Abstract
Hummingbirds can maintain the highest wingbeat frequencies of any flying vertebrate – a feat accomplished by the large pectoral muscles that power the wing strokes. An unusual feature of these muscles is that they are activated by one or a few spikes per cycle as revealed by electromyogram recordings (EMGs). The relatively simple nature of this activation pattern provides an opportunity to understand how motor units are recruited to modulate limb kinematics. Hummingbirds made to fly in low-density air responded by moderately increasing wingbeat frequency and substantially increasing the wing stroke amplitude as compared with flight in normal air. There was little change in the number of spikes per EMG burst in the pectoralis major muscle between flight in normal and low-density heliox (mean=1.4 spikes cycle−1). However the spike amplitude, which we take to be an indication of the number of active motor units, increased in concert with the wing stroke amplitude, 1.7 times the value in air. We also challenged the hummingbirds using transient load lifting to elicit maximum burst performance. During maximum load lifting, both wing stroke amplitude and wingbeat frequency increased substantially above those values during hovering flight. The number of spikes per EMG burst increased to a mean of 3.3 per cycle, and the maximum spike amplitude increased to approximately 1.6 times those values during flight in heliox. These results suggest that hummingbirds recruit additional motor units (spatial recruitment) to regulate wing stroke amplitude but that temporal recruitment is also required to maintain maximum stroke amplitude at the highest wingbeat frequencies.
Keywords: Anna's hummingbirds, Calypte anna, electromyography, heliox, load lifting, stroke amplitude, wingbeat frequency
INTRODUCTION
Two important mechanisms for varying muscle power during flight are modulation of wing stroke amplitude and of wingbeat frequency (Ellington, 1984; Rayner, 1979). Power output can be held constant over a range of kinematics as long as an increase in either stroke amplitude or wingbeat frequency is offset by a decrease in the other variable. Modulation of these wingbeat kinematics can also lead to changes in power output, with maximum values constrained either by a morphological limit to stroke amplitude, physiological limits to contractile frequency or by a necessary trade-off between stroke amplitude and wingbeat frequency (Lehmann and Dickinson, 1997).
Aerodynamic theory predicts a U-shaped relationship between power requirements and forward flight speed because induced costs decline but profile and parasitic costs increase from hovering to progressively faster speeds. Metabolic requirements should follow a similar pattern if muscle efficiency does not vary substantially with flight speed. Experimental measurements of metabolic and muscle power output generally support this hypothesis (Askew and Ellerby, 2007; Clark and Dudley, 2009; Ellerby and Askew, 2007a; Pennycuick, 1968; Tobalske et al., 2003; Tucker, 1968), although the power requirements at the fastest forward velocities can be substantially lower than those during hovering flight in some species (Dial et al., 1997). These power requirements correlate strongly with electromyogram (EMG) intensity (the area under a rectified EMG signal) of the pectoralis major, which is the muscle that powers the avian downstroke (Dial and Biewener, 1993; Ellerby and Askew, 2007b; Hedrick et al., 2003; Tobalske, 1995; Tobalske and Dial, 1994; Tobalske et al., 2005). The EMG intensity is also highest during the slowest and fastest speeds, and lowest during intermediate speeds. Tests of other flight behaviors further indicate that both EMG intensity and duration are correlated with different flight modes including forward flight, take-off, ascending and descending flights (Dial, 1992a; Dial, 1992b; Tobalske and Biewener, 2008; Tobalske et al., 2005; Williamson et al., 2001). Thus, the ability of birds to perform different modes of flight in the laboratory has provided clear examples of how muscle activity is regulated to match power requirements (Dial and Biewener, 1993). The EMG burst frequencies of the two opposing pectoral muscles (the pectoralis major and the supracoracoideus) always determine the wingbeat frequency but it is not known how muscle activation patterns in these or other wing muscles produce specific changes in wing kinematics such as stroke amplitude.
In 1968, Susumu Hagiwara and colleagues recorded the EMGs from the hummingbird pectoralis major (Hagiwara et al., 1968). It was noted at the time that the EMGs in this muscle appeared as discrete events, strongly resembling spikes from an extracellular recording of a single motor unit. However, unlike a single unit recording, the EMG waveform amplitude varied as the hummingbirds engaged in different flight behaviors. The hummingbirds were also capable of changing the number of spikes per EMG burst but no formal descriptions of the flight behaviors or measurements of the wingbeat kinematics were available to help interpret these distinctive patterns of muscle activity.
Subsequent research on the kinematics of hovering hummingbirds has revealed that these animals can adjust wingbeat frequency and stroke amplitude with a degree of independence. When maximally challenged to fly in low-density air using physically variable gas mixtures, hummingbirds respond by systematically increasing wing stroke amplitude with only slight increases in wingbeat frequency (Chai and Dudley, 1995). These trends have also been demonstrated within and among species in the field along natural elevational gradients (Altshuler, 2006; Altshuler and Dudley, 2003). However, it is not known if hummingbirds actively increase wing stroke amplitude through neural input to the muscle or if stroke amplitude is passively regulated because the wing is able to travel over a wider arc in less dense air given the same neural input. When loaded with relatively large external weights, hummingbirds briefly but substantially increase both stroke amplitude and wingbeat frequency (Altshuler and Dudley, 2003; Chai and Millard, 1997).
In this study, we recorded EMGs from the pectoralis major of Anna's hummingbirds (Calypte anna) during hovering in normal air, hovering in low-density air and maximum load-lifting trials. We determined the number and amplitude of EMG spikes, as well as the stroke periods and stroke amplitudes for individual wingbeats.
MATERIALS AND METHODS
All measurements were made with adult male Anna's hummingbirds (Calypte anna Lesson 1829), captured in Los Angeles and Riverside County, CA, USA. Research was conducted under permits from the California Department of Fish and Game, and the United States Fish and Wildlife Department. All animal procedures were approved by the Institutional Animal Care and Use Committee at the California Institute of Technology and the University of California, Riverside.
The muscle activation patterns and wingbeat kinematics of five hummingbirds were studied during preliminary experiments in which only free flight in normal air was examined. Following these initial trials, four new hummingbirds were measured successively using three experimental conditions: (1) hovering at an artificial feeder in normal air, (2) during experimental reductions in air density, and (3) brief flight bouts while transiently lifting maximum loads (Fig. 1). For conditions 1 and 2, hummingbirds were trained to feed every 20 min from an artificial feeder made of a 10 ml syringe in an airtight, acrylic chamber. Access to the feeder was restricted between feeding bouts and was only permitted while the bird fed or remained hovering directly in front of the feeder.
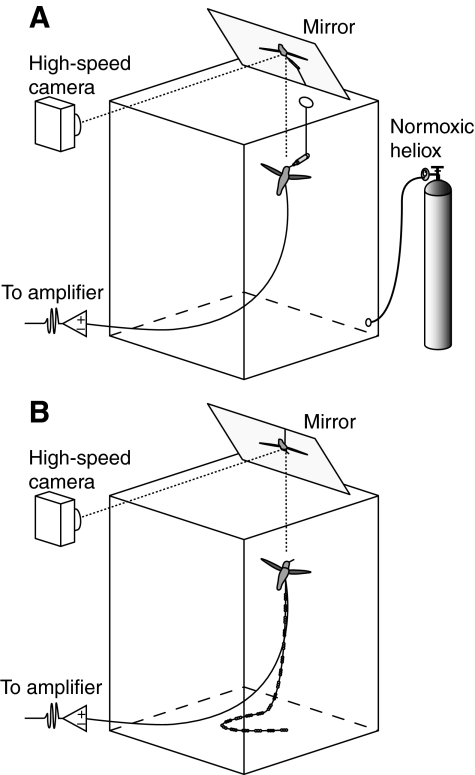
Fig. 1.
Experimental set-up for in vivo recordings of muscle activation and wingbeat kinematics. (A) Hovering performance in normal air and in normoxic heliox was recorded while the birds fed from an artificial feeder. A high-speed camera filmed through a mirror held at 45 deg to attain horizontal wingbeat kinematics. A bipolar electrode was inserted into the pectoralis major on one or both sides of the animal. Replacing normal air with normoxic heliox gradually lowered air density within the airtight chamber. (B) Maximum load lifting performance in normal air was recorded immediately following density reduction trials. A harness was placed around the neck of the bird, which was attached to a string with color-coded beads. As the hummingbird ascended while attempting to escape, it lifted progressively more weight until reaching its maximum burst performance at which point the animal hovered briefly.
Prior to experimental procedures, hummingbirds were anesthetized using isoflurane and underwent a brief surgery for implantation of EMG wires. Each bipolar electrode was made from a pair of silver wires (0.08 mm diameter) insulated with heavy polyimide (HML, California Fine Wire, Grover Beach, CA, USA) that were isolated electrically but bonded together. The tips were offset by 0.5 mm and stripped of insulation for the first 0.5 mm of each lead. A bipolar electrode was inserted in each of the pectoralis major muscles by feeding the tip into a 25-gauge needle and bending back the wire to create a hook. The needle and wire were inserted into the muscle and the needle was subsequently removed. The wires were sutured to the skin above the pectoralis major and again to the intervertebral fascia on the dorsal side of the animal. A ground electrode was inserted above the vertebral column and underneath the skin. One of the birds in the preliminary studies had two EMG electrodes placed in the left pectoralis major and a third EMG electrode placed in the underlying left supracoracoideus. The four focal hummingbirds had one EMG electrode placed in each of the left and right pectoralis major muscles.
The EMG signals were amplified 1000× with an extracellular amplifier (A-M Systems, 1700, Sequim, WA, USA). The online analog filters of the amplifier were set with low and high frequency cut-offs of 1 Hz and 10 kHz, respectively. The analog signals were acquired using an analog-to-digital converter (Molecular Devices, Digidata 1440, Sunnyvale, CA, USA) sampling at 10 kHz. The EMG signals were synchronized to the high-speed images (see below) by recording the camera trigger signal in the A-D converter. For comparisons among individuals and related statistical analyses, the signals from the amplifier were post-processed offline in several ways. We used a zero-phase, fourth-order high-pass Butterworth filter with a cut-off frequency set at 12× the wingbeat frequency to remove movement artifacts and set the mean of the inactive portions of the signal to zero. We then determined the number of EMG spikes per wingbeat and the amplitudes of those spikes. The EMG spikes recorded in the hummingbird pectoralis major are ~1 ms in duration on average and our algorithm counted spikes as distinct events if the peaks were separated by more than 0.5 ms. We determined the single maximum spike amplitude for each bird from all of its recordings in different trials, and then calculated the normalized EMG amplitudes for the spikes in all of that bird's trials by dividing all spike amplitudes by the maximum spike amplitude. Offline filtering and other signal analyses were performed using custom software written in Matlab (Mathworks Inc., Natick, MA, USA).
The air density was reduced by systematically replacing normal air at Riverside, CA, USA (elevation=350 m, density=1.18 kg m−3) with normoxic heliox (21% oxygen, balance helium; density=0.41 kg m−3). Air density was calculated using measured barometric pressure, temperature and humidity, and reduction in air density was calculated using frequency changes recorded from a Galton whistle within the chamber. The air density was reduced in several stages until the hummingbirds were unable to fly or feed without losing vertical position and gradually descending to the floor of the chamber. However, we considered the successful feeding bout just before this obvious inability to fly, and at a slightly higher air density, as the successful flight closest to aerodynamic failure. We used this procedure because in this study we were focused on attaining wingbeat kinematics and corresponding EMG recordings over a range of air densities rather than on determining the specific density of aerodynamic failure for hummingbirds with implanted EMG wires. Thus, the values for air densities and corresponding kinematics at the category listed as ‘failure’ do not represent the values at true aerodynamic failure. Immediately following true aerodynamic failure, we opened the door to return the chamber to normal air density. Within seconds, the hummingbirds fully recovered normal flying ability.
Load-lifting trials followed air-density trials and began 10 minutes after a hummingbird had finished the last of several recovery feeding bouts during hovering in normal air. We placed a rubber harness over the neck of the animal and attached the harness to a color-coded weight chain. Upon release from the floor of the chamber, the hummingbirds displayed their natural escape response, attempting to fly vertically away from the floor thereby lifting progressively more weight until reaching the maximum elevation and therefore the maximum load. These lifts were repeated several times until we were confident that we had recorded the maximum effort for each preparation. Immediately following load-lifting trials, the hummingbirds were again tested during hover feeding in normal air. The EMGs from the first hover feeding trial, the recovery hover feeding bout in between air density and load-lifting trials, and the last hover feeding trial were compared to determine if the EMG wires had shifted during experiments.
During all flight trials, the hummingbird were filmed using a Troubleshooter high-speed camera (Fastec Imaging, San Diego, CA, USA) recording at 1000 frames s−1 with a shutter speed of 1/5000. The camera filmed through a mirror held at an angle of 45 deg above the chamber to provide a perspective of the horizontal plane. The wingbeat frequency was calculated over the frames that the hummingbird maintained a stationary position. The stroke amplitude was measured for those wing angles at the extreme forward and backward strokes in the middle of supination and pronation, respectively. At these positions, the wings were in the midst of rotation and appeared as thin lines from the above-bird perspective.
The body mass was measured before and after experiments on a digital balance (Ohaus Scout Pro, Pine Brook, NJ, USA) with a resolution of 0.01 g. The mean of these two values was used. Following the second mass measurement, we captured an image of the bird's wing in an outstretched position against a white background with a distance scale. The wing length, area and shape were analyzed using additional software written in Matlab. The mean mass of the pectoralis major muscle in C. anna was determined from separate individuals used in other terminal experiments. The morphological, kinematic and environmental variables were incorporated in an aerodynamic model to estimate the power requirements (Ellington, 1984).
The kinematic and electrophysiological variables were compared across treatments using the repeated-measures general linear model in the program SPSS (SPSS Inc., Chicago, IL, USA). Mauchly's test of sphericity indicated that the requirement for equality of variances was violated for all variables. Three corrections for the violation of sphericity are the Greenhouse–Geisser, Huynh–Feldt and Lower Bound, which accordingly adjust the degrees of freedom and the significance value of the F-ratio. All of the repeated-measures analyses of variance (ANOVAs) were highly significant after using any of the three corrections. The degrees of freedom and probabilities for the most conservative correction, the lower bound, are reported below.
RESULTS
The EMG traces from the pectoralis major muscle of Anna's hummingbirds (C. anna) appear as a small number of discrete spikes per burst during hovering flight as has been previously reported in this and some other small North American hummingbirds (Hagiwara et al., 1968). A sample recording of two electrodes placed in the left pectoralis major and a third electrode placed in the underlying left supracoracoideus of a hovering C. anna male is depicted in Fig. 2. The traces in the lower left (Fig. 2C) are the direct output from the amplifier with analog filter cut-offs of 1 Hz and 10 kHz. The panels in the lower right (Fig. 2D) are the same traces following offline filtering. Because all three signals contain crosstalk (especially electrode iii), the activations of each of the two muscles are recorded from three distinct sites. The supracoracoideus activation was composed of one distinct spike in all three traces. The pectoralis major activation bursts were composed of 1–3 spikes, and the number of spikes per burst was consistent between the two recordings from the pectoralis major muscle. However, the relative spike amplitudes within a burst did differ depending upon recording site. The spike numbers and the relative spike amplitudes did not differ between the output signals from the amplifier and the signals that were post-processed offline. Thus, the synchronized activations of EMG bursts in hovering hummingbirds did not derive from offline filtering, smoothing or other signal processing.
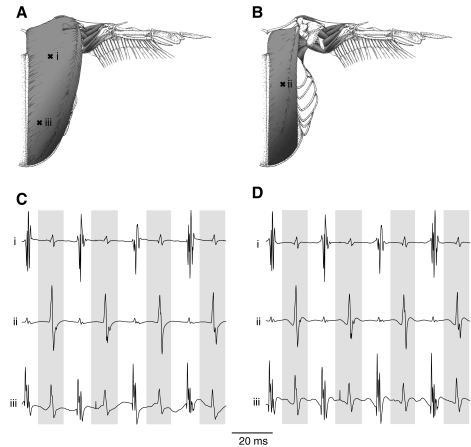
Fig. 2.
Representative electromyograms (EMGs) from the pectoralis major and supracoracoideus of an individual Anna's hummingbird from a preliminary experiment. Electrodes i and iii were placed in the left pectoralis major (A), and electrode ii was placed in the underlying supracoracoideus (B) at the sites indicated by ‘x’. Hummingbird musculoskeletal drawings were adapted from Welch and Altshuler (Welch and Altshuler, 2009). Representative traces from the three EMG electrodes are presented as direct output from the amplifier with filter cut-offs of 1 Hz and 10 kHz (C). Upstrokes are indicated by the white spaces in between the gray bars, which indicate the downstrokes. The same traces are presented following offline filtering (D). The time scale applies to both raw amplifier output and post-processed signals.
Representative traces from one of the four focal hummingbirds are presented in the left panels of Fig. 3. These traces are also a direct output from the extracellular amplifier with only the broad online filter applied. During each of these activities there is notable cycle-to-cycle consistency in the EMG pattern. For all recordings, an EMG burst was recorded in the pectoralis major with the first or only spike appearing 5 ms before the beginning of the subsequent downstroke, i.e. midway through the upstroke. The mean (±s.d.) compilations on the left side of Fig. 3 were calculated from post-processed signals.
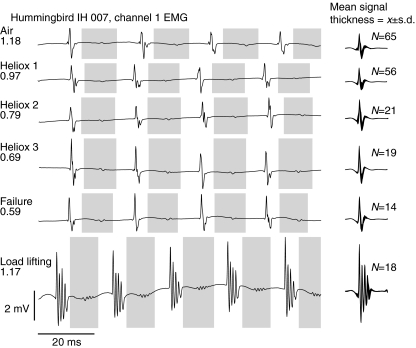
Fig. 3.
Representative electromyograms (EMGs) from the pectoralis major of an individual Anna's hummingbird (IH 007). The panels on the left-hand side depict the raw amplifier output from a bipolar electrode pair, with online filters set at 1 Hz and 10 kHz. No additional signal processing was applied. Gray bars correspond to downstrokes and the white spaces in between correspond to upstrokes. Six traces are presented to represent a sample from each of the hovering, heliox and load-lifting categories with the air density provided below the treatment name. The mean signal is presented on the right-hand side. These traces were generated by applying an offline high-pass filter to the amplifier output and aligning all traces (sample sizes in figure) to the maximum spike. The thickness of the signal represents the mean ± standard deviation (s.d.) at each time point relative to the spike.
We assume that either the amplitude or the size of the recorded EMG signal is a measure of the number of motor units in the muscle that become activated (McComas et al., 1971). Because hummingbird EMG bursts are composed of distinct spikes, we accordingly measured spike amplitudes instead of integrating the rectified signal to describe changes in spatial recruitment of motor units, and present spike number rather than burst duration to describe changes in temporal recruitment. However, we also analyzed our results using both spike size and integrated amplitude and the statistical patterns and significance trends are identical for the two approaches. The EMG patterns exhibited during hovering in air and in heliox differed primarily in spike amplitudes, with higher spike amplitudes at lower air densities (Fig. 3). Compared with hovering in normal air, the activation patterns during maximum load lifting showed both an increase in spike number and an increase in spike amplitude. With respect to the latter, the first spike in the burst typically had the highest and most consistent amplitude with subsequent spikes exhibiting greater variation. The mean interspike intervals were 1.05 ms for hovering in normal air or heliox and 1.14 ms during transient load lifting.
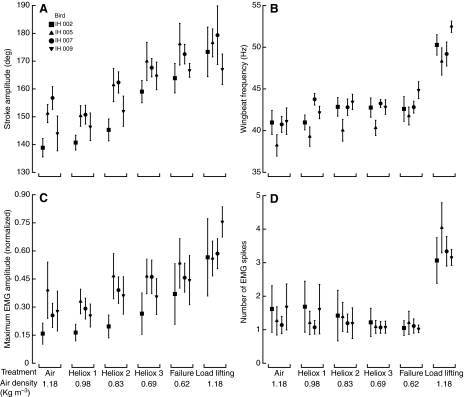
The mean values for wing kinematics (stroke amplitude and frequency) and EMG properties (spike amplitudes and numbers) are presented for all four individuals in Fig. 4. Hummingbirds increased stroke amplitude as density decreased (repeated-measures ANOVA F1,3=63.18, P<0.005) until approaching values near 180 deg. The value listed for ‘failure’ actually represents density values somewhat above true aerodynamic failure (see Materials and methods). During maximum load lifting, hummingbirds used stroke amplitudes that were greater than maximum recorded performance in heliox by occasionally overextending their wings until they touched during stroke reversal. Wingbeat frequency increased modestly across decreasing air density but increased substantially during maximum load lifting compared with all free-flight trials at either normal or reduced air density (F1,3=69.62, P<0.005). The normalized maximum EMG spike amplitude increased throughout density reduction and during maximum load lifting (F1,3=19.94, P<0.05). The number of EMG spikes remained unchanged during density reduction, and then increased substantially during maximum load lifting (F1,3=47.30, P<0.01). Hummingbirds used either one or two EMG spikes during hovering in air or heliox, and used between three and five EMG spikes during maximum load lifting.
Fig. 4.
Regulation of wingbeat kinematics [(A) stroke amplitudes; (B) wingbeat frequencies] and electromyogram (EMG) features [(C) maximum EMG spike amplitude; (D) number of EMG spikes per excitation] in relation to experimental treatments. Treatment densities represent median values. Symbols represent means (±s.d.) of individual hummingbirds. Over the course of several hours, hummingbirds were recorded hover feeding in air, across decreasing air densities and during maximum load lifting. Note that EMG spike amplitudes are normalized such that the single highest spike for each individual across all trials would have a value of 1.
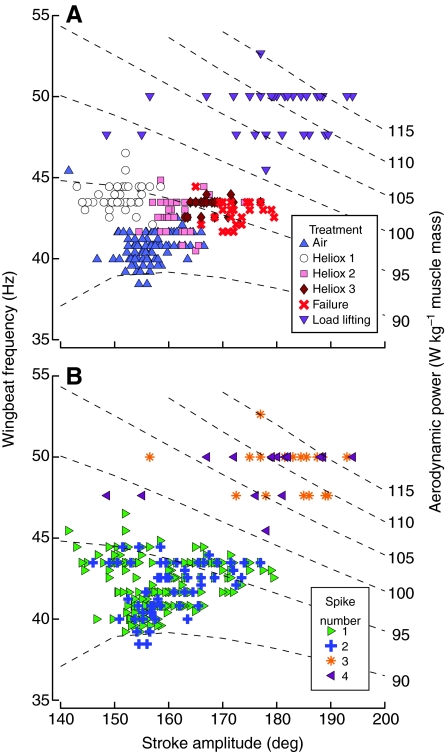
A combined analysis of the kinematics and EMGs are provided for a representative hummingbird in Fig. 5. These plots include isoclines for the aerodynamic (induced + profile) power, which was estimated using equations in Ellington (Ellington, 1984) in combination with mean values of morphology, kinematics and environmental variables for the four hummingbirds in the study. One notable difference from the Ellington (Ellington, 1984) model was that we used a profile drag coefficient of 0.139 based upon spinner measurements with a real hummingbird wing held at an angle of attack of 15 deg (Altshuler et al., 2004). These isoclines are provided not as definitive calculations based on a full kinematic analysis but rather as relative strata to consider how adjustments to wing stroke amplitude and wingbeat frequency are likely to shift the requirements for hovering flight. The hummingbirds are able to vary wing stroke amplitudes from 140 deg to 180 deg with concomitant decreases in wingbeat frequency from 47 Hz down to 43 Hz using 1–2 spikes per burst. Achieving kinematic combinations that involve stroke amplitudes greater than ~155 deg at wingbeat frequencies greater than ~46 Hz was associated exclusively with three of more spikes per burst.
Fig. 5.
Wingbeat frequency as a function of stroke amplitude in an individual Anna's hummingbird (IH 007). Each point represents the values for a single wingbeat in each of six behavioral treatments (A). The same data are replotted with new symbols and a new color map representing the number of spikes per excitation burst within the wingbeat (B). The broken lines represent the aerodynamic power isoclines, with power expressed in watts per kilogram of muscle mass listed on the right-hand side of each line. The isoclines assume normal body mass and thus do not include the additional requirements from added weights in the load-lifting trial.
DISCUSSION
During hovering flight, hummingbirds initiate each wing stroke with 1–3 highly synchronous bursts of motoneuron action potentials that result in corresponding spiking muscle action potentials in the pectoral muscles (Fig. 2) (Hagiwara et al., 1968). Increasing demands on the flight system brought about by flight in air of lowered density were met by increasing wing stroke amplitude along with more modest increases in wingbeat frequency (Fig. 4), as has previously been shown for four other hummingbird taxa (Altshuler and Dudley, 2003; Chai and Dudley, 1995). The increase in wing stroke amplitude is related to an increase in the number of motor units activated on each cycle, as indicated by increased EMG amplitude in the pectoralis major (Fig. 3). At very high demands tested via maximum load lifting, the cycle frequency increased as has been demonstrated for many other hummingbird taxa (Altshuler et al., 2010; Chai et al., 1997; Chai and Millard, 1997). During peak lifting, there were more spikes per EMG burst, indicating that the burst duration increased as the overall cycle length decreased. These results suggest that hummingbirds recruit additional motor units (spatial recruitment) to regulate wing stroke amplitude but that temporal recruitment is also required to maintain maximum stroke amplitude at the highest wingbeat frequencies.
During hovering in reduced-density gas and while transiently carrying maximal loads hummingbirds achieve both increased rates of aerodynamic and mechanical power output as well as increased rates of flight muscle metabolism, provided mechanical efficiency does not change substantially when compared with hovering in normal air. Sustained hovering flight is assumed to be aerobically powered because associated high ATP consumption rates cannot be met for long by anaerobic metabolism. Support for this assumption lies, in part, in the fact that the maximal activities of several enzymes associated with anaerobic glycolysis of intramuscular glycogen and stores are relatively low (Suarez et al., 2009) and that the stores themselves are relatively small, given the high rates of hovering metabolism that must be supported (Suarez et al., 1990). That hummingbirds can sustain hovering flight at a feeder for >2 s at reduced air densities suggests that this behavior is largely or exclusively powered by aerobic metabolism. By contrast, the hovering flight of hummingbirds lifting maximal loads was never sustained for more than 1–2 s before the hummingbird descended to the chamber floor. The transient nature of this hovering performance, as well as the fact that hummingbirds exhibited panting or noticeably heavier breathing for a few seconds following a maximal lift, strongly indicate that the energetic costs of this level of mechanical performance could not be borne alone by aerobic metabolism. This difference in the apparent ability of aerobic metabolism to support each level of mechanical performance is notable when considered alongside differences in associated EMG patterns. Our electrophysiology results indicate that activation of a subset of pectoralis muscle fibers once or twice per contraction cycle at wingbeat frequencies of up to ≈45 Hz is associated with rates of ATP consumption that can be primarily or entirely met by the aerobic machinery of the muscle fibers and associated oxygen delivery pathways. However, ATP consumption rates associated with simultaneous activation of a greater proportion of pectoralis muscle fibers, with at least some of these fibers being activated 2–4 times per wingbeat, at frequencies near 50 Hz significantly exceed the rate at which ATP can be produced solely via aerobic metabolism. These results provide insights into the sustainability of hummingbird muscle performance at extremes of both operating frequency and intensity.
The muscles that power hummingbird flight are composed exclusively of fast oxidative-glycolytic (FOG or IIA) fibers (Suarez, 1992; Welch and Altshuler, 2009). Selection in hummingbirds has favored fibers that respond rapidly and are fatigue resistant. In their homogeneity, the hummingbird flight muscles (and other small birds) are rather different from the muscles of larger birds, which are made up of a collection of different fiber types (Rosser and George, 1986; Rosser et al., 1996; Schiaffino and Reggiani, 1994). Fiber recruitment in muscles with heterogeneous fibers can result in qualitative changes in the muscle performance achieved. For example, tasks requiring modest force generation and contraction velocities, sustained over relatively long durations may be accomplished primarily via recruitment of motor units, most of which include slow-twitch, fatigue-resistant fibers. By contrast, briefly sustained tasks requiring high levels of force generation or contraction velocities involve progressive recruitment of motor units containing fast-twitch fibers, increasingly susceptible to fatigue (Hodson-Tole and Wakeling, 2009). By contrast, fiber recruitment in the hummingbird muscles involves a quantitative increase in the number of physiologically similar fibers contributing to the task.
The unitary nature of the muscle action potentials recorded from the hummingbirds' pectoral muscles indicates a high degree of synchrony in the arriving motoneuron action potentials. This, despite the fact that the hummingbird pectoralis major is innervated by a large motor pool spanning several spinal segments, as are the pectoral muscles of other birds (E. R. Donovan, B. K. Kenney and D.L.A., unpublished observations) (Sokoloff et al., 1989). Hummingbirds are therefore synchronizing hundreds of motoneurons during the behaviors reported here. Brief excitations are likely to occur whenever muscles operate at especially high frequencies, when the kinetics of turning the muscle on and off become limiting. If the available window for activation becomes small enough then the motoneuron action potentials must necessarily become synchronized. Single spikes in muscles are often recorded in arthropods but, in these cases, there are a small number of motoneurons and the muscle potential reflect the activity of individual units (Josephson et al., 2000). In addition to the hummingbird pectoral muscles, distinct spikes have also been observed in the rattlesnake shaker muscle (Martin and Bagby, 1973) but not in some other fast vertebrate muscles (Elemans et al., 2008; Goller and Suthers, 1996; Rome et al., 1996).
The hummingbird pectoralis major does differ from that of all other birds in one neuroanatomical feature. Whereas other birds have regular grid-like spacing of motor endplates (MEPs) in the muscle, hummingbirds have a unique pattern of two curvilinear bands that concentrate the MEPs in the middle of the muscles (E. R. Donovan, B. K. Kenney and D.L.A., unpublished observations) (Gaunt and Gans, 1993). Such different arrangements in MEP distributions should influence how EMG wires record muscle potentials. It is possible that other muscles operating at high frequency have some degree of synchronized activation. If so, these patterns may be obscured by placement of individual EMG electrodes in muscles with distributed activation locations. To date, the EMGs and the MEP arrangements have only been documented in the small North American hummingbirds. It would be highly informative to attain similar measurements from hummingbirds that span the full range of body sizes and wingbeat frequencies. The Cuban Bee Hummingbird (Mellisuga helenae) weighs less than half of the Anna's hummingbirds used here but is estimated to flap its wings at double the cycle frequency whereas the Giant Hummingbird (Patagona gigas) can weigh 5–6 times that of the C. anna and flaps its wings at one third of the cycle frequency (Altshuler and Dudley, 2003).
For hummingbirds hovering in normal air, in low-density gas and while carrying loads, our results support the hypotheses that spatial recruitment of motor units determines the wing stroke amplitude. At the most rapid wingbeat frequencies, temporal recruitment of motor units is further required to reach maximum stroke amplitude. Additional control of wingbeat kinematics during hovering certainly comes from the opposing upstroke muscle, the supracoracoideus, which makes a significant contribution to the total aerodynamic force (Warrick et al., 2005). It is unknown how other muscles in the wing may contribute to regulating the wingbeat kinematics described here. For example, smaller wing muscles may act to modify finer features of wing position related to deviation angle, angle of attack and timing of pronation and supination, and/or act as power elements that help produce large wing stroke amplitudes at high wing frequencies. Nonetheless, the synchronized excitation signal in the pectoralis major and its highly structured motor code are likely solutions to the problem of developing high force at high contraction/relaxation frequencies. Constraints imposed by high contractile frequencies also appear to influence muscle fiber types and gross anatomy of the skeleton and muscles (Ridgway, 1890; Savile, 1950). Distinct motor codes for regulating muscle strain across different cycling frequencies may be a general feature that is otherwise difficult to observe for more complex and slower operating skeletal muscles.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Scott Currie and Bob Josephson for comments on the manuscript and Qing Liu for assistance with digitization. Funding for this research was provided by a University of California Regents Faculty Fellowship, a National Institutes of Health National Research Service Award (5F32NS046221), and a National Science Foundation Award (IOS 0923849) to D.L.A., and by a National Science Foundation Award (IOS 0217229) to M.H.D. Deposited in PMC for release after 12 months.
REFERENCES
- Altshuler D. L. (2006). Flight performance and competitive displacement of hummingbirds across elevational gradients. Am. Nat. 167, 216-229 [DOI] [PubMed] [Google Scholar]
- Altshuler D. L., Dudley R. (2003). Kinematics of hovering hummingbird flight along simulated and natural elevational gradients. J. Exp. Biol. 206, 3139-3147 [DOI] [PubMed] [Google Scholar]
- Altshuler D. L., Dudley R., Ellington C. P. (2004). Aerodynamic forces of revolving hummingbird wings and wing models. J. Zool. 264, 327-332 [Google Scholar]
- Altshuler D. L., Dudley R., Heredia S. M., McGuire J. A. (2010). Allometry of hummingbird lifting performance. J. Exp. Biol. 213, 725-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew G. N., Ellerby D. J. (2007). The mechanical power requirements of avian flight. Biol. Lett. 3, 445-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai P., Dudley R. (1995). Limits to vertebrate locomoter energetics suggested by hummingbirds hovering in heliox. Nature 377, 722-725 [Google Scholar]
- Chai P., Millard D. (1997). Flight and size constraints: hovering performance of large hummingbirds under maximal loading. J. Exp. Biol. 200, 2757-2763 [DOI] [PubMed] [Google Scholar]
- Chai P., Chen J. S. C., Dudley R. (1997). Transient hovering performance of hummingbirds under conditions of maximal loading. J. Exp. Biol. 200, 921-929 [DOI] [PubMed] [Google Scholar]
- Clark C. J., Dudley R. (2009). Flight costs of long, sexually selected tails in hummingbirds. Proc. R. Soc. Lond. B. Biol. Sci. 276, 2109-2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial K. P. (1992a). Activity patterns of the wing muscles of the pigeon (Columba livia) during different modes of flight. J. Exp. Zool. 262, 357-373 [Google Scholar]
- Dial K. P. (1992b). Avian forelimb muscles and nonsteady flight: can birds fly without using the muscles in their wings?. Auk 109, 874-885 [Google Scholar]
- Dial K. P., Biewener A. A. (1993). Pectoralis muscle force and power output during different modes of flight in pigeons (Columba livia). J. Exp. Biol. 176, 31-54 [Google Scholar]
- Dial K. P., Biewener A. A., Tobalske B. W., Warrick D. R. (1997). Mechanical power output of bird flight. Nature 390, 67-70 [Google Scholar]
- Elemans C. P. H., Mead A. F., Rome L. C., Goller F. (2008). Superfast vocal muscles control song production in songbirds. PLoS ONE 3, e2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby D. J., Askew G. N. (2007a). Modulation of flight muscle power output in budgerigars Melopsittacus undulatus and zebra finches Taeniopygia guttata: in vitro muscle performance. J. Exp. Biol. 210, 3780-3788 [DOI] [PubMed] [Google Scholar]
- Ellerby D. J., Askew G. N. (2007b). Modulation of pectoralis muscle function in budgerigars Melopsitaccus undulatus and zebra finches Taeniopygia guttata in response to changing flight speed. J. Exp. Biol. 210, 3789-3797 [DOI] [PubMed] [Google Scholar]
- Ellington C. P. (1984). The aerodynamics of hovering insect flight. VI. Lift and power requirements. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 305, 145-181 [Google Scholar]
- Gaunt A. S., Gans C. (1993). Variations in the distribution of motor endplates in the avian pectoralis. J. Morphol. 215, 65-88 [DOI] [PubMed] [Google Scholar]
- Goller F., Suthers R. A. (1996). Role of syringeal muscles in controlling the phonology of bird song. J. Neurophysiol. 76, 287-300 [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Chichibu S., Simpson N. (1968). Neuromuscular mechanisms of wing beat in hummingbirds. Zeitschrift fur Vergleichende Physiologie 60, 209-218 [Google Scholar]
- Hedrick T. L., Tobalske B. W., Biewener A. A. (2003). How cockatiels (Nymphicus hollandicus) modulate pectoralis power output across flight speeds. J. Exp. Biol. 206, 1363-1378 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E. F., Wakeling J. M. (2009). Motor unit recruitment for dynamic tasks: current understanding and future directions. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 179, 57-66 [DOI] [PubMed] [Google Scholar]
- Josephson R. K., Malamud J. G., Stokes D. R. (2000). Asynchronous muscle: a primer. J. Exp. Biol. 203, 2713-2722 [DOI] [PubMed] [Google Scholar]
- Lehmann F. O., Dickinson M. H. (1997). The changes in power requirements and muscle efficiency during elevated force production in the fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1133-1143 [DOI] [PubMed] [Google Scholar]
- Martin J. H., Bagby R. M. (1973). Properties of rattlesnake shaker muscle. J. Exp. Zool. 185, 293-300 [DOI] [PubMed] [Google Scholar]
- McComas A. J., Fawcett P. R. W., Campbell M. J., Sica R. E. P. (1971). Electrophysiological estimation of the number of motor units within a human muscle. J. Neurol. Neurosurg. Psychiatry 34, 121-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycuick C. J. (1968). Power requirements for horizontal flight in the pigeon, Columba livia. J. Exp. Biol. 49, 527-555 [Google Scholar]
- Rayner J. M. V. (1979). Vortex theory of animal flight. Part 2. The forward flight of birds. J. Fluid Mech. 91, 731-763 [Google Scholar]
- Ridgway R. (1890). The Hummingbirds. In Report of the U.S. National Museum for 1890, pp. 253-383 Washington, DC: Smithsonian Institution United States National Museum; [Google Scholar]
- Rome L. C., Syme D. A., Hollingworth S., Lindstedt S. L., Baylor S. M. (1996). The whistle and the rattle: The design of sound producing muscles. Proc. Natl. Acad. Sci. USA 93, 8095-8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser B. W. C., George J. C. (1986). The avian pectoralis: histochemical characterization and distribution of muscle fiber types. Can. J. Zool. 64, 1174-1185 [Google Scholar]
- Rosser B. W. C., Wick M., Waldbillig D. M., Bandman E. (1996). Heterogeneity of myosin heavy-chain expression in fast twitch fiber types of mature avian pectoralis muscle. Biochem. Cell Biol. 74, 715-728 [DOI] [PubMed] [Google Scholar]
- Savile D. B. O. (1950). The flight mechanism of swifts and hummingbirds. Auk 67, 499-504 [Google Scholar]
- Schiaffino S., Reggiani C. (1994). Myosin isoforms in mammalian skeletal muscle. J. Appl. Physiol. 77, 493-501 [DOI] [PubMed] [Google Scholar]
- Sokoloff A., Deacon T., Goslow G. E. (1989). Musculotopic innervation of the primary flight muscles, the Pectoralis (Pars-Thoracicus) and Supracoracoideus, of the pigeon (Columba livia): A WGA-HRP study. Anat. Rec. 225, 35-40 [DOI] [PubMed] [Google Scholar]
- Suarez R. K. (1992). Hummingbird flight: sustaining the highest mass-specific metabolic rates among vertebrates. Experientia 48, 565-570 [DOI] [PubMed] [Google Scholar]
- Suarez R. K., Lighton J. R. B., Moyes C. D., Brown G. S., Gass C. L., Hochachka P. W. (1990). Fuel selection in rufous hummingbirds: ecological implications of metabolic biochemistry. Proc. Natl. Acad. Sci. USA 87, 9207-9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K., Welch K. C., Hanna S. K., Herrera M. L. G. (2009). Flight muscle enzymes and metabolic flux rates during hovering flight of the nectar bat, Glossophaga soricina: further evidence of convergence with hummingbirds. Comp. Biochem. Physiol. A 153, 136-140 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W. (1995). Neuromuscular control and kinematics of intermittent flight in the European Starling (Sturnus vulgaris). J. Exp. Biol. 198, 1259-1273 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Biewener A. A. (2008). Contractile properties of the pigeon supracoracoideus during different modes of flight. J. Exp. Biol. 211, 170-179 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Dial K. P. (1994). Neuromuscular control and kinematics of intermittent flight in Budgerigars (Melopsittacus undulatus). J. Exp. Biol. 187, 1-18 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Hedrick T. L., Dial K. P., Biewener A. A. (2003). Comparative power curves in bird flight. Nature 421, 363-366 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Puccinelli L. A., Sheridan D. C. (2005). Contractile activity of the pectoralis in the zebra finch according to mode and velocity of flap-bounding flight. J. Exp. Biol. 208, 2895-2901 [DOI] [PubMed] [Google Scholar]
- Tucker V. A. (1968). Respiratory exchange and evaporative water loss in the flying budgerigar. J. Exp. Biol. 48, 67-87 [Google Scholar]
- Warrick D. R., Tobalske B. W., Powers D. R. (2005). Aerodynamics of the hovering hummingbird. Nature 435, 1094-1097 [DOI] [PubMed] [Google Scholar]
- Welch K. C., Altshuler D. L. (2009). Fiber type homogeneity of the flight musculature in small birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 152, 324-331 [DOI] [PubMed] [Google Scholar]
- Williamson M. R., Dial K. P., Biewener A. A. (2001). Pectoralis muscle performance during ascending and slow level flight in mallards (Anas platyrhynchos). J. Exp. Biol. 204, 495-507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.