction
Carcinoid tumors are neoplasms that arise from the disseminated neuroendocrine (NE) cells of the gastrointestinal (GI) tract, lungs, and other organs. The incidence of carcinoid tumors in the United States is estimated at 1.5 cases per 100,000 people, or approximately 2,500 new cases of carcinoid tumors per year [1,2]. While they are slow growing compared to adenocarcinomas, carcinoids frequently metastasize to the liver, and can cause debilitating symptoms due to tumor secretion of bioactive amines and peptides such as serotonin. Surgery is the only potentially curative treatment, but complete resection is often impossible due to widespread disease [3-5]. Other conventional cancer treatments such as chemotherapy and radiation therapy are generally not effective for carcinoids. Therefore, there is a pressing need for new therapeutic approaches for advanced carcinoid disease.
An understanding of the molecular biology of carcinoid tumor cells may lead to novel targeted therapies for these malignancies. Several signal transduction pathways have been implicated in the growth and hormone production of carcinoids. These include the Notch1-HES-ASCL1 pathway, Ras-Raf-MEK-MAPK pathway, PI3K-Akt -MAPK pathway, and the glycogen synthase-3β (GSK- 3β) pathway [6-10]. Strategies to target these pathways in carcinoids have been described [11]. Treatment of pheochromocytomas and medullary thyroid cancer cells with lithium chloride (LiCl) resulted in significant growth reduction. However, the effects of LiCl in carcinoid cells are not known. Based on our earlier data on other neuroendocrine tumors, we hypothe-sized that LiCl treatment would inhibit growth of carcinoid cells via inactivation of GSK-3. In this study we demonstrate that treatment of GI and bronchopulmonary carcinoid tumor cells with LiCl results in suppression of neuroendocrine hormones and tumor markers such as serotonin and chromogranin A. Importantly, treatment also leads to inhibition of carcinoid cancer cell growth in vitro. Finally, we show that the mechanism of growth inhibition is the induction of apoptosis. These results suggest that LiCl has potential as a new therapeutic approach for advanced carcinoid tumor disease.
Materials and methods
Cell culture
BON human pancreatic carcinoid tumor cells, kindly provided by Drs. Mark Evers and Courtney Townsend, Jr. (University of Texas, Galveston, TX, USA), and H727 human bronchopulmonary carcinoid cells (ATCC, Manassas, VA, USA) were maintained as previously described [12;13]. Carcinoid cells were treated with varying concentration of LiCl (0 to 30 mM).
Immunoblot analysis
After treatment with LiCl, carcinoid cell lysates were prepared as previously described [12,13]. Total protein concentrations were quantified with a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). Denatured cellular extracts were resolved by 10% SDS-PAGE, transferred to nitro-cellulose membranes (Schleicher and Schuell, Keene, NH, USA), blocked in milk, and incubated with appropriate antibodies. The following antibody dilutions were used: 1:1,000 for GSK-3β, phospho-GSK-3β, poly (ADP-ribose) poly-merase (PARP; Cell Signaling, Beverly, MA, USA), chromogranin A (CgA; Zymed Laboratories, San Francisco, CA, USA), and mammalian achaete-scute homolog 1 (ASCL1; BD Pharmingen, San Diego, CA, USA), and 1:10,000 for glyceralde-hyde-3-phosphate dehydrogenase (GAPDH; Trevigen, Gaithersburg, MD, USA). Horseradish peroxidase conjugated goat antirabbit or goat antimouse secondary antibodies (Pierce, Rockford, IL, USA) were used depending on the source of the primary antibody. For visualization of the protein signal, Immunstar (Bio-Rad Laboratories, Hercules, CA, USA) or SuperSignal West Femto Kit (Pierce) were used for all immunoblots as described previously [9,10].
Cell proliferation assay
Cell proliferation was measured by the MTT (thiazolyl blue tetrazolium bromide; Sigma-Aldrich, St. Louis, MO, USA) rapid colorimetric assay as previously described [13]. In brief, cells were seeded in triplicate on 24-well plates and incubated for 24 h under standard conditions to allow cell attachment. The cells were then treated with various concentrations of LiCl and incubated for up to 6 days. The MTT assay was performed by replacing the standard medium with 250 μL of serum-free medium containing MTT (0.5 mg/mL) and incubated at 37° C for 3 h. After incubation, 750 μL of dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) was added to each well and mixed thoroughly. The plates were then measured at 540 nm using a spectrophotometer (µQuant, Bio-Tek Instruments, Winooski, VT, USA).
Cell death detection ELISA
To confirm that carcinoid cell growth inhibition after LiCl treatment is mediated by apoptosis, a cell death detection ELISA kit (Roche, Indianapolis, IN, USA) was used for BON cells treated with various concentrations of LiCl as indicated. Total cellular extracts from the LiCl treated cells were analyzed for the presence of mono- and oligonucleosomes in the fractions as described by the manufacturer’s protocol and the absorbance was measured at 405 nm.
Serotonin ELISA
To measure the effect of LiCl treatment on carcinoid cell production of serotonin, a hormone implicated in the carcinoid syndrome, we used a serotonin ELISA (Research Diagnostics Inc., Flanders, NJ) as described previously [7].
Statistical analysis
Analysis of variance (ANOVA) with Bonferroni post hoc testing was performed using a statistical analysis software package (SPSS version 10.0, SPSS, Chicago, IL, USA). A p value of < 0.05 was considered statistically significant.
Results
Lithium chloride inhibits the growth of carcinoid cells
To determine the effects of LiCl on carcinoid cell growth, two different carcinoid cell lines, BON and H727, were utilized. BON GI carcinoid cells were initially treated with LiCl in concentrations up to 50 mM for 2 and 4 days, and the MTT assay was used to quantify cell proliferation. As shown in Figure 1A, the half-maximal inhibitory concentration (IC50) of LiCl in BON cells was determined to be approximately 25 mM at 4 days. Based on this finding, concentrations of LiCl ranging from 0 to 20 mM were used to treat BON and H727 cells for a 6-day proliferation assay. Treatment of BON cells with LiCl resulted in a dose-dependent inhibition of growth (Figure 1B). At a LiCl concentration of 10 mM, modest growth inhibition was seen. However, at higher doses such as 15 and 20 mM, the growth suppression effect of LiCl was pronounced. Similarly, H727 bronchopulmonary carcinoid cells showed decreased cell viability in a dose-dependent manner, with a maximal effect between 15 and 20 mM of LiCl treatment (Figure 1C). The differences in the rate of carcinoid tumor cell growth seen with different concentrations of LiCl treatment were statistically significant (p <0.005).
Figure 1.
Lithium inhibits growth of carcinoid cells. BON and H727 carcinoid cells were treated with the indicated concentrations of lithium chloride (LiCl) and the MTT assay was performed to measure cell proliferation. A. The half- maximal inhibitory concentration (IC50) of LiCl in GI carcinoid BON cells was determined in a 4-day experiment. The IC50 of LiCl for BON was 25 mM at day 4. B. Concentrations of LiCl up to the IC50 were then used to perform a 6-day growth proliferation assay. There was a dose-dependent reduction of cellular proliferation with LiCl. C. Bronchopulmonary carcinoid H727 cells were treated with similar concentrations of LiCl as indicated. Like BON cells, H727 cells also showed growth reduction in a dose-dependent manner.
Lithium chloride phosphorylates GSK-3β and suppresses chromogranin A in carcinoid tumor cells
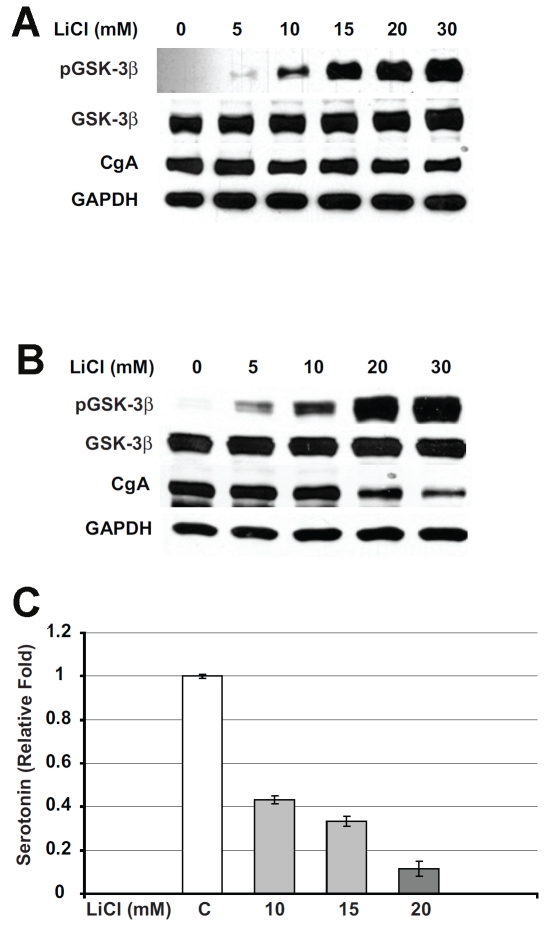
We next confirmed that LiCl-mediated growth inhibition in carcinoid cells is due to the inactivation of GSK- 3β by phosphorylation at the serine 9 residue. Western blot analysis on cell extracts from BON cells treated with increasing concentrations of LiCl showed a small increase in regular GSK-3β. However, a significant dose-dependent increase in phosphorylated GSK-3β was seen after LiCl treatment (Figure 2A). No phosphorylated GSK-3β was present in untreated control BON cells. Similarly, H727 cells treated with LiCl also showed an increase in phosphorylated GSK-3β in a dose-dependent manner (Figure 2B).
Figure 2.
Lithium treatment leads to GSK-3β phos-phorylation and suppression of neuroendocrine tumor markers in carcinoid cells. Carcinoid cells were treated with the indicated concentrations of lithium chloride (LiCl) for 2 days and immunoblotting was performed on whole cell lysates for phosphorylated GSK-3β, total GSK-3β, and the neuroendocrine markers chromogranin A (CgA) and serotonin. A. LiCl treatment increased the level of phosphorylated GSK-3β, whereas total GSK-3β was relatively unchanged in BON cells. GAPDH was used as a loading control. B. Total cellular extracts from H727 cells treated with LiCl were analyzed by Western blot for the levels of phosphorylated GSK-3β and neuroendocrine markers. LiCl treatment led to an increase in phosphorylated GSK-3β protein. Note that in control cells there was no detectable level of phosphorylated GSK-3β. In contrast to this, regular active GSK -3β was present at high levels in both control and treated cells with no change in the levels in treated cells. The increase in the levels of phosphorylated GSK-3β was associated with a significant reduction in CgA. GAPDH was used to confirm equal protein loading. C. Serotonin levels were measured by ELISA in LiCl-treated BON carcinoid cell lysates. Lithium treatment dose-dependently reduced the levels of serotonin in BON cells.
Having demonstrated that phosphorylation of GSK-3β with LiCl is associated with growth inhibition in carcinoid tumor cells, we then analyzed its effect on cellular production of the neuroendocrine tumor marker chromogranin A (CgA). CgA was reduced with increasing amounts of phosphorylated GSK-3β in both BON and H727 cells. However, H727 cells showed a more marked reduction in CgA than BON cells.
Patients with carcinoid tumors frequently suffer from the carcinoid syndrome, a constellation of debilitating symptoms which include diarrhea, wheezing, and flushing. The carcinoid syndrome is caused by tumor overproduction of hormones such as serotonin. We measured the levels of serotonin in BON cells after treatment with LiCl. As shown in Figure 3A, there was a significant reduction in relative serotonin levels in cells treated with LiCl.
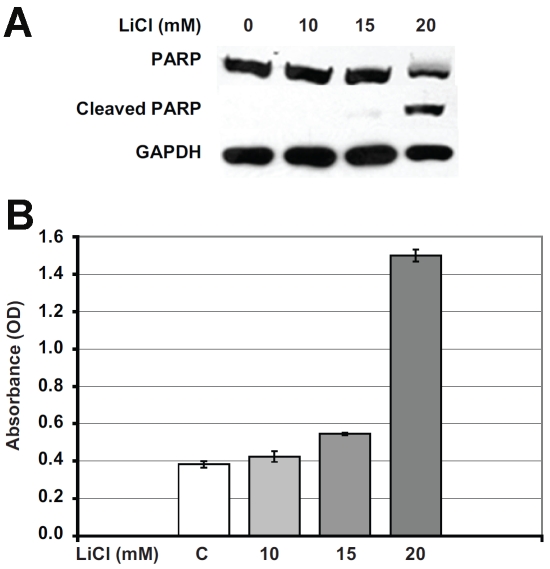
Figure 3.
Apoptosis-mediated carcinoid cell growth inhibition by lithium treatment. A. To determine the mechanism of growth inhibition, BON cells were treated with LiCl and assayed for apoptosis, as measured by cleavage of poly (ADP-ribose) polymerase (PARP). At 20 mM LiCl concentration, there was a significant cleavage of PARP protein indicating that the growth inhibition is mediated by apoptosis. GAPDH was used as a protein loading control in all Western blots. B. To further confirm that growth inhibition is mediated by apoptosis, a cell death detection ELISA was performed using LiCl treated BON cell extracts. At 20mM LiCl concentration, there was a marked increase in absorbance due to the increase in mono- and oligonucelosomes in the cytoplasmic fraction of the cell lysates, indicating the activation of apoptosis.
LiCl treatment leads to apoptosis in carcinoid cells
We were interested to determine if the reduction in cellular proliferation of lithium-treated carcinoid cells was due to apoptosis. One of the processes of apoptosis is carried out by activation of a cascade of proteolytic enzymes belonging to the caspase family. Caspase-mediated cleavage of proteins can result in activation of nuclear poly (ADP-ribose) polymerase (PARP), which plays an important role in both DNA synthesis and repair, and is cleaved early in the apoptotic process. We performed Western analysis on cell extracts treated with LiCl for protein expression of markers of apoptosis. As shown in Figure 3B, 4-day treatment with 20 mM of LiCl led to PARP cleavage, indicating the presence of apoptosis. To independently validate the activation of the apoptotic process, we performed an apoptosis-detecting ELISA on cell lysates after LiCl treatment. As shown in Figure 3C, there was clearly an increase in the absorbance of the 20 mM LiCl treated lysates, indicating apoptosis. These results indicate that the decrease in cellular proliferation of lithiumtreated carcinoid cells is mediated by apoptosis.
Discussion
The element lithium was isolated in the early 19th century. After the observation was made that solutions of lithium dissolve uric acid crystals, lithium salts were used to treat a variety of diseases such as uremia, kidney stones, gout, and rheumatism. In the 1870s, Carl Lange in Denmark and William Hammond in New York used lithium to treat patients with mania. In 1949 this treatment for mania was rediscovered by the Australian psychiatrist John Cade. After noting that guinea pigs became lethargic when given lithium, Cade performed a trial of lithium in patients with severe mania. Further trials led to the approval in 1970 by the United States Food and Drug Administration of lithium for the treatment of mania, and today the drug is widely used for this indication [14-17].
Inactivation of GSK-3β with specific inhibitors has been shown to reduce tumor growth in several malignancies including those of the pancreas [18, 19], prostate, [20] and colon [21]. GSK-3β is also known to interact with other important signal transduction pathways in cancer. In two recent studies, Wang and colleagues reported that GSK-3 inhibition in colon cancer cells resulted in activation of ERK1/2 and induction of COX-2 and IL-8 (20), and Dong et al. showed that GSK-3β plays a key role in rapamycin-mediated cell cycle regulation and chemo-sensitivity in breast cancer cells [22]. We found that raf-1 pathway activation in medullary thyroid cancer cells leads to GSK-3β phosphorylation and inhibition of cell growth [9]. In 1996, Klein and Melton reported that lithium was a potent inhibitor of GSK3β [23]. Therefore, based on these observations, GSK-3β inhibition with lithium and similar drugs is an attractive chemotherapeutic target.
In the present study we report that LiCl treatment leads to a dose-dependent inhibition of proliferation in carcinoid cancer cells. Cell lysates from LiCl treated carcinoids resulted in induction of inactive, phosphorylated GSK-3β.
The mechanism of Li-mediated growth suppression in carcinoid cells is induction of apoptosis. This is in contrast to medullary thyroid cancer cells, in which GSK-3β-related growth inhibition is due to cell cycle arrest. It seems that inactivation of GSK-3β affects cellular proliferation in many cancer cell lines but the mechanism of action is cell type specific. In addition to inhibition of growth, lithium treatment also caused a marked reduction in neuroendocrine tumor markers and hormones such as CgA and serotonin.
In summary, the present study extends our understanding of the importance of GSK signaling in cancer and the potential of lithium as treatment for neuroendocrine tumor disease. We have demonstrated that lithium treatment in gastrointestinal and bronchopulmonary carcinoid tumor cells leads to suppression of the neuroendocrine phenotype and inhibition of cell proliferation in vitro. Lithium has been used for decades in the treatment of mania, and its safety profile is well established. Based on these studies, a clinical trial of lithium for patients with carcinoid tumor disease is underway at our institution.
Acknowledgments
This research was supported by grant P30 CA014520 from the National Cancer Institute, a Research Scholars Grant from the American Cancer Society (HC), National Institutes of Health grants DK063015, DK064735, DK066169, and CA109053 (HC), the George H.A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons (HC), a grant from the Vilas Foundation (HC), the Robert Draper Technology Innovation Award (MK), and research grants from the Carcinoid Cancer Foundation (HC & MK).
References
- 1.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102(7):1464–1473. doi: 10.1111/j.1572-0241.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 3.Lal A, Chen H. Treatment of advanced carcinoid tumors. Curr Opin Oncol. 2006;18(1):9–15. doi: 10.1097/01.cco.0000198018.53606.62. [DOI] [PubMed] [Google Scholar]
- 4.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13(12):1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am. 2006;15(3):463–478. doi: 10.1016/j.soc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138(6):1137–1142. doi: 10.1016/j.surg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kunnimalaiyaan M, Chen H. The Raf-1 pathway: a molecular target for treatment of select neuro-endocrine tumors? Anticancer Drugs. 2006;17(2):139–142. doi: 10.1097/00001813-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase -3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6(3):1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 10.Kunnimalaiyaan M, Ndiaye M, Chen H. Neuroendocrine tumor cell growth inhibition by ZM336372 through alterations in multiple signaling pathways. Surgery. 2007;142(6):959–964. doi: 10.1016/j.surg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinchot SN, Pitt SC, Sippel RS, Kunnimalaiyaan M, Chen H. Novel targets for the treatment and palliation of gastrointestinal neuroendocrine tumors. Curr Opin Investig Drugs. 2008;9(6):576–582. [PMC free article] [PubMed] [Google Scholar]
- 12.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 13.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4(6):910–917. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 14.Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2(10):349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 15.Cade JF. Recent advances in the use of lithium. Aust N Z J Psychiatry. 1971;5(1):3–4. doi: 10.3109/00048677109159340. [DOI] [PubMed] [Google Scholar]
- 16.Cade JF. Lithium–when, why and how? Med J Aust. 1975;1(22):684–686. [PubMed] [Google Scholar]
- 17.McIntyre RS, Mancini DA, Parikh S, Kennedy SH. Lithium revisited. Can J Psychiatry. 2001;46(4):322–327. doi: 10.1177/070674370104600402. [DOI] [PubMed] [Google Scholar]
- 18.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kap-paB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 19.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12(17):5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene. 2004;23(47):7882–7892. doi: 10.1038/sj.onc.1208068. [DOI] [PubMed] [Google Scholar]
- 21.Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, et al. Inhibition of GSK-3beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 2007 doi: 10.1111/j.1349-7006.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong J, Peng J, Zhang H, Mondesire WH, Jian W, et al. Role of glycogen synthase kinase 3beta in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 2005;65(5):1961–1972. doi: 10.1158/0008-5472.CAN-04-2501. [DOI] [PubMed] [Google Scholar]
- 23.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]