Abstract
Cytoplasmic lipid droplets (LDs) are organelles in which cells store neutral lipids for use as an energy source in times of need, but they also play important roles in the regulation of key metabolic processes. Although LDs are essential for normal cell function, excess accumulation of intracellular lipid is associated with several metabolic diseases, including obesity, type 2 diabetes, and atherosclerosis. The function of LDs is regulated by their associated proteins, including the members of the PAT family: perilipin, adipophilin/adipose differentiation–related protein, tail-interacting protein 47, S3-12, and OXPAT/myocardial LD protein/lipid-storage droplet protein 5. In this review we discuss the PAT proteins in two cardiovascular contexts: 1) in the atherosclerotic vessel wall, where LDs within macrophage foam cells store cholesteryl esters derived from modified lipoproteins, and 2) in the myocardium, where LDs store fatty acids, the major energy substrate for normal heart function, as triglyceride.
Introduction
Previously considered intracellular depots where cells store neutral lipids, mostly triglycerides (TG) and cholesteryl esters (CE) to be used in times of need, we currently understand lipid droplets (LDs) as dynamic functional organelles. LDs play crucial roles in cellular energy balance and in the regulation of other important cellular processes [1–4]. The structure of LDs resembles that of plasma lipoproteins, with a core of lipid esters (neutral lipid) coated and stabilized in the cytoplasm by an interface composed of a monolayer of phospholipids, free cholesterol (FC), and proteins. Proteomic analyses have identified many LD-associated proteins, including enzymes, lipid transporters, and caveolins [5,6], but the most abundant proteins in LDs are the PAT family proteins, which are characterized by sequence similarity and localization on lipid droplets [7,8]. The PAT family takes its name from the founding member, perilipin, as well as adipose differentiation–related protein (ADFP) and tail-interacting protein of 47 kDa (TIP47). The remaining two mammalian family members are S3-12 and OXPAT. Although perilipin, TIP47, and S3-12 are generally the only names for these proteins used in the LD literature, ADFP is also known as adipophilin (the original name for the human ortholog), ADPH, and ADRP. OXPAT is also known as myocardial LD protein (MLDP) and as lipid-storage droplet protein 5 (LSDP5). For consistency and clarity in this review, we refer to these proteins as perilipin, ADFP, TIP47, S3-12, and OXPAT. The fundamental importance of PAT protein function is reflected in the fact that members of the family have also been identified in insects, fungi, and slime molds [8,9].
Perilipin and ADFP are exclusively associated with LDs and are degraded by proteosomal and/or lysosomal pathways when not bound to LDs. In contrast, TIP47, S3-12 and OXPAT exchange on and off LDs depending on the cell's metabolic state and are stable both when associated with LDs or when in the cytoplasm [10–12]. ADFP and TIP47 have a ubiquitous tissue distribution, and ADFP is the most abundant LD-associated protein in cells that do not express perilipin. Expression of perilipin is restricted to white and brown adipose tissue, but lower levels are also found in steroidogenic tissues. S3-12 is expressed mostly in white adipose tissue, but lower levels are also found in heart and skeletal muscle. The expression of OXPAT is enriched in tissues capable of high rates of fatty acid β-oxidation, such as brown adipose tissue, fasted liver, heart and slow-twitch muscles [13••,14••,15••].
The best-characterized member of the PAT family is perilipin, which has a dual function in adipocytes [16,17]. Under basal conditions perilipin limits access of cellular lipases, such as hormone-sensitive lipase (HSL) and adipocyte triglyceride lipase (ATGL), to the core of TG within LDs, thereby preventing uncontrolled TG hydrolysis and limiting release of free fatty acids and glycerol. However, activation of protein kinase A by rising levels of cyclic adenosine monophosphate leads to polyphosphorylation of perilipin and stimulation of TG lipase activity. The molecular mechanisms of this activation are still being investigated but involve interactions between perilipin, HSL, ATGL, and CGI-58, an activator of ATGL [7]. Consistent with this model, perilipin-knockout mice have increased basal lipolysis but reduced stimulated lipolysis in isolated adipocytes and in the whole animal [18,19].
In healthy cells, LDs play a very important role in the control of lipid homeostasis, serving as energy reservoirs and providing substrates to fuel cellular oxidative processes when needed, and providing lipids for membranes, membrane trafficking, signaling, and protein modification. However, the hypercaloric and fat-rich diet characteristic of Western countries has resulted in an epidemic of metabolic disorders, such as obesity, insulin resistance, diabetes, and cardiovascular disease, which are associated with excessive or aberrant lipid accumulation in adipose and nonadipose tissues. Understanding the mechanisms by which lipids are stored in and mobilized from LDs will illuminate both the physiology of healthy cells and tissues and the basis of some important metabolic diseases. In this review, we provide an overview of the role of the PAT family proteins in LD metabolism in different cells of the cardiovascular system.
Vascular Cells: Foam Cell Formation and Atherosclerosis
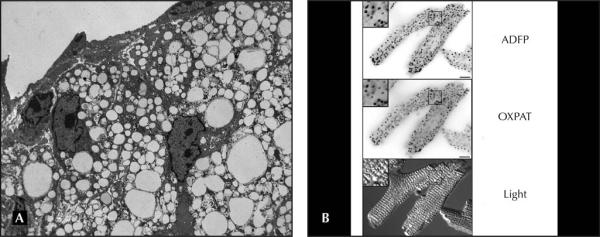
Atherosclerosis is the main underlying pathology of cardiovascular disease and stroke, which are the number one and number three causes of death in the developed countries [20]. Atherosclerosis has its origins in the response of the arteries to subendothelial accumulation of modified low-density lipoproteins (mLDL). Circulating monocytes migrate to the subendothelial space and take up mLDL via scavenger receptor (SR)–mediated endocytosis. Interestingly, unlike the LDL receptor, the SRs are not down-regulated by intracellular lipids, which leads to unfettered uptake of cholesterol-rich mLDL by subendothelial macrophages. Once internalized, the CE carried by the mLDL is hydrolyzed in lysosomes by the acid lipase, and FC is exported to the cytosol. Part of the FC can be effluxed to extracellular acceptors or used as a structural component of cell membranes, but a large proportion of this FC is re-esterified in the endoplasmic reticulum (ER) by the acyl-coenzyme A (CoA):cholesterol acyltransferase-1 (ACAT-1) and stored in LDs. The accumulation of LDs in the cytoplasm of lipid-laden macrophages gives them a foamy appearance; therefore, lipid-laden macrophages in atherosclerotic lesions are commonly known as “foam cells” (Fig. 1A). Foam cells are a hallmark of atherosclerosis through all stages of lesion development [21]. Furthermore, plaque rupture, a key event in arterial thrombosis, usually happens in foam cell–rich areas of the lesions [22].
Figure 1.
A, Electron microscopy image of lipid-laden macrophages resident in a mouse atherosclerotic lesion. The foamy appearance of the cytoplasm is due to abundant lipid droplets (LDs). Similar images have been reported previously [24••]. B, Photomicrographs of isolated mouse cardiomyocytes that contain scattered LDs coated by adipose differentiation–related protein (ADFP) and oxidative tissues–enriched PAT protein (OXPAT), as detected by immunofluorescence microscopy. Similar images have been reported previously [14••].
Among the proteins of the PAT family, ADFP plays a major role in foam cell formation and in the pathogenesis of atherosclerosis. ADFP expression increased in response to different forms of lipid loading in primary human monocytes [23], in thioglycollate-elicited mouse peritoneal macrophages [24••], and in several macrophage/monocytic cell lines [25,26•,27]. Further, ADFP overexpression increased lipid accumulation in THP-1 macrophages upon incubation with acetylated (ac) LDL, whereas depletion of ADFP using small interfering (si) RNA reduced lipid accumulation [26•]. Peritoneal macrophages isolated from ADFP-deficient mice displayed a substantial reduction in the accumulation of cytoplasmic LDs following incubation with oxidized (ox) LDL and acLDL [24••]. Analysis of human carotid endarterectomy specimens and coronary arteries by reverse transcription polymerase chain reaction (RT-PCR) and in situ hybridization demonstrated high ADFP expression localized mostly in a subset of lipid-rich macrophages [28]. Furthermore, RT-PCR analysis of carotid endarterectomy specimens showed that ADFP expression was 3.5-fold higher in atherosclerotic plaques than in healthy areas of the same artery [26•]. Interestingly, the expression of ADFP in the atherosclerosis-studded arteries of apolipoprotein E-deficient mice in C57BL/6J background was also about 3.5-fold higher than in the atherosclerosis-free arteries of wild-type C57BL/6J mice [24••]. Thus, lipid accumulation and ADFP expression in macrophages positively correlate with one another, regardless of whether lipid accumulation is being driven by lipid loading or whether ADFP levels are being manipulated by overexpression or RNA interference.
These data have been extended in a murine model of ADFP deficiency. The effects of Adfp gene inactivation in apolipoprotein E–deficient mice significantly reduced the number of LDs in foam cells in atherosclerotic lesions, and both the global inactivation of ADFP and the specific inactivation in bone marrow–derived cells protected mice against atherosclerosis [24••]. From a more mechanistic viewpoint, ADFP overexpression increased lipid accumulation by preventing cholesterol efflux from THP-1 macrophages [26•]. Although siRNA knockdown of ADFP did not result in the expected increase in cholesterol efflux from THP-1 macrophages, peritoneal macrophages isolated from ADFP-deficient mice displayed greater cholesterol efflux than those isolated from their wild-type litter mates [24••]. These data suggest that, by facilitating CE confinement in the LDs, ADFP hinders the reverse transport of cholesterol out of atheromatous lesions.
How does ADFP promote macrophage LD accumulation and foam cell development? Clues are available from studies of triglyceride lipolysis in nonmacrophages. In those studies, forced ADFP expression promoted TG accumulation by inhibition of TG hydrolysis that was associated with decreased association of the major murine TG lipase, ATGL, with LDs [29]. It is possible that in macrophages ADFP excludes one or more of the proposed cholesteryl ester hydrolases from LDs. However, the rate of CE hydrolysis was similar in mouse macrophages whether they expressed ADFP or not. On the other hand, one group has reported that ADFP overexpression led to increased TG content in THP-1 macrophages by stimulating the incorporation of fatty acyl CoAs into TG and by inhibiting fatty-acid oxidation [30].
Although ADFP clearly plays a significant role in regulating foam cell formation, it is still a controversial issue whether the other PAT proteins also play a role. Initial studies restricted the expression of perilipin to adipose and steroidogenic tissues, but there are more recent reports of perilipin expression in human macrophage cell lines and in human atherosclerotic lesions. Perilipin protein has been detected in THP-1 and human monocyte-derived macrophages [31••,32–34]. In human atherosclerotic lesions, Faber et al. [35] showed perilipin expression in foam cells adjacent to ruptured plaques, but it was completely absent in stable plaques. Forcheron et al. [36] reported the presence of perilipin in atheromata, but also in healthy arterial wall. In contrast, Hofnagel et al. [31••] observed strong perilipin staining in regions rich in intimal macrophages and weak staining in regions of medial smooth muscle cells of advanced atherosclerotic lesions, but they did not detect perilipin in coronary arteries with early and intermediate lesions.
The strongest evidence supports perilipin expression in advanced or ruptured human atherosclerotic lesions, where it might influence the metabolism of LDs in macrophages or even replace ADFP. A scenario in which a PAT protein is replaced by another family member would not be new. For example, Adfp gene expression is induced early at day 1 of adipocyte differentiation and ADFP coats the initial LDs in early adipocytes; but after the onset of perilipin expression, ADFP is displaced from the LDs and, despite high ADFP mRNA expression, ADFP protein is hardly detectable in the mature adipocyte [37]. However, ADFP coats the surface of LDs of mouse perilipin-null adipocytes [19], and it is possible that, in case of ADFP depletion, perilipin could eventually take over its role in foam cells. In at least one other pathologic condition of lipid accumulation, human hepatic steatosis, perilipin appears to be ectopically expressed in hepatocytes [38]. However, several arguments exist against the possibility that perilipin plays a major role in foam cell formation or that it could become a substitute for ADFP. In contrast to ADFP expression in macrophages, perilipin expression is constitutive: perilipin was not up-regulated by lipid loading [32,34], nor in response to knockdown of ADFP by siRNA [16,32]. Furthermore, perilipin was not detected in mouse lipid-laden macrophages that do not express ADFP [24••].
Another strong candidate to play a role in foam cell formation is TIP47, the PAT protein with the highest structural similarity and the most similar tissue distribution to ADFP [7]. In fact, TIP47 has been shown to be up-regulated in mouse fibroblasts isolated from ADFP-deficient embryos [39], but no compensatory up-regulation was observed in livers and atherosclerotic lesions of ADFP-deficient mice [24••,40]. Furthermore, in the mouse macrophage cell line RAW 264.7 and in thioglycollate-elicited mouse peritoneal macrophages, TIP47 was not up-regulated by lipid loading, and TIP47 expression did not increase in peritoneal macrophages isolated from ADFP-deficient mice cultured under basal conditions or upon incubation with oxLDL [9]. TIP47 is also expressed in human THP-1 macrophages [41–43], but no detailed studies on the role of TIP47 in foam cell formation have been performed in human macrophages or in human atherosclerotic lesions. With respect to S3-12, the protein was not detected in mouse macrophages and mouse atherosclerotic lesions [24••], but no studies have been performed using human samples. Similarly, to our knowledge the role of OXPAT in foam cell formation has not been tested yet. It will be important to clarify whether other PAT proteins play a role in foam cell formation, or are able to replace ADFP in foam cells, in order to better understand lipid homeostasis in foam cells and to discover novel therapeutic interventions to prevent foam cell formation. Regarding the issue of compensation in the setting of PAT protein deficiencies, future studies should not only assess the total cellular expression of candidate compensating proteins, but also test the protein composition of LDs under different metabolic conditions.
Cardiomyocytes
The heart is an organ of high energy turnover that can oxidize both fat and carbohydrates. In the fasted state, the energy needs of the cardiomyocyte are predominantly met by β-oxidation of long-chain fatty acids [44]. Although the heart needs to burn large amounts of “fuel” to provide energy for contraction, the healthy heart has a relatively low TG reserve, which is stored in multiple minute LDs distributed throughout the sarcoplasm. However, compared with other tissues such as liver and white adipose tissue, the heart has an extremely high rate of lipolytic turnover. Inhibition of this high turnover by gene knockout of ATGL in the mouse resulted in severe lipid overload of myocardium to the point of mechanical disruption of the contractile elements and early death due to heart failure [45].
Among the PAT proteins that are expressed in the myocardium (ADFP, TIP47, S3-12, and OXPAT), OXPAT deserves particular attention. OXPAT is the fifth and most recently described member of the PAT family, and has been characterized independently by three groups [13••,14••,15••]. These groups agreed on several points that support a physiologically relevant role of this protein as a regulator of lipid homeostasis in the myocardium, where it has been shown to coat the LDs in isolated mouse cardiomyocytes [14••] (Fig. 1B). For example, the three groups found that OXPAT expression is particularly high in tissues that oxidize fat as a primary source of energy and that its expression is upregulated by fasting, a condition of increased free fatty acid (FFA) mobilization from adipose tissue and increased FFA utilization in heart, liver, and skeletal muscle. In addition, they also agreed that OXPAT expression is upregulated by peroxisome proliferator-activated receptor α (PPARα) agonists, and it is well documented that there are relatively high levels of PPARα expression in the heart, where it acts as a critical transcriptional regulator of cardiac lipid and energy substrate metabolism [46]. An interesting point is that the genes coding for the three exchangeable PAT proteins (TIP47, S3-12, and OXPAT) are clustered within 200-kb pairs, in the human, mouse, and rat genomes. Other cases of gene clustering, such as that observed between certain apolipo-proteins, have been shown to facilitate transcriptional and functional interplay between different proteins. An argument against this being the case with the exchangeable PAT proteins is the fact that, although TIP47 is ubiquitously expressed, the tissue distribution of S3-12 and OXPAT is noticeably different, with higher S3-12 expression in white adipose tissue, a tissue specialized in lipid storage, while OXPAT is predominantly expressed in “lipid-consuming” tissues. Nevertheless, since S3-12 is also expressed in heart, albeit at a relatively low level, it is possible that different combinations of exchangeable PAT proteins on the surface of LDs in cardiomyocytes might facilitate the storage or use of TG depending on the myocardial energy requirements under different physiologic situations. Furthermore, certain pathologies, such as diabetic cardiomyopathy, are associated with increased lipid accumulation in the myocardium [47]. Understanding the LD protein composition in the diseased heart may also help to shed light on the pathophysiology of these diseases and perhaps eventually result in new therapeutic approaches.
Conclusions
Despite the relevance of intracellular lipid storage and mobilization to atherosclerosis and cardiomyopathy, studies of the major coat proteins of cytoplasmic LDs, the PAT proteins, in the heart and vascular cells are in the early stages. Most attention in the PAT protein field has centered on triglyceride-containing LDs in adipocytes and the liver. The next decade of PAT protein research will no doubt build on the provocative data that have emerged in the cardiovascular system as reviewed here. Manipulation of ADFP expression dramatically affects the degree of cholesteryl ester accumulation in macrophages and the amount of atherosclerosis in a murine model. Another key finding is that, of all murine tissues, the myocardium expresses the highest levels of OXPAT, the PAT protein that appears to package triglyceride so that it is available for use by β-oxidation. Future work will no doubt explore additional murine models of PAT protein deficiency and excess for their cardiovascular phenotype. Additionally, many other proteins outside the PAT family also coat LDs. Just as OXPAT is particularly enriched on myocardial LDs, so there may be other cardiac-enriched LD proteins that influence cardiac lipid metabolism. The most important priority for the field will be to extend the findings about PAT proteins made in cell- and animal-based systems to human cardiovascular disease. Do genetic variations in the PAT genes account for differences in susceptibility to atherosclerosis or cardiomyopathy? Can we pharmacologically or nutritionally manipulate myocardial and vascular PAT protein levels to protect against lipid overload in these common human diseases? The tools and talent are now available to address these important questions.
Acknowledgments
Supported by National Institutes of Health grants HL-51586 (to Dr. Chan) and DK-068046 (to Dr. Bickel). Dr. Chan was supported in part by the Betty Rutherford Chair from the St. Luke's Episcopal Hospital and Baylor College of Medicine. Dr. Paul was supported in part by a Scientist Development Grant from the American Heart Association, National Research Program, 0535118N. Dr. Bickel thanks Dr. Nathan Wolins and Lora Staloch for expert immunostaining of mouse cardiomyocytes and Ilya Treskov and Dr. Anthony Muslin for help isolating mouse cardiomyocytes.
Footnotes
Disclosures No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Beckman M. Cell biology. Great balls of fat. Science. 2006;311:1232–1234. doi: 10.1126/science.311.5765.1232. [DOI] [PubMed] [Google Scholar]
- 2.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto T, Ohsaki Y, Cheng J, et al. Lipid droplets: a classic organelle with new outfits. Histochem Cell Biol. 2008;130:263–279. doi: 10.1007/s00418-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nature reviews. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 5.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Ying Y, Zhao Y, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 7.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Miura S, Gan JW, Brzostowski J, et al. Functional conservation for lipid storage droplet association among perilipin-, ADRP-, and TIP 47-related proteins in mammals, drosophila, and dictylostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Leger RJ. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J Biol Chem. 2007;282:21110–21115. doi: 10.1074/jbc.M609592200. [DOI] [PubMed] [Google Scholar]
- 10.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Wolins NE, Quaynor BK, Skinner JR, et al. S3-12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 12.Wolins NE, Skinner JR, Schoenfish MJ, et al. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 13••.Dalen KT, Dahl T, Holter E, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]; This is one of three independent reports of the fifth PAT protein, MLDP/OXPAT/LSDP5, as being a PPAR-regulated LD protein expressed in tissues with high capacity for fatty acid oxidation, especially heart. It uniquely established that, when ectopically expressed, LSDP5 limits both basal and stimulated lipolysis.
- 14••.Wolins NE, Quaynor BK, Skinner JR, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]; This is one of three independent reports of the fifth PAT protein, MLDP/OXPAT/LSDP5, as being a PPAR-regulated LD protein expressed in tissues with high capacity for fatty acid oxidation, especially heart. It uniquely established that OXPAT coats LDs in cardiomyocytes and, when ectopically expressed, promotes both lipid accumulation and utilization through β-oxidation.
- 15••.Yamaguchi T, Matsushita S, Motojima K, et al. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]; This is one of three independent reports of the fifth PAT protein, MLDP/OXPAT/LSDP5, as being a PPAR-regulated LD protein expressed in tissues with high capacity for fatty acid oxidation, especially heart. It uniquely established that a 33-amino acid repeat motif shared with the other PAT proteins is required for LD binding of MLDP.
- 16.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Tansey JT, Sztalryd C, Hlavin EM, et al. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Lepr (db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 19.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 21.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galis ZS, Sukhova GK, Kranzhofer R, et al. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A. 1995;92:402–406. doi: 10.1073/pnas.92.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buechler C, Ritter M, Duong CQ, et al. Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim Biophys Acta. 2001;1532:97–104. doi: 10.1016/s1388-1981(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 24••.Paul A, Chang BH, Li L, et al. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res. 2008;102:1492–1501. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report established that modulation of a PAT protein (ADFP) can attenuate atherosclerosis in a susceptible murine model.
- 25.Chen JS, Greenberg AS, Tseng YZ, Wang SM. Possible involvement of protein kinase C in the induction of adipose differentiation-related protein by Sterol ester in RAW 264.7 macrophages. J Cell Biochem. 2001;83:187–199. doi: 10.1002/jcb.1225. [DOI] [PubMed] [Google Scholar]
- 26•.Larigauderie G, Furman C, Jaye M, et al. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:504–510. doi: 10.1161/01.ATV.0000115638.27381.97. [DOI] [PubMed] [Google Scholar]; This report showed that ADFP overexpression in a macrophage cell line can increase the capacity for lipid accumulation upon incubation with acetylated LDL.
- 27.Wei P, Taniguchi S, Sakai Y, et al. Expression of adipose differentiation-related protein (ADRP) is conjointly regulated by PU.1 and AP-1 in macrophages. J Biochem. 2005;138:399–412. doi: 10.1093/jb/mvi136. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Reape TJ, Li X, et al. Induced expression of adipophilin mRNA in human macrophages stimulated with oxidized low-density lipoprotein and in atherosclerotic lesions. FEBS Lett. 1999;462:145–150. doi: 10.1016/s0014-5793(99)01521-5. [DOI] [PubMed] [Google Scholar]
- 29.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, et al. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Larigauderie G, Cuaz-Perolin C, Younes AB, et al. Adipophilin increases triglyceride storage in human macrophages by stimulation of biosynthesis and inhibition of beta-oxidation. FEBS J. 2006;273:3498–3510. doi: 10.1111/j.1742-4658.2006.05357.x. [DOI] [PubMed] [Google Scholar]
- 31••.Hofnagel O, Buers I, Schnoor M, et al. Expression of perilipin isoforms in cell types involved in atherogenesis. Atherosclerosis. 2007;190:14–15. doi: 10.1016/j.atherosclerosis.2006.06.010. author reply 16–17. [DOI] [PubMed] [Google Scholar]; This report extended the relevance of PAT proteins to human atherosclerosis. The authors found perilipin expression in human macrophages and smooth muscle cells, and in advanced coronary atherosclerotic lesions, but not in early or intermediate lesions.
- 32.Larigauderie G, Bouhlel MA, Furman C, et al. Perilipin, a potential substitute for adipophilin in triglyceride storage in human macrophages. Atherosclerosis. 2006;189:142–148. doi: 10.1016/j.atherosclerosis.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Fisher BJ, Clair RW, et al. Redistribution of macrophage cholesteryl ester hydrolase from cytoplasm to lipid droplets upon lipid loading. J Lipid Res. 2005;46:2114–2121. doi: 10.1194/jlr.M500207-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Persson J, Degerman E, Nilsson J, Lindholm MW. Perilipin and adipophilin expression in lipid loaded macrophages. Biochem Biophys Res Commun. 2007;363:1020–1026. doi: 10.1016/j.bbrc.2007.09.074. [DOI] [PubMed] [Google Scholar]
- 35.Faber BC, Cleutjens KB, Niessen RL, et al. Identification of genes potentially involved in rupture of human atherosclerotic plaques. Circ Res. 2001;89:547–554. doi: 10.1161/hh1801.096340. [DOI] [PubMed] [Google Scholar]
- 36.Forcheron F, Legedz L, Chinetti G, et al. Genes of cholesterol metabolism in human atheroma: overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler Thromb Vasc Biol. 2005;25:1711–1717. doi: 10.1161/01.ATV.0000174123.19103.52. [DOI] [PubMed] [Google Scholar]
- 37.Brasaemle DL, Barber T, Wolins NE, et al. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- 38.Straub BK, Stoeffel P, Heid H, et al. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 39.Sztalryd C, Bell M, Lu X, et al. Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J Biol Chem. 2006;281:34341–34348. doi: 10.1074/jbc.M602497200. [DOI] [PubMed] [Google Scholar]
- 40.Chang BH, Li L, Paul A, et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robenek H, Lorkowski S, Schnoor M, Troyer D. Spatial integration of TIP47 and adipophilin in macrophage lipid bodies. J Biol Chem. 2005;280:5789–5794. doi: 10.1074/jbc.M407194200. [DOI] [PubMed] [Google Scholar]
- 42.Robenek H, Robenek MJ, Buers I, et al. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280:26330–26338. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- 43.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–1338. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–116. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 45.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 46.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 47.Ruberg FL. Myocardial lipid accumulation in the diabetic heart. Circulation. 2007;116:1110–1112. doi: 10.1161/CIRCULATIONAHA.107.721860. [DOI] [PubMed] [Google Scholar]