Abstract
Extracellular matrix (ECM) is a complex cellular environment consisting of proteins, proteoglycans, and other soluble molecules. ECM provides structural support to mammalian cells and a regulatory milieu with a variety of important cell functions, including assembling cells into various tissues and organs, regulating growth and cell–cell communication. Developing a tailored in vitro cell culture environment that mimics the intricate and organized nanoscale meshwork of native ECM is desirable. Recent studies have shown the potential of hydrogels to mimic native ECM. Such an engineered native-like ECM is more likely to provide cells with rational cues for diagnostic and therapeutic studies. The research for novel biomaterials has led to an extension of the scope and techniques used to fabricate biomimetic hydrogel scaffolds for tissue engineering and regenerative medicine applications. In this article, we detail the progress of the current state-of-the-art engineering methods to create cell-encapsulating hydrogel tissue constructs as well as their applications in in vitro models in biomedicine.
Keywords: biopatterning, cell-encapsulating microfluidic hydrogels, cell microenvironment, extracellular matrix, tissue engineering
Mimicking the extracellular matrix
Cells and tissues are routinely cultured in vitro on 2D substrates [1–3]. However, it has been demonstrated that cells or tissues cultured on 2D substrates (e.g., tissue culture plates or flasks) do not mimic cell growth in vivo, and fail to express certain tissue-specific genes and proteins at levels comparable to those found in vivo. For instance, it has been found that cell–drug interactions in a 2D culture system do not represent the actual working mechanism in vivo. Thus, 2D culture is not appropriate to be used in in vitro drug testing models. This is due to the fact that cells and tissues in vivo are immersed within a 3D network constituting a complex extracellular environment with a highly porous nanotopography, while a 2D culture system is too simple to mimic the native environment (Table 1).
Table 1.
A comparison of cell/tissue behavior under 2D and 3D culture conditions.
| Feature/function | In 2D | In 3D | Ref. |
|---|---|---|---|
| Tissue-specific architecture | Poor | Rich | [220] |
| Cell morphology | Flat, extended | Round, contracted | [17,221] |
| Interactions | Limited | Multiple | [38] |
| Cell motility | Fast, free | Slow, restricted | [6] |
| Cell adhesion | Weak | Strong | [222] |
| Cell growth | Directional | In all directions | [6,223] |
| Cell proliferation | High | Low | [5,6] |
| Apoptosis | Induced | Tissue-like | [223,224] |
| Intracellular stiffness | An order-of-magnitude higher in 3D | [4] | |
| Cell polarization | Partly | Full | [6] |
| extracellular matrix remodeling | Absent or poor | Present | [5] |
| Fluid perfusion | 1D | 3D | [170] |
| Signaling and diffusion | Asymmetric | Nearly symmetric | [225] |
| Metabolic rate | High | Low | [223] |
| Cell survival when exposed to cytotoxic agents | Low | High | [226] |
From a tissue engineering (TE) standpoint, constructing a culture environment that closely mimicks the native tissue, which is composed of the extracellular matrix (ECM), soluble bioactive factors, and products of homo- and hetero-typical cell–cell interactions, is desirable to replicate tissue functions in vitro. However, this remains as one of the major challenges in TE, given the complexity of cell–ECM interactions as well as multicellular architectural features such as repeating tissue units and proper vascular structure. Cells commit to their fate by deriving a vast amount of information from this environment. As a part of the cell environment, ECM has been the most emulated component in TE studies. In native tissue, ECM is mainly a mixture of two classes of macromolecules, glycosaminoglycans and fibrous proteins (e.g., collagen, elastin, fibronectin and laminin), which self-assemble into nanofibrillar supramolecular networks that fill the extracellular space between cells [4]. ECM is a dynamic structure, which provides structural and anchoring support to the cells to improve tissue architecture. It also contributes to signaling, directing cell fate and function through cell–matrix interactions. In addition, the ECM is constantly remodeled by cells during development, homeostasis and wound healing by balancing its synthesis and degradation by a variety of enzymes (e.g., matrix metalloproteinases) [5–6].
Significant advances in the design of artificial matrices have led to an evolution from a simple supporting scaffold to a more complex dynamic biomaterial environment. Ideally, the artificial matrices should: support cell growth and maintenance; provide appropriate mechanical, chemical and biological characteristics mimicking native ECM; and facilitate effective nutrient transfer, gas exchange (i.e., O2 and CO2), metabolic waste removal and signal transduction. Scaffolds in various forms, such as, hydrogel and nanofibers, have been studied and employed for different tissue regeneration purposes.
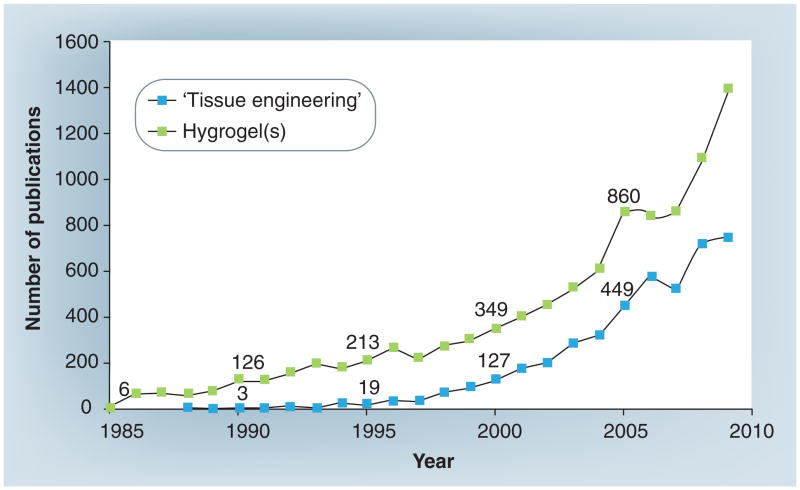
A significant growth of interest in hydrogels started around the 1990s, partly due to the rapid emergence of the TE field, as hydrogels possess characteristics of native ECM [6–16], paving the way for functional tissues (Figure 1) [10,17–19]. The biocompatibility of various hydrogels (e.g., collagen, agarose and polyethylene glycol) is well characterized and the state-of-the-art nano- and microfabrication technologies (e.g., lithography, nano- and micro-fluidics, micromolding and biopatterning) provide the techniques to engineer scaffolds with intricate structures [11,20–25]. However, challenges remain when it comes to engineering functional tissues. Hydrogel-based cell-encapsulating constructs with embedded microchannels have recently been investigated, and became a promising tool to generate active tissue mimics by improving nutrient and gas transport [7,8]. Such cell-encapsulating hydrogel platforms could be employed for other applications, such as in vitro models for drug testing and toxicological assays. Given the intricate nature of the problem, the ultimate success of all these applications requires an interdisciplinary approach involving engineering, chemistry, materials science and cell biology.
Figure 1. The total number of publications with ‘tissue engineering’ and ‘hydrogel’ or ‘hydrogels’ in the title.
The exact numerical values are provided at 5-year intervals (Source: Science Citation Index Expanded [SCI-EXPANDED]). The interest in hydrogels has significantly increased since 1990.
In this article, we present hydrogels as scaffolds to mimic native ECM. Then, we provide a comprehensive description of state-of-the-art technologies by addressing the existing challenges with a focus on cell-encapsulating microfluidic hydrogels. Furthermore, the potential applications of such engineered cell microenvironments are discussed.
Engineered hydrogel scaffolds as ECM mimics
The efforts to engineer a cell microenvironment that mimics the dynamic native ECM have been driven by the clinical demand for tissue (or organ) repair and replacement [18,26]. Construction of functional tissues relies on the structural environment, cell–biomaterial interactions and incorporated biological signals (e.g., growth factors encapsulated in hydrogels) [27]. Thus, the scaffolds must offer properties (i.e., mechanical and chemical) that lead to cellular function in a native manner. In this sense, hydrogels have advantages when utilized as scaffolds for TE as one can easily adjust their physico-chemical (electrical charge and pore size) [28–32], and mechanical (stiffness, tensile strength) [33–34] properties to levels that are desirable for tissue scaffolds [7–9,35–36], cell encapsulation [37–39,227], immobilization [40] and drug delivery [41–44].
Hydrogels are 3D cross-linked insoluble, hydrophilic networks of polymers that partially resemble the physical characteristics of native ECM [16]. Polymers in hydrogel format can absorb a large amount of water or biological fluid (up to 99%) due to the presence of interconnected microscopic pores. Some hydrogels possess features of fluid transport and stimulus responsive characteristics (e.g., pH, temperature and light) [45]. Another appealing feature of hydrogels as scaffolds for TE is their biomechanical similarity to native ECM. The limitation of hydrogel mechanical properties is well known [46]. A hydrogel with the desired mechanical properties (in terms of stiffness and tensile strength [33–34]) can be achieved by adjusting various parameters including the type of polymers used, their concentrations and the crosslinking density [34]. Biocompatible hydrogel scaffolds can be obtained by selecting bio-compatible synthetic or natural polymers and crosslinkers [47].
A variety of natural and synthetic polymers have been used to fabricate hydrogels. Collagen [48], hyaluronic acid [49], chondroitin sulfate [50], fibrin [51], fibronectin [52], alginate [53], agarose [8], chitosan [54] and silk [55] have been the most commonly used natural polymers for TE and regenerative medicine applications. Among all these natural polymers, collagen has been the most widely investigated since it is the most abundant structural protein of ECM in multiple tissues [56], including bladder [57], heart valve [58], blood vessel [59], skin [60] and the liver [61]. Synthetic biodegradable polymers, such as poly(ethylene glycol) [7,62], poly(lactic acid) [36], poly(glycolic acid) [63], and a copolymer poly(lactic-glycolic) acid [64] have also been used for engineered scaffolds. To increase the biological (e.g., hydrophilicity, cell-adhesiveness, degradability), biophysical (e.g., porosity, branched vasculature) and mechanical (e.g., stiffness, viscoelasticity) properties of tissue scaffolds, combinations of natural or synthetic hydrogels (i.e., hybrid hydrogels) have also been utilized [65]. Such ‘bioartificial’ scaffolds possess desirable mechanical properties and biocompatibility due to the coexistence of both synthetic and biological components. The biological properties of such scaffolds can further be improved by surface chemistry as the biomaterial composition makes them amenable to surface modification and biomimetic coatings [66–68].
Several approaches have been utilized to examine the mechanical (e.g., tension, compression, indentation, swelling) [33,69–70] and physicochemical (e.g., porosity, interconnectivity) [30] properties of both natural and synthetic hydrogels, including extensiometry [71], compression test [72] and bulge test [70]. However, these techniques are invasive and destructive. They are not appropriate to characterize mechanical properties of cell-encapsulating hydrogels during culture [69]. To overcome these specific problems, two techniques involving spherical indentation have been developed [73]; long focal microscopy-based spherical microindentation and optical-coherence tomography-based spherical microindentation techniques. Both monitoring techniques can be utilized to determine the mechanical properties of cell-encapsulating hydrogels for in vitro engineering of soft tissues [69]. While the former involves the central indention of a circumferentially suspended hydrogel using a sphere of a known weight and measurement of the resulting central deformation displacement, the latter is a noninvasive imaging technique based on Hertz contact theory, where the depth of indentation of a sphere into a hydrogel resting on a substrate can be used to calculate the mechanical properties of the hydrogel.
Challenges associated with hydrogels
The conventional approach in TE involves the process of seeding cells onto a 3D scaffold and inducing them to proliferate, differentiate and eventually to develop into a tissue construct [74]. A combination of chemical cues are also used to improve the outcome [75,76]. There are examples of successful clinical translations of engineered tissues [77–81], such as tissue engineered bladders [78], lung tracheal segments [82] and the lamina propria of human vocal fold developed from highly elastic gels of double-crosslinked hyaluronic acid microparticles [83]. However, challenges still exist with conventional scaffolding methods in mimicking native ECM [84–89]:
Progress in scaffolding techniques enables the manufacture of scaffolds with complex architecture [90–95]. However, poor cell penetration and noneven cell seeding still exist due to the lack of appropriate spatial and temporal control [95–99];
Success in emulating artificial matrices [100–102] for relatively simple tissues composed of a single cell type [55,99,103–105] has been demonstrated. Engineering complex tissues with multiple cell types and unique ECM composition has been challenging although successful cases exist;
Unlike native tissues, most engineered tissues lack a complex microvascular system, which is essential for certain tissues to maintain their viability and function through the transport of nutrients and a plethora of signaling molecules.
Engineering of avascular tissues, such as bone [103], skin [104] and cartilage [64,65] has been successful. Furthermore, the most successful implants are those positioned close to a rich host vascular network [78]. To date, maintaining the viability of the seeded cells at high cell densities (107–109 cells/cm3) within a large in vitro construct is a challenge [106]. Thus, replicating the inherent microvascular network of complex tissues represents one of the most fundamental challenges in tissue regeneration [26,29,35,107]. As a future prospect, a functional vascular system incorporated into tissue constructs is critical for the development of thick and complex tissues [108,109].
Construction of an artificial 3D ECM
Microscale engineering is a method that can be used to control the cellular microenvironment [48,110–114] and has the potential to construct matrices mimicking the inhomogeneous and anisotropic properties of native tissues [48,115–117]. In addition, this method can also be applied to modify and control cell–cell and cell–ECM interactions [113]. Using microengineering technologies, hydrogel scaffolds mimicking in vivo ECM have been developed to support cell growth, proliferation and to promote tissue generation. These techniques include lithography [31,40,118,119], nano- and microfluidics [48,120–130], micromolding [10,131–133], and biopatterning [134–137], all of which offer the potential to address the aforementioned existing challenges in engineering a predesigned cell microenvironment.
Among all the microscale TE methods, ‘top-down’ or ‘bottom-up’ approaches have recently emerged as forefront technologies to create tissue-like constructs of a higher complexity (e.g., microarchitecture made of multiple cell types and ECM components) [10,96,138]. Top-down approach emphasizes control of microscale features (i.e., shape and size) of relatively large hydrogel constructs [8,139]. Despite significant advances, there are several challenges with top-down approaches (e.g., the intricate microstructural features of native tissues such as cell density and microarchitecture) [84–89]. On the other hand, the modular bottom-up approach aims to generate larger tissue constructs with controllable and repeatable patterns by assembling smaller building blocks (e.g., cell-encapsulating microgels). Bottom-up approaches are exciting since they mimic native tissues composed of repeating functional units [10] (e.g., the lobule in the liver [9]). In this article, we will focus on bottom-up approaches where the newest technologies and methods are emerging. The bottom-up approach requires methods to fabricate functional units and assemble them together at high throughput. Some nano- and microscale technologies have been employed to assemble cell-encapsulating microgels including manual handling [108,131,140,141], microfluidics [8,142] or directed assembly [7] to build layers of microgels. Furthermore, enhanced perfusion has also been observed in hydrogels created through a random assembly of micro-modules encapsulating endothelial cells. [106,143,144]. However, diffusion through hydrogels is limited to relatively small constructs due to inefficient mass transport [145,146]. To sustain cell growth, a microenvironment must provide effective nutrient transfer, gas exchange (i.e., O2 and CO2) and metabolic waste removal. To address this issue, the microfluidic channels formed within cell-encapsulating hydrogels may be used to maximize the perfusion capacity of the constructs [8,35,147–149,179].
Microengineering of cell-encapsulating hydrogels
Micromolding and photolithography are two cutting-edge technologies that have been used to generate 3D cell-laden hydrogel microstructures with controlled features (e.g., shape and size) [131,150]. In generating micromolded hydrogels, precursors are first molded and then gelled to generate structures of a variety of shapes and sizes (Figure 2). Using fluoro-based materials with higher surface energies and enhanced fabrication resolution it is possible to micromold nano-scale particles that have applications in drug delivery. However, the ability to engineer the nanoscale topography of hydrogels will also be important in generating improved 3D TE scaffolds with microstructures [10]. Micromolding has become particularly appealing due to soft lithography, which has enabled easy fabrication of poly(dimethyl siloxane) (PDMS) molds from prefabricated silicon wafers. Photolithography provides reliable shape definition, typically by using photomasks for patterning multiple cells with materials to facilitate the selective adhesion of one cell type to a specific regions (Figure 3). Using this technology, photo-crosslinkable hydrogels are placed underneath a mask that controls the exposure of light to particular regions of a film of hydrogel precursors. Where the light is exposed, the photo-crosslinkable hydrogel will crosslink to generate structures that are in the shape of the mask. Advances in soft lithography have enabled [8,151–152] and microfabrication of features as small as a few micrometers. In particular, silicon-based elastomers have been widely used in microfluidics due to their simple fabrication and material properties, such as gas permeability, optical transparency and flexibility [153]. However, the hydrophobic nature of PDMS requires surface treatments, such as plasma treatment, to increase cell adhesion [154,155].
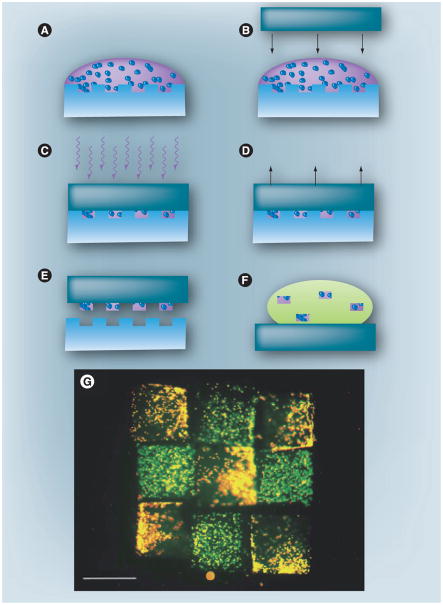
Figure 2. Fabrication of a 3D cell-laden microscale hydrogels using micromolding.
(A) Liquid prepolymer is deposited onto a hydrophilic poly(dimethyl siloxane) (PDMS) pattern. (B) A hydrophobic PDMS cover slip is placed on top of the prepolymer, forming a reversible seal. (C) Polymer liquid is crosslinked using UV light or heat. (D) The PDMS cover slip is removed. (E) Hydrogels are washed from pattern. (F) Hydrogels are free of the pattern. (G) Flourescent image of microscale tissue constructs comprised of cell-laden hydrogels containing hepatocytes and fibroblasts. Scale bar: 400 μm.
Reproduced with permission from [131].
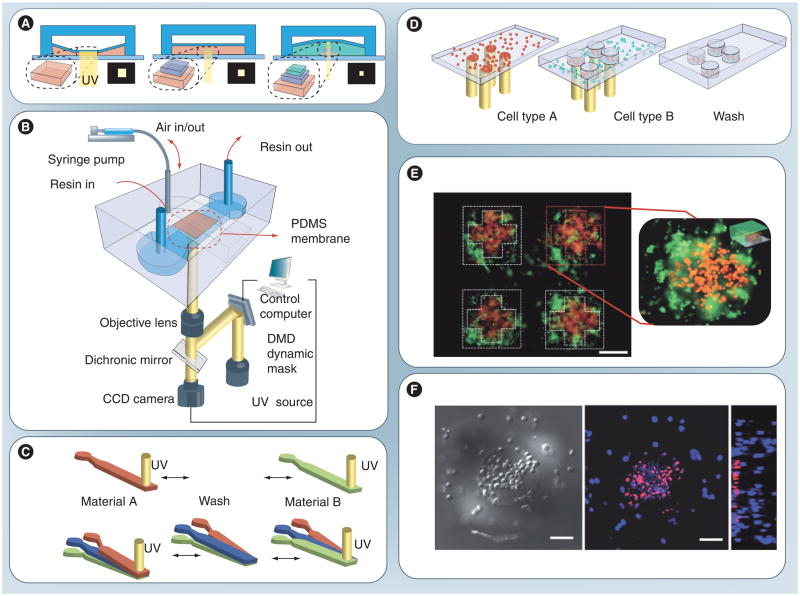
Figure 3. Fabrication of a 3D cell-laden microscale hydrogel using optofluidic maskless lithography method.
(A) The 3D-Optofluidic maskless lithography (OFML). OFML is performed in a two-layered PDMS microfluidic channel with UV curable resin placed in the bottom. The height of the bottom channel is controlled via deformation of the PDMS membrane under pneumatic pressure of the top chamber. Projection patterns are sequentially launched and projected onto the channel. (B) The 3D-OFML system is composed of two parts; the height-tunable PDMS microfluidic channel and the optical system. Pneumatic pressure is applied with a syringe pump and the UV projection for each layer is computer controlled. (C) Microfluidic control methods for exchanging resin materials to generate hybrid microstructures. Solutions can be injected sequentially through the same inlet (top) or multilaminar flow can be exploited (bottom). (D, F) Generation of 3D hydrogel tissue-like microstructures containing different living cells in each part of the structure. HeLa cells stained with red and blue fluorescence (D) are used for heterogeneous cell patterning. (F) Confocal microscope images of the hydrogel block containing cells. Differential interference contrast transmission image shows the circular and the rectangular pattern of hydrogel structure (left). (E) An array of patterned hydrogel with two different fluorescent microspheres. First layer is patterned in cross-shape and the other beads in the square-shape. Images were taken from the bottom of the structure with an inverted optical microscope.
Scale bars in (E) and (F) indicate 100 μm.
DMD: Digital micromirror-array device; PDMS: Poly(dimethyl siloxane).
Reproduced with permission from [218].
A combination of photopatterning and electropatterning offers the capability to encapsulate cells with a high spatial resolution (< 10 μm) within a microscale hydrogel (microgel). These microgels can later be organized into specific geometries to generate larger structures [156,157]. Several other microengineering techniques have also been utilized to pattern cells in 3D gels, such as laser scanning lithography [158–159] and dielectrophoresis [160]. A study reported the construction of a functional 3D hepatic tissue using poly-ethylene glycol, in which nutrient delivery was achieved by convective flow. This demonstrates that lithographic arraying methods can be efficiently used to construct 3D cell-encapsulating hydrogel scaffolds with complex internal architecture [9]. A flow photolithographic method, which combines the advantages of both microscope projection photolithography and microfluidics, has been used to continuously synthesize microgels while controlling particle size and shape [142]. However, there are several limitations with this method, such as the cytotoxicity of the photoinitiator, cell damage induced by UV light exposure and low throughput. Furthermore, this technique requires an additional system to assemble microscale building blocks into a desired 3D architecture through self-assembly [10,26], or by other means such as manual handling [131,141], and microfluidics [8,142]. The three most frequently used methods to generate cell encapsulating microgels are compared in Table 2. Novel methods to assemble these microgels into 3D constructs need to be further developed.
Table 2.
A comparison of different methods to fabricate 3D cell-laden hydrogels.
| Feature/function | Method | Ref. | ||
|---|---|---|---|---|
| Micromolding | Photolithography | Bioprinting | ||
| Hydrogel type | Multiple | Photo-crosslinkable hydrogel (e.g., PEG) | Injectable hydrogel (e.g., collagen, agarose, alginate) | |
| Hydrogel shape | Multiple (e.g., cylinder, cubic) | Multiple (limited in thickness) | Spherical | [132] |
| Hydrogel size | 40 μm to mm scale | 120 μm to mm scale | 37–400 μm | [131,132,142, 193,227] |
| Fabrication throughput | Medium | Low | High | |
| Cell viability† | <50 to >99% | ~60 to 100% | ~65 to ~100% | [7,87,193,131,132, 142, 227–231] |
| Coculture capability | Yes | Yes | Yes | [232] |
| Spatial deposition control | Not applicable | Not applicable | Yes, with microscale resolution | |
| Single cell encapsulation | Yes | Yes | Yes | [193] |
Cell viability relative to culture.
PEG: Polyethylene glycol.
Microfluidic cell-encapsulating hydrogels
Efficient nutrient and metabolite diffusion has only been observed in relatively small hydrogel-based tissue constructs due to transport limitations [145–146,161–163]. Currently, vascularization remains a challenge and materials strategies that attempt to induce or organize vessel formation, either de novo (vasculogenesis) or by sprouting of existing vessels (angiogenesis) will be beneficial [164]. To address this issue, emerging micro- and nanofluidic technologies [165–167] have been utilized to construct microchannels that resemble the vascular system of native tissues [8,127,168–172]. A variety of techniques including lithography [119], micromolding [38,132], microfluidic emulsification [26,120] and hot embossing [121] have been used to build such capillary networks in tissue scaffolds or polymer microchips. More recently, micro- and nano-fluidics have also emerged as powerful technologies to generate microengineered tunable hydrogel scaffolds with tissue-like motifs [45,173–175].
An alternative approach to using cell-encapsulating microfluidic hydrogels to facilitate nutrient and soluble factor exchange within 3D constructs [7–8,35,131,176–182] is to integrate microfluidic channels within 3D cell-encapsulating hydrogel matrices. This could provide in vitro tissue constructs with spatial and temporal complexity mimicking native tissues. Biocompatibility of these devices is considered to be an important issue when applied to biomedical and biochemical analysis. The ability to tailor the chemical and structural properties of biomedical devices to control cell attachment, survival, proliferation and differentiation is of crucial importance [183].
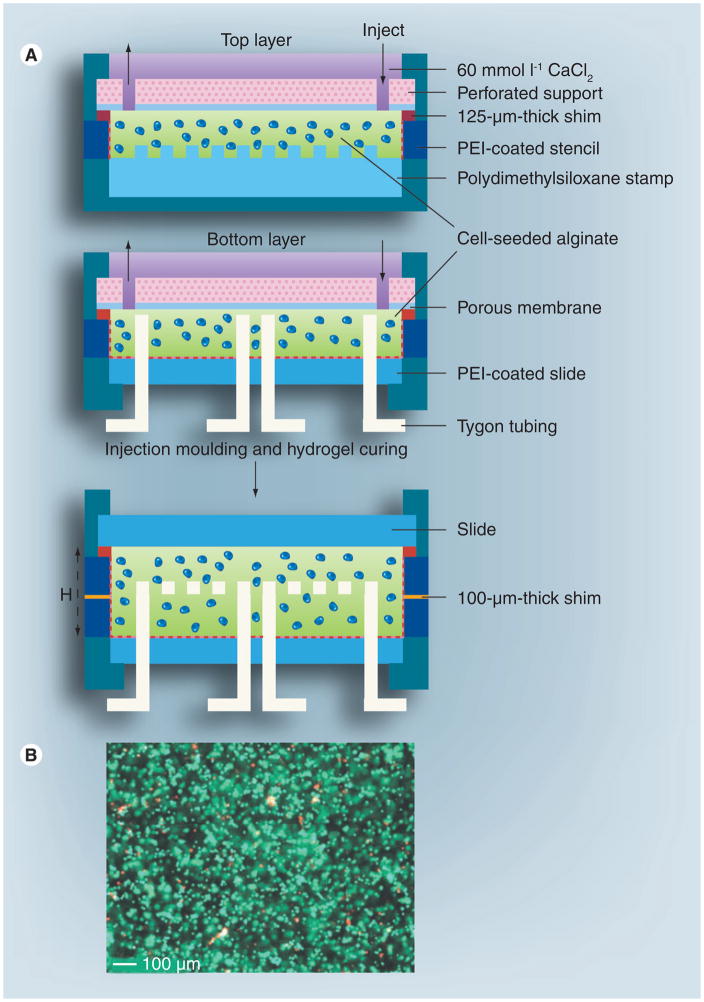
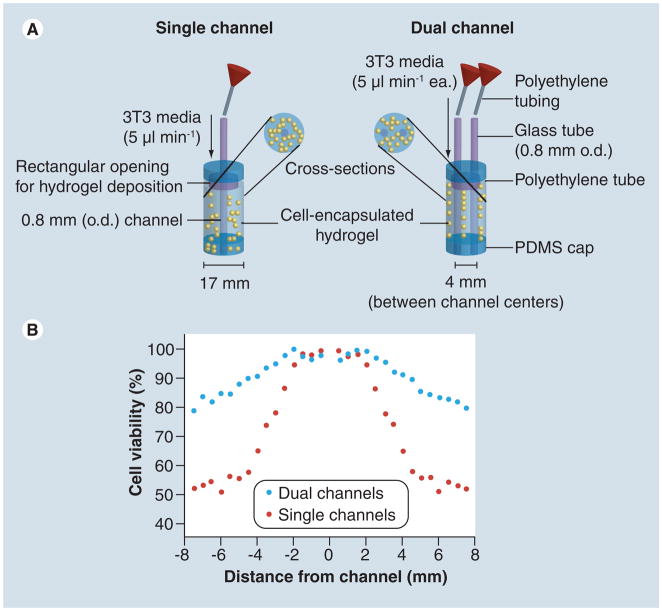
Micromolding is another simple approach that has been used to construct microfluidic cell-encapsulating hydrogels, [8]. With this method, cells are first suspended in a gel precursor and then molded on a patterned wafer (generally silicon) to generate microchannels of different sizes and shapes (i.e., square cross-section with different aspect ratios). It has been shown that cells encapsulated within such microfluidic hydrogels remained viable [8] due to the perfused network of microchannels, through which nutrients and oxygen can be efficiently delivered. In another study, microfluidic networks were directly embedded within a cell-encapsulating hydrogel construct (Figure 4) where high cell viability was observed and the distribution of solutes within the 3D matrix was regulated in a spatio-temporal fashion [35]. A related study shows that the cell viability across the hydrogel was highly correlated to the nutrient perfusion profiles (Figure 5) [179].
Figure 4. Fabrication of cellular microfluidic hydrogels using a molding method.
(A) The fabrication process. The dotted line indicates PEI coating. (B) Cell viability results using live/dead staining.
H: Height (mm in thickness); PEI: polyethylenimine.
Reproduced with permission from [35]
Figure 5. A schematic perfusion bioreactor.
(A) 2D microchannel perfusion/viability model: single- and dual-channeled hydrogel constructs were prepared using cell-encapsulating agarose. (B) Fractional cell viability with single- and dual-channel set-ups taken at day 1, 2 and 3, respectively, after the channel constructs were built.
3T3: Mouse embryonic fibroblast cell line; o.d.: outer diameter;
PDMS: Poly(dimethyl siloxane).
Reproduced with permission from [170].
Cell printing has been used to pattern cells within scaffolding materials (e.g., natural hydrogels such as collagen). 3D printing methods can be employed for microfluidic cell-encapsulating constructs [35,107,134–135,180–181,184–187]. This approach offers the ability to deposit cell-encapsulating microgels at predetermined locations with multiple cell types at high throughput [107,137]. Currently, several printing techniques have been explored to generate cell-encapsulating gel droplets, such as inkjet [136,188–189], laser [190–192] and acoustic printing [193–195]. Furthermore, a scaffold-free approach has been developed to build cell encapsulating hydrogels with customized tubular structures of defined topology (both linear and branched tubular structures) (Figure 6) [187]. In this method, agarose rods were used as a temporary space occupier of the lumen of branched tubes, which was later manually removed [187]. However, this approach is difficult to adapt for more complex structures due to the need for manual handling. In another study, a thermal inkjet printer was used to simultaneously deposit human microvascular endothelial cells and fibrin (bio-ink) into fibrinogen solution (bio-paper) to form a cell-encapsulating microfluidic hydrogel (Figure 7) [186]. 3D bioprinting is thus capable of manipulating cellular components with precise spatial and temporal control to create 3D constructs [227]. These controls and high-throughput aspects make printing an attractive cell-encapsulating gel deposition technology. However, the viability and function of the printed cells need to be characterized for each printing technology. Factors, such as shear at the nozzle, heat in the ejection reservoirs and impact on the surface need to be optimized separately for each technology for maximal cell viability and function.
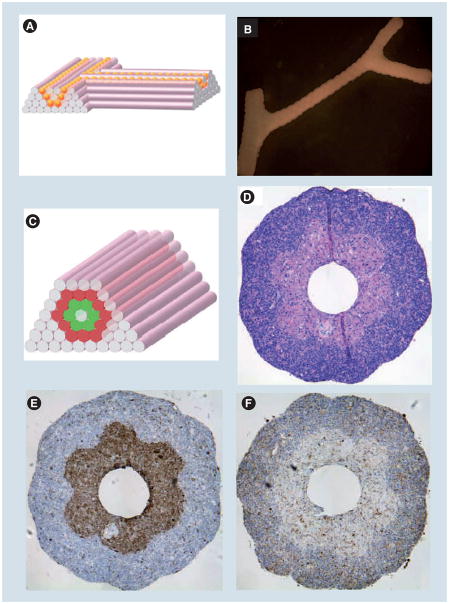
Figure 6. Printing cellular microfluidic hydrogels.
(A) Design template for building branching tubular structures by depositing agarose rods and multicellular spheroids of the same diameter. (B) Fusion patterns of multicellular spheroids (300 μm HSF spheroids) assembled into tubular structures after 6 days of deposition. (C–F) Building a double-layered vascular wall by assembling human umbilical vein smooth muscle cells and HSF multicellular cylinders according to specific patterns (C). (D–F) show the results of H&E (D), smooth muscle α-actin (E) and caspase-3 (F) straining of the structure after 3 days of fusion.
Reproduced with permission from [219].
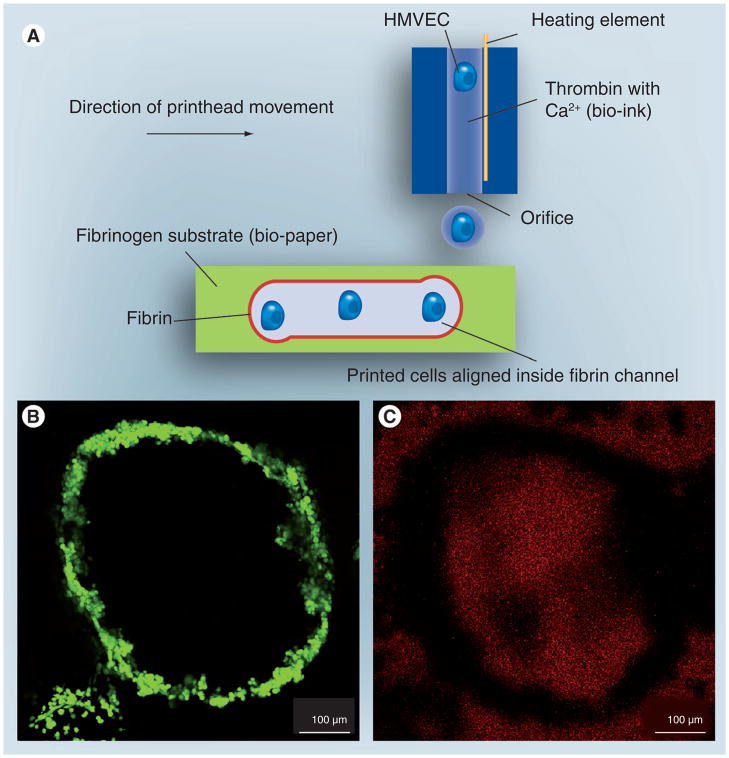
Figure 7. Fabrication of cellular microfluidic hydrogels using printing.
(A) Proliferation-ready endothelial cells aligned inside the fibrin channels using a thermal inkjet printer. The technology may enable building human microvasculature. (B & C) Channel structure of printed microvasculature cultured for 21 days. (B) Printed ring shaped microvasculature cultured for 21 days (C) Integrity of printed structure cultured for 21 days.
HMVEC: Human microvascular endothelial cells.
Reproduced with permission from [186].
Biomedical applications of hydrogels & 3D cell-encapsulating hydrogels
As the cell encapsulating 3D hydrogels provide cells with a tissue-like extracellular environment, they offer potential applications such as in vitro model systems for drug screening, diagnostics and toxicological assays.
As drug discovery & delivery devices
Various polymeric drug-delivery systems have been recently introduced and evaluated [196]. Macromolecular drugs, such as proteins or hydrophilic oligonucleotides, are inherently compatible with hydrogels. Various formulations of hydrogel materials with known mechanical, physical and chemical properties can be prepared to construct drug-delivery vehicles with finely tuned drug release kinetics [41,42,44,197,198]. The highly porous structure of hydrogels enables the introduction of a relatively large load of drugs [199]. In addition, the porosity of hydrogels can be tuned by controlling affinity towards the swelling environment and the crosslinking density. The porosity can also be adjusted to facilitate drug release [200,201]. In drug discovery, miniaturized hydrogel platforms can be used to control the fluid flow, enable high-throughput screening and minimize sample and reagent volumes [196].
As cell-based biosensors & diagnostic tools
Ethical considerations, limited portability and robustness for practical large-scale applications preclude the use of living animals as biosensors. Although other methods (e.g., immunochemical or nucleic-acid based methods) are available for pathogen detection, they often take hours or days to provide meaningful results [202]. Thus, the need for reliable, high-throughput in vitro human cell-based alternative methods are attractive for basic research in the fields of safety and risk assessment [203]. Given their easily customizable features, hydrogels (e.g., microgels) could be utilized as components of integrated sensors within microdevices [204]. Cell-based biosensors, employing both prokaryotic and eukaryotic cells as primary transducers, have emerged as powerful tools for rapid detection of hazards and threats associated with food, agriculture, the environment and biosecurity [205,206]. Providing cells with an in vivo-like physiological environment for interaction and communication, 3D cell-encapsulating hydrogels have attracted interest in the cell-based chem–bio sensing [207]. Such native-like 3D synthetic microenvironments may provide a better understanding of disease and organ failure caused by various biotic or abiotic agents such as chemical toxins, bacterial and viral pathogens [208].
As stem cell niches
It is generally accepted that adult stem cells in vivo reside in special microenvironments (i.e., niches) contributed by other cells, ECM components, molecules such as cytokines and chemokines and physicochemical conditions (e.g., the physical environment, pH, O2) [199]. Once displaced from this native niche, these cells will begin to differentiate into a diverse range of specialized cell types (pluripotency). The major factor determining stem cell fate is the interplay between stem cells and their niches, which creates a dynamic system necessary for tissue maintenance and function in vivo [101,209]. Thus, the control of ECM composition in engineered constructs has proven to be a valuable tool to guide the development and commitment of stem cells during new tissue generation [101].
Clinical trials for stem cell therapies have so far exceeded 2000 cases and there is an urgent need to develop technologies that can control stem cell behavior in culture. Cell therapy alone is a poorly controlled process [164] and a recent study has revealed that when injected into a post-myocardial infarction heart in mice, stem cells mineralized, probably due to the new mechanical environment in the scar tissue [210,211]. Thus, replicating stem cell ‘niche’ conditions in vitro would enable the design of stem-cell therapeutics, treatment of genetic disorders and cancer, and regenerative medicine [101]. Control of cell fate is perhaps the most limiting factor in the translation of embryonic stem-cell therapy. Although, microscale technologies have been developed for TE and a wide range of natural and synthetic materials have been successfully used for stem cell cultures [212–214], the complexity of the stem cell niche is still difficult to reproduce [209,215], and the controlled use of stem cells in TE remains a challenging goal.
Conclusion
Given the ease of execution, experiments in biosciences have traditionally been carried out in 2D environments [216]. In vivo, mammalian cells reside in a 3D microenvironment that provides physical support, chemical growth factors for cell adhesion, survival and growth. These factors are interconnected through a complex network of microvasculature that provides nutrient delivery, gas exchange and signaling cues; all with profound effect on cell behavior, assembly and organization of tissues. This 3D microenvironment plays a critical role in regulating cell fate, ranging from proliferation and migration to apoptosis [217]. Mimicking such a 3D cell microenvironment in vitro is crucial for various TE applications (e.g., constructing tissues for repair and replacement). On the other hand, one should consider that heterogeneities in cell behavior would be further exaggerated in richer chemistry and formulation of 3D scaffolds. Furthermore, cells in these synthetic environments must grow at the expense of the scaffold, proliferate, self-assemble, secrete their own ECM and eventually take the shape of scaffold, which resembles the structure of tissue of interest. Thus, engineering tissues with a complex vascular structure and made of many cell types whose organization is crucial for function is an important challenge. Transmission and receipt of complex molecular information involved in cell sorting, boundary formation in tissues and cell movement can be effected through cell–cell and cell–matrix interactions.
Micro- and nano-fluidic technologies have offered the capability to construct hydrogels with complex and delicate structures. Emerging microscale technologies (e.g., micromolding, lithography, biopatterning) have enabled researchers to engineer cell-encapsulate microscale hydrogels with incorporated microchannels that mimic the microvascular system. Multimicrochannel approaches may allow larger tissue constructs to have better diffusion characteristics. Challenges still remain with these technologies, such as:
Fluid transport limitations;
Regularity of microchannels, which do not represent the irregular microvascular system and the complexity of the endothelial branched nature of vascularization in native tissues;
Problems associated with patterning various cell types spatially (i.e., layered tissue motif comprising various cell types).
Although multiple challenges are facing the field, emerging microscale technologies (e.g., micromolding and biopatterning) are exciting and they may enable the engineering of tissue constructs that better mimic native tissues.
Acknowledgments
Hikmet Geckil gratefully acknowledges the support of the J William Fulbright Foundation, as a Fulbright Scholar at the BAMM Labs, Center for Biomedical Engineering, Brigham and Women’s Hospital (MA, USA), Harvard Medical School.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was partially supported by the NIH-R21-EB007707 and the Randolph Hearst Foundation, Brigham and Women’s Hospital Department of Medicine Young Investigator in Medicine Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Ni Y, Chen R. Extracellular recombinant protein production from Escherichia coli. Biotechnol Lett. 2009;31(11):1661–1670. doi: 10.1007/s10529-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 2.Porro D, Sauer M, Branduardi P, Mattanovich D. Recombinant protein production in yeasts. Mol Biotechnol. 2005;31(3):245–259. doi: 10.1385/MB:31:3:245. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Reagan MR, Kaplan DL. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):988–1006. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker EL, Bonnecaze RT, Zaman MH. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J. 2009;97(4):1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong H, McCullough CM, Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28(26):3824–3833. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Nat Acad Sci USA. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling Y, Rubin J, Deng Y, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7(6):756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 9.Liu Tsang V, Chen AA, Cho LM, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21(3):790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 10.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28(34):5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Lutolf MP. Integration column: artificial ECM: expanding the cell biology toolbox in 3D. Integr Biol. 2009;1:235–241. doi: 10.1039/b902243k. [DOI] [PubMed] [Google Scholar]
- 12.Baroli B. Hydrogels for tissue engineering and delivery of tissue-inducing substances. J Pharm Sci. 2007;96(9):2197–2223. doi: 10.1002/jps.20873. [DOI] [PubMed] [Google Scholar]
- 13.Federovich NE, Alblas J, Dewijn JR, Hennink WE, Verbout AJ, Dhert WJA. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905–1925. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 14.Jabbarzadeh E, Starnes T, Khan YM, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci USA. 2008;105(32):11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jay SM, Saltzman WM. Shining light on a new class of hydrogels. Nat Biotechnol. 2009;27(6):543–544. doi: 10.1038/nbt0609-543. [DOI] [PubMed] [Google Scholar]
- 16.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345. [Google Scholar]
- 17.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mano JF, Silva GA, Azevedo HS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seunarine K, Gadegaard N, Tormen M, Meredith DO, Riehle MO, Wilkinson CD. 3D polymer scaffolds for tissue engineering. Nanomedicine (Lond) 2006;1(3):281–296. doi: 10.2217/17435889.1.3.281. [DOI] [PubMed] [Google Scholar]
- 20.Kopecek J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28(34):5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders BR, Laajam N, Daly E, Teow S, Hu X, Stepto R. Microgels: from responsive polymer colloids to biomaterials. Adv Colloid Interface Sci. 2009;147–148:251–262. doi: 10.1016/j.cis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Lee WH, Shin SJ, Park Y, Lee S-H. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small. 2009;5(11):1264–1268. doi: 10.1002/smll.200801667. [DOI] [PubMed] [Google Scholar]
- 23.Seidlits SK, Lee JY, Schmidt CE. Nanostructured scaffolds for neural applications. Nanomedicine (Lond) 2008;3(2):183–199. doi: 10.2217/17435889.3.2.183. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Cabello JC, Prieto S, Arias FJ, Reguera J, Ribeiro A. Nanobiotechnological approach to engineered biomaterial design: the example of elastin-like polymers. Nanomedicine (Lond) 2006;1(3):267–280. doi: 10.2217/17435889.1.3.267. [DOI] [PubMed] [Google Scholar]
- 25.Madurantakam PA, Cost CP, Simpson DG, Bowlin GL. Science of nanofibrous scaffold fabrication: strategies for next generation tissue-engineering scaffolds. Nanomedicine (Lond) 2009;4(2):193–206. doi: 10.2217/17435889.4.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224–230. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 28.Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. The effect of hydrogel charge density on cell attachment. Biomaterials. 2004;25(15):3023–3028. doi: 10.1016/j.biomaterials.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 29.Ford MC, Bertram JP, Hynes SR, et al. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci USA. 2006;103(8):2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dadsetan M, Hefferan TE, Szatkowski JP, et al. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials. 2008;29(14):2193–2202. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 2007;28(19):2978–2986. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M, Berkland C, Detamore MS. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng Part B Rev. 2008;14(4):341–366. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 34.Wenger MP, Bozec L, Horton MA, Mesquida P. Mechanical properties of collagen fibrils. Biophys J. 2007;93(4):1255–1263. doi: 10.1529/biophysj.106.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6(11):908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 36.Tu C, Cal Q, Yang J, et al. The fabrication and characterization of poly(lactic acid) scaffolds for tissue engineering by improved solidliquid phase separation. Polym Adv Technol. 2003;14:565–573. [Google Scholar]
- 37.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mcguigan AP, Bruzewicz DA, Glavan A, Butte M, Whitesides GM. Cell encapsulation in sub-mm sized gel modules using replica molding. PLoS ONE. 2008;3:e2258. doi: 10.1371/journal.pone.0002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan W-H, Takeuchi S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv Mater. 2007;19:2696–2701. [Google Scholar]
- 40.Liu J, Gao D, Li HF, Lin JM. Controlled photopolymerization of hydrogel microstructures inside microchannels for bioassays. Lab Chip. 2009;9(9):1301–1305. doi: 10.1039/b819219g. [DOI] [PubMed] [Google Scholar]
- 41.Gao D, Xu H, Philbert MA, Kopelman R. Bio-eliminable nano-hydrogels for drug delivery. Nano Lett. 2008;8:3320–3324. doi: 10.1021/nl8017274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 43.Soppimath KS, Aminabhavi TM, Dave AM, Kumbar SG, Rudzinski WE. Stimulus-responsive ‘smart’ hydrogels as novel drug delivery systems. Drug Dev Ind Pharm. 2002;28:957–974. doi: 10.1081/ddc-120006428. [DOI] [PubMed] [Google Scholar]
- 44.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Bettinger CJ, Weinberg EJ, Kulig KM, et al. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv Mater. 2005;18(2):165–169. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdick JA. Cellular control in two clicks. Nature. 2009;460:469–470. doi: 10.1038/460469a. [DOI] [PubMed] [Google Scholar]
- 47.Rivest C, Morrison DWG, Ni B, et al. Microscale hydrogels for medicine and biology: synthesis, characterisation and applications. J Mechanics Materials Structures. 2007;2:1103–1119. [Google Scholar]
- 48.Gillette BM, Jensen JA, Tang B, et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7(8):636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 49.Sahoo S, Chung C, Khetan S, Burdick JA. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9(4):1088–1092. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Williams CG, Sun DD, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68(1):28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 51.Eyrich D, Brandl F, Appel B, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28(1):55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 52.Fukuda J, Khademhosseini A, Yeh J, et al. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 54.Azab AK, Orkin B, Doviner V, et al. Crosslinked chitosan implants as potential degradable devices for brachytherapy: in vitro and in vivo analysis. J Control Release. 2006;111:281–289. doi: 10.1016/j.jconrel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Kim UJ, Kim HS, et al. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42(6):1226–1234. doi: 10.1016/j.bone.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Black LD, Allen PG, Morris SM, Stone PJ, Suki B. Mechanical and failure properties of extracellular matrix sheets as a function of structural protein composition. Biophys J. 2008;94(5):1916–1929. doi: 10.1529/biophysj.107.107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolland F, Korossis S, Wilshaw SP, et al. Development and characterization of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061–1070. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Schenke-Layland K, Vasilevski O, Opitz F, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143(3):201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Uchimura E, Sawa Y, Taketani S, et al. Novel method of preparing acellular cardiovascular grafts by decellularization with poly(ethylene glycol) J Biomed Mater Res A. 2003;67(3):834–837. doi: 10.1002/jbm.a.10097. [DOI] [PubMed] [Google Scholar]
- 60.Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25(13):2679–2686. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 61.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10(7–8):1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 62.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10(3–4):515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 63.Hiraoka Y, Kimura Y, Ueda H, Tabata Y. Fabrication and biocompatibility of collagen sponge reinforced with poly(glycolic acid) fiber. Tissue Eng. 2003;9(6):1101–1112. doi: 10.1089/10763270360728017. [DOI] [PubMed] [Google Scholar]
- 64.Uematsu K, Hattori K, Ishimoto Y, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional polylacticglycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 65.Chen GP, Sato T, Ushida T, Ochiai N, Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng. 2004;10:323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 66.Savina IN, Dainiak M, Jungvid H, Mikhalovsky SV, Galaev IY. Biomimetic macroporous hydrogels: protein ligand distribution and cell response to the ligand architecture in the scaffold. J Biomater Sci. 2009;20(12):1781–1795. doi: 10.1163/156856208X386390. [DOI] [PubMed] [Google Scholar]
- 67.Teo WE, He W, Ramakrishna S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol J. 2006;1(9):918–929. doi: 10.1002/biot.200600044. [DOI] [PubMed] [Google Scholar]
- 68.Hasirci V, Kenar H. Novel surface patterning approaches for tissue engineering and their effect on cell behavior. Nanomedicine (Lond) 2006;1(1):73–90. doi: 10.2217/17435889.1.1.73. [DOI] [PubMed] [Google Scholar]
- 69.Ahearne M, Yang Y, Liu K-K. Mechanical characterisation of hydrogels for tissue engineering applications. Topics Tissue Eng. 2008;4:1–16. [Google Scholar]
- 70.Ahearne M, Yang Y, El Haj AJ, Then KY, Liu KK. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J R Soc Interface. 2005;2:455–463. doi: 10.1098/rsif.2005.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 72.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 73.Ahearne M, Liu KK, Yang Y. Dual-Camera Spherical Indentation System for Examining the Mechanical Characteristics of Hydrogels. Springer-Verlag; Berlin, Germany: 2009. pp. 2011–2014. [Google Scholar]
- 74.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salvay DM, Shea LD. Inductive tissue engineering with protein and DNA-releasing scaffolds. Mol Biosyst. 2006;2(1):36–48. doi: 10.1039/b514174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deforest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8(8):659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atala A. Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert Opin Biol Ther. 2005;5(7):879–892. doi: 10.1517/14712598.5.7.879. [DOI] [PubMed] [Google Scholar]
- 78.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 79.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 80.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100(22):12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Griffith LG, Naughton G. Tissue engineering – current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 82.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 83.Sahiner N, Jha AK, Nguyen D, Jia X. Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration. J Biomater Sci. 2008;19(2):223–243. doi: 10.1163/156856208783432462. [DOI] [PubMed] [Google Scholar]
- 84.Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A Discov Mol Cell Evol Biol. 2003;272(2):497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 85.Jakab K, Norotte C, Damon B, et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. 2008;14(3):413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 86.Burg T, Groff R, Burg K, Hill M, Boland T. Systems engineering challenges in inkjet biofabrication. SoutheastCon; Proceedings IEEE; 2007.2007. [Google Scholar]
- 87.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Fedorovich NE, Alblas J, De Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13(8):1905–1925. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 89.Varghese D, Deshpande M, XUT, Kesari P, Ohri S, Boland T. Advances in tissue engineering: cell printing. J Thorac Cardiovasc Surg. 2005;129(2):470–472. doi: 10.1016/j.jtcvs.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 90.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–39. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 91.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22(7):354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24:2363–2378. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 93.Sun W, Lal P. Recent development on computer aided tissue engineering – a review. Comput Methods Programs Biomed. 2002;67:85–103. doi: 10.1016/s0169-2607(01)00116-x. [DOI] [PubMed] [Google Scholar]
- 94.Landers R, Hubner U, Schmelzeisen R, Mulhaupt R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials. 2002;23(23):4437–4447. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 95.Yang S, Leong KF, DUZ, Chua CK. The design of scaffolds for use in tissue engineering. Part II Rapid prototyping techniques. Tissue Eng. 2002;8:1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 96.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glicklis R, Shapiro L, Agbaria R, Merchuk JC, Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol Bioeng. 2000;67(3):344–353. doi: 10.1002/(sici)1097-0290(20000205)67:3<344::aid-bit11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 98.Jakab K, Damon B, Neagu A, Kachurin A, Forgacs G. Three-dimensional tissue constructs built by bioprinting. Biorheology. 2006;43(3–4):509–513. [PubMed] [Google Scholar]
- 99.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 100.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote HMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, Wang X, Keshav V, Johanas JT, Leisk GG, Kaplan DL. Dynamic culture conditions to generate silk-based tissue-engineered vascular grafts. Biomaterials. 2009;30(19):3213–3223. doi: 10.1016/j.biomaterials.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mauney JR, Sjostorm S, Blumberg J, et al. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74(5):458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 104.Boucard N, Viton C, Agay D, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28:3478–3488. doi: 10.1016/j.biomaterials.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 105.Fan HB, Hu YY, Zhang CL, et al. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27:4573–4580. doi: 10.1016/j.biomaterials.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 106.McGuigan AP, Sefton MV. Modular tissue engineering: fabrication of a gelatin-based construct. J Tissue Eng Regen Med. 2007;1(2):136–145. doi: 10.1002/term.14. [DOI] [PubMed] [Google Scholar]
- 107.Moon S, Hasan SK, Song YS, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods. 2010;16(1):157–166. doi: 10.1089/ten.TEC.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mcguigan AP, Leung B, Sefton MV. Fabrication of cell-containing gel modules to assemble modular tissue-engineered constructs [corrected] Nat Prtotoc. 2006;1(6):2963–2969. doi: 10.1038/nprot.2006.443. [DOI] [PubMed] [Google Scholar]
- 109.Nomi M, Atala A, Coppi PD, Soker S. Principles of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 110.Bhatia SN. Customizing cellular microenvironments for hepatic tissue engineering. Abstr Pap Am Chem Soc. 2001;221:U127. [Google Scholar]
- 111.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2(2):119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 112.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22(7):863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 113.Nelson CM, Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518–523. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 114.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marenzana M, Kelly DJ, Prendergast PJ, Brown RA. A collagen-based interface construct for the assessment of cell-dependent mechanical integration of tissue surfaces. Cell Tissue Res. 2007;327:293–300. doi: 10.1007/s00441-006-0316-z. [DOI] [PubMed] [Google Scholar]
- 116.Lee CS. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials. 2007;28:2987–2993. doi: 10.1016/j.biomaterials.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 117.Mikos AG. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5(5):365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 119.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Ann Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 120.Tumarkin E, Kumacheva E. Microfluidic generation of microgels from synthetic and natural polymers. Chem Soc Rev. 2009;38(8):2161–2168. doi: 10.1039/b809915b. [DOI] [PubMed] [Google Scholar]
- 121.Xu F, Datta P, Wang H, et al. Polymer microfluidic chips with integrated waveguides for reading microarrays. Analyt Chem. 2007;79(23):9007–9013. doi: 10.1021/ac7016597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Godin J, Chen CH, Cho SH, Qiao W, Tsai F, Lo YH. Microfluidics and photonics for bio-system-on-a-chip: a review of advancements in technology towards a microfluidic flow cytometry chip. J Biophotonics. 2008;1(5):355–376. doi: 10.1002/jbio.200810018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Z, Psaltis D. Optofluidic dye lasers. Microfluid Nanofluidics. 2008;4:145. [Google Scholar]
- 124.Kim YG, Moon S, Kuritzkes DR, Demirci U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens Bioelectron. 2009;25(1):253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moon SJ, Lin R, Demirci U. CD4+ T-lymphocyte capture using a disposable microfluidic chip for HIV. J Vis Exp. 2007;8:315. doi: 10.3791/315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Demirci U. Using micro-electro-mechanical systems (MEMS) to develop diagnostic tools. J Vis Exp. 2007;8:314. doi: 10.3791/314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8(1):98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 128.Cheng X, Irimia D, Dixon M, et al. A microchip approach for practical label-free CD4+ T-cell counting of HIV-infected subjects in resource-poor settings. J Acquir Immun Syndr. 2007;45(3):257–261. doi: 10.1097/QAI.0b013e3180500303. [DOI] [PubMed] [Google Scholar]
- 129.Cheng X, Irimia D, Dixon M, et al. A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip. 2007;7(2):170–178. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moon S, Keles HO, Ozcan A, et al. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens Bioelectron. 2009;24(11):3208–3214. doi: 10.1016/j.bios.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeh J, Ling Y, Karp JM, et al. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27(31):5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 132.Khademhosseini A, Eng G, Yeh J, et al. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79(3):522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 133.Dang TT, Xu Q, Bratlie KM, et al. Microfabrication of homogenous, asymmetric cell-laden hydrogel capsules. Biomaterials. 2009;30:6896–6902. doi: 10.1016/j.biomaterials.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lam CXF, Mo XM, Teoh SH, Hutmacher DW. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C Biomim Supramol Syst. 2002;20:49–56. [Google Scholar]
- 135.Pfister A, Landers R, Laib A, Hubner U, Schmelzeisen R, Mulhaupt R. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J Polym Sci Part A Polym Chem. 2004;42:624–638. [Google Scholar]
- 136.Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1(9):910–917. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 137.Mironov V, Boland T, Trusk T, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 138.Nahmias Y, Berthiaume F, Yarmush ML. Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol. 2007;103:309–329. doi: 10.1007/10_029. [DOI] [PubMed] [Google Scholar]
- 139.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11(1–2):302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 140.Mcguigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McGuigan AP, Sefton MV. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007;13(5):1069–1078. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 142.Panda P, Ali S, Lo E, et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8(7):1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mcguigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA. 2006;103(31):11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mcguigan AP, Sefton MV. Design criteria for a modular tissue-engineered construct. Tissue Eng. 2007;13(5):1079–1089. doi: 10.1089/ten.2006.0245. [DOI] [PubMed] [Google Scholar]
- 145.Doraiswamy A, Narayan RJ, Harris ML, Qadri SB, Modi R, Chrisey DB. Laser microfabrication of hydroxyapatite-osteoblast-like cell composites. J Biomed Mater Res A. 2006;80A(3):635–643. doi: 10.1002/jbm.a.30969. [DOI] [PubMed] [Google Scholar]
- 146.Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4(4):415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 147.Nahmias Y, Kramvis I, Barbe L, Casali M, Berthiaume F, Yarmush ML. A novel formulation of oxygen-carrying matrix enhances liver-specific function of cultured hepatocytes. FASEB J. 2006;20:2531–2533. doi: 10.1096/fj.06-6192fje. [DOI] [PubMed] [Google Scholar]
- 148.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106(37):15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu T, Gregory CA, Molnar P, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27(19):3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 150.Franzesi GT, Ni B, Ling Y, Khademhosseini A. A controlled-release strategy for the generation of cross-linked hydrogel microstructures. J Am Chem Soc. 2006;128(47):15064–15065. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 151.Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Analyt Chem. 2003;75(23):6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 152.Weibel DB, Diluzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Microbiol. 2007;5:209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 153.Regehr KJ, Domenech M, Koepsel JT, et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009;9(15):2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rettig JR, Folch A. Large-scale single-cell trapping and imaging using microwell arrays. Anal Chem. 2005;77(17):5628–5634. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 155.Ochsner M, Dusseiller MR, Grandin HM, et al. Micro-well arrays for 3D shape control and high resolution analysis of single cells. Lab Chip. 2007;7(8):1074–1077. doi: 10.1039/b704449f. [DOI] [PubMed] [Google Scholar]
- 156.Albrecht DR, Tsang VL, Sah RL, Bhatia SN. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip. 2005;5:111–118. doi: 10.1039/b406953f. [DOI] [PubMed] [Google Scholar]
- 157.Nichol JW, Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009;5:1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater. 2006;18:2679–2684. [Google Scholar]
- 159.Revzin A, Rajagopalan P, Tilles AW, Berthiaume F, Yarmush ML, Toner M. Designing a hepatocellular microenvironment with protein microarraying and poly(ethylene glycol) photolithography. Langmuir. 2004;20(8):2999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 160.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 161.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 162.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1877. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 163.Rowley JA, Madlambayan G, Money DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 164.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 165.Han D, Gouma P-I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomedicine (Lond) 2006;2:37–41. doi: 10.1016/j.nano.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 166.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Borenstein J, Khademhosseini A, Toner M, Takayama S. Micro and Nanoengineering of the Cell Microenvironment: Technologies and Applications. Artech House; Boston, MA, USA: 2009. [Google Scholar]
- 168.Du Y, Cropek D, Mofrad MRK, Weinberg EJ, Khademhosseini A, Borenstein J. Microfluidic systems for engineering vascularized tissue constructs. In: Tian W-C, Finehout E, editors. Microfluidics for Biological Applications. Springer Science and Business Media, LLC; NY, USA: 2008. [Google Scholar]
- 169.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 170.Song YS, Lin RL, Montesano G, et al. Engineered 3D tissue models for cell-laden microfluidic channels. Anal Bioanal Chem. 2009;395(1):185–193. doi: 10.1007/s00216-009-2935-1. [DOI] [PubMed] [Google Scholar]
- 171.Demirci U, Yaralioglu GG, Haeggstrom E, Percin G, Ergun S, Khuri-Yakub BT. Acoustically actuated flextensional si/sub x/n/sub y/and single-crystal silicon 2-D micromachined ejector arrays. IEEE Transactions on Semiconductor Manufacturing. 2004;17(4):517–524. [Google Scholar]
- 172.Demirci U, Yaralioglu GG, Haeggstrom E, Khuri-Yakub BT. Femtoliter to picoliter droplet generation for organic polymer deposition using single reservoir ejector arrays. IEEE Transactions on Semiconductor Manufacturing. 2005;18(4):709–715. [Google Scholar]
- 173.Cabodi M. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 174.Fidkowski C. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 175.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 176.Gottwald E, Giselbrecht S, Augspurger C, et al. A chip-based platform for the in vitro generation of tissues in three-dimensional organization. Lab Chip. 2007;7(6):777–785. doi: 10.1039/b618488j. [DOI] [PubMed] [Google Scholar]
- 177.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 178.Beebe DJ, Moore JS, Bauer JM, et al. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404(6778):588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 179.Song YS, Lin RL, Montesano G, et al. Engineered 3D tissue models for cell-laden microfluidic channels. Analyt Bioanalyt Chem. 2009;395:185–193. doi: 10.1007/s00216-009-2935-1. [DOI] [PubMed] [Google Scholar]
- 180.Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325–1335. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 181.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nature Mater. 2003;2:265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 182.Xu F, Moon S, Zhang X, Shao L, Song YS, Demirci U. Multi-scale heat and mass transfer modelling of cell and tissue cryopreservation. Philos Transact A Math Phys Eng Sci. 2010;368(1912):561–583. doi: 10.1098/rsta.2009.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang L, Hu W. Molecular determinants of biocompatibility. Expert Rev Med Devices. 2005;2(4):493–500. doi: 10.1586/17434440.2.4.493. [DOI] [PubMed] [Google Scholar]
- 184.Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12(5):1325–1335. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 185.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat Mater. 2003;2(4):265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 186.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 187.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ. Jet-based methods to print living cells. Biotechnol J. 2006;1(9):930–948. doi: 10.1002/biot.200600058. [DOI] [PubMed] [Google Scholar]
- 189.Sanjana NE, Fuller SB. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J Neurosci Methods. 2004;136(2):151–163. doi: 10.1016/j.jneumeth.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 190.Stuhrmann B, Jahnke HG, Schmidt M, et al. Versatile optical manipulation system for inspection, laser processing, and isolation of individual living cells. Rev Sci Instrum. 2006;77:063116. [Google Scholar]
- 191.Pirlo RK, Dean DM, Knapp DR, Gao BZ. Cell deposition system based on laser guidance. Biotechnol J. 2006;1(9):1007–1013. doi: 10.1002/biot.200600127. [DOI] [PubMed] [Google Scholar]
- 192.Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–136. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 193.Demirci U, Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip. 2007;7(9):1139–1145. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 194.Demirci U, Montesano G. Cell encapsulating droplet vitrification. Lab Chip. 2007;7(11):1428–1433. doi: 10.1039/b705809h. [DOI] [PubMed] [Google Scholar]
- 195.Demirci U. Acoustic picoliter droplets for emerging applications in semiconductor industry and biotechnology. J Microelectromech Syst. 2006;15(4):957–966. [Google Scholar]
- 196.Chung BG, Kang L, Khademhosseini A. Micro- and nanoscale technologies for tissue engineering and drug discovery applications. Expert Opin Drug Discov. 2007;2:1–16. doi: 10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- 197.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26(2):125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 198.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 199.Jagur-Grodzinski J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym Adv Technol. 2010;21(1):27–47. [Google Scholar]
- 200.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 201.Ali M, Horikawa S, Venkatesh S, Saha J, Hong JW, Byrn ME. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J Control Release. 2007;124(3):154–162. doi: 10.1016/j.jconrel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 202.Banerjee P, Bhunia AK. Mammalian cell-based biosensors for pathogens and toxins. Trends Biotechnol. 2009;27:177–189. doi: 10.1016/j.tibtech.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 203.Mazzoleni G, Lorenzo DD, Steimberg N. Modelling tissues in 3D: the next future of pharmacotoxicology and food research? Genes Nutr. 2009;4:13–22. doi: 10.1007/s12263-008-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Peppas N, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine. Adv Mater. 2006;18:1–17. [Google Scholar]
- 205.Sung JH, Shuler ML. A micro cell culture analog (microcca) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip. 2009;9(10):1385–1394. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- 206.Mahler GJ, Esch MB, Glahn RP, Shuler ML. Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol Bioeng. 2009;104(1):193–205. doi: 10.1002/bit.22366. [DOI] [PubMed] [Google Scholar]
- 207.Banerjee P, Lenz D, Robinson JP, Rickus JL, Bhunia AK. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab Invest. 2008;88(2):196–206. doi: 10.1038/labinvest.3700703. [DOI] [PubMed] [Google Scholar]
- 208.Nickerson CA, Richter EG, Ott CM. Studying host-pathogen interactions in 3-D: organotypic models for infectious disease and drug development. J Neuroimmune Pharmacol. 2007;2(1):26–31. doi: 10.1007/s11481-006-9047-x. [DOI] [PubMed] [Google Scholar]
- 209.Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19(5):534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Breitbach M. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 211.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Anderson D, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 213.Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60(2):215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 214.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol Ther. 2007;15(3):467–480. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Placzek MR, Chung I-M, Macedo HM, et al. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6:209–232. doi: 10.1098/rsif.2008.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures Science. 2007;316(5828):1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 217.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee SA, Chung SE, Park W, Lee SH, Kwon S. Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography. Lab Chip. 2009;9:1670–1675. doi: 10.1039/b819999j. [DOI] [PubMed] [Google Scholar]
- 219.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gelain F. Novel opportunities and challenges offered by nanobiomaterials in tissue engineering. Int J Nanomedicine. 2008;3(4):415–424. [PMC free article] [PubMed] [Google Scholar]
- 221.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14(5):633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 222.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 223.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14(1):71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 224.Grossmann J. Molecular mechanisms of “Detachment-induced apoptosis–anoikis”. Apoptosis. 2002;7(3):247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 225.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11(4):381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jeong MK, Choi MJ, Kwon SJ, et al. Ultrasonic characterization of thermal distribution in vicinity for a cylindrical thermal lesion in a biological tissue. Key Eng Mater. 2006;321–323(II):1133–1138. [Google Scholar]
- 227.Moon S, Hasan SK, Song YS, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods. 2010;16(1):157–166. doi: 10.1089/ten.TEC.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Song YS, Adler D, Xu F, Kayaalp E, Nureddin A, Anchan RM, Maas RL, Demirci U. Vitrification and levitation of a liquid droplet on liquid nitrogen. Proc Natl Acad Sci U S A. 2010;107(10):4596–4600. doi: 10.1073/pnas.0914059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Arumuganathar S, Irvine S, McEwan JR, Jayasinghe SN. Aerodynamically assisted bio-jets: the development of a novel and direct non-electric field driven methodology for engineering living organisms. Biomed Mater Mater Tissue Eng Regen Med. 2007;2:158–168. doi: 10.1088/1748-6041/2/2/015. [DOI] [PubMed] [Google Scholar]
- 230.Chen CY, Barron JA, Ringeisen BR. Cell patterning without chemical surface modification: cell–cell interactions between printed bovine aortic endothelial cells (BAEC) on a homogeneous cell-adherent hydrogel. Appl Surf Sci. 2006;252(24):8641–8645. [Google Scholar]
- 231.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29(2):193–203. doi: 10.1016/j.biomaterials.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 232.Barron JA, Wu P, Ladouceur HD, Ringeisen BR. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices. 2004;6(2):139–147. doi: 10.1023/b:bmmd.0000031751.67267.9f. [DOI] [PubMed] [Google Scholar]
- 233.Fukuda J, Khademhosseini A, Yeo Y, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27(30):5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]