Abstract
Elephant seals are naturally adapted to survive up to three months of absolute food and water deprivation (fasting). Prolonged food deprivation in terrestrial mammals increases reactive oxygen species (ROS) production, oxidative damage and inflammation that can be induced by an increase in the renin–angiotensin system (RAS). To test the hypothesis that prolonged fasting in elephant seals is not associated with increased oxidative stress or inflammation, blood samples and muscle biopsies were collected from early (2–3 weeks post-weaning) and late (7–8 weeks post-weaning) fasted seals. Plasma levels of oxidative damage, inflammatory markers and plasma renin activity (PRA), along with muscle levels of lipid and protein oxidation, were compared between early and late fasting periods. Protein expression of angiotensin receptor 1 (AT1), pro-oxidant (Nox4) and antioxidant enzymes (CuZn- and Mn-superoxide dismutases, glutathione peroxidase and catalase) was analyzed in muscle. Fasting induced a 2.5-fold increase in PRA, a 50% increase in AT1, a twofold increase in Nox4 and a 70% increase in NADPH oxidase activity. By contrast, neither tissue nor systemic indices of oxidative damage or inflammation increased with fasting. Furthermore, muscle antioxidant enzymes increased 40–60% with fasting in parallel with an increase in muscle and red blood cell antioxidant enzyme activities. These data suggest that, despite the observed increases in RAS and Nox4, an increase in antioxidant enzymes appears to be sufficient to suppress systemic and tissue indices of oxidative damage and inflammation in seals that have fasted for a prolonged period. The present study highlights the importance of antioxidant capacity in mammals during chronic periods of stress to help avoid deleterious systemic consequences.
Keywords: antioxidant enzyme, cachexia, fasting, inflammation, oxidative stress, renin–angiotensin system
INTRODUCTION
Food deprivation is a stressful physiological condition that activates the hypothalamic–pituitary–adrenal axis, leading to an increased release of adrenocorticotropic hormone and glucocorticoids and to subsequent alterations in fluid balance (Munck et al., 1984), cardio-respiratory function (Sapolsky et al., 2000) and pro-oxidant/antioxidant systems (Mitsui et al., 2002; Sorensen et al., 2006). While moderate caloric restriction has beneficial effects on animal health (Chandrasekar et al., 2001; Ahmet et al., 2005; Mattson and Wan, 2005), prolonged food deprivation leads to the depletion of antioxidant stores and to an increase in production of reactive oxygen species (ROS) and oxidative damage in a variety of animals, including humans (Martensson, 1986).
The harmful effects of prolonged food deprivation are consistent among various vertebrate species (Pascual et al., 2003; Morales et al., 2004). Prolonged food deprivation in the rat increases hepatic mitochondrial ROS production, protein oxidative damage, lipid peroxidation and lipoperoxidation-derived protein modifications (Sorensen et al., 2006). Food deprivation also increases the activity of tumor necrosis factor-α (TNF-α) in rat adipose tissue (Wu et al., 2004) and the oxidatively modified DNA content in peripheral blood mononuclear cells (De Souza Rocha et al., 2008). Likewise, prolonged food deprivation increases myocardial hydrogen peroxide production and hepatic lipid peroxidation and decreases liver and muscle glutathione (GSH) content and antioxidant enzyme activities (Crescimanno et al., 1989; Di Simplicio et al., 1997; Grattagliano et al., 2000; Kondoh et al., 2003).
Northern elephant seals (Mirounga angustirostris Gill 1866) maintain electrolyte and fluid homeostasis during prolonged food and water deprivation (fasting) by activating the renin–angiotensin system (RAS) (Ortiz et al., 2000; Ortiz et al., 2006). In human and rat, the chronic activation of RAS is associated with several pathological conditions, such as inflammatory disease, hypertension, heart and kidney disease, endothelial dysfunction and insulin resistance (Mancini et al., 1996; Kimi and Iwao, 2000; Wang et al., 2000; McFarlane et al., 2001; Cooper et al., 2007; Ortiz et al., 2007). Increased RAS stimulates production of ROS by increasing the expression/activity of NADPH oxidases. ROS react with lipids, proteins or nucleic acids, in a variety of tissues and cells, leading to oxidative damage and inflammation (Rajagopalan et al., 1996; Cai and Harrison, 2000; Landmesser et al., 2002; Lambeth, 2004; Bedard and Krause, 2007).
Northern elephant seals naturally experience prolonged periods (up to three months) of absolute food and water deprivation while breeding, nursing, molting or weaning, with no apparent detrimental effects (Ortiz et al., 1978; Crocker et al., 1998; Ortiz et al., 2001; Ortiz et al., 2006). Unlike hibernators, elephant seals remain normothermic during their postweaning fast, with relatively high metabolic rates compared with mammals of similar size (Rea and Costa, 1992; Crocker et al., 1998). Because prolonged fasting is a natural component of the life history of the elephant seal, we hypothesized that fasting is not associated with increased oxidative damage or inflammation despite an increase in RAS. In order to test our hypothesis, we compared systemic and tissue levels of oxidative stress and inflammation, along with selected RAS components, between early- and late-fasted northern elephant seal pups, in an effort to contribute to the elucidation of the mechanisms evolved by these animals to cope with this potentially detrimental behavior. Elephant seals have clearly evolved robust physiological mechanisms that have allowed them to adapt to extreme behaviors or environmental conditions, making them an ideal and interesting model to study the physiological mechanisms by which mammals contend with the detrimental effects of fasting-induced oxidative stress.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California Merced and Sonoma State University (CA, USA). All work was carried out under the National Marine Fisheries Service marine mammal permit no. 87-1743.
Animals and sample collection
Postweaned northern elephant seal pups of known age (within 2–3 days of weaning) from Año Nuevo State Reserve (30 km north of Santa Cruz, CA, USA) were sampled at two periods: early (2–3 weeks postweaning; N=10, 5 males, 5 females) and late (7–8 weeks postweaning; N=9, 5 males, 4 females). Each sampling group was independent, but, because their age was known with relative accuracy, they represented early and late fasting. Pups were weighed at weaning, and the percentage of body mass loss over the fasting period was calculated. On the day of sampling, animals were initially sedated with 1 mg kg−1 telazol (tiletamine/zolazepam HCl, Fort Dodge Labs, Ft Dodge, IA, USA) administrated intramuscularly. Once immobilized, a 16-gauge, 3.5 inch spinal needle was inserted into the extradural spinal vein, and blood samples were collected in pre-chilled EDTA-treated vacutainer sample tubes and placed on ice. Immobilization was maintained with a 100 mg bolus of intravenous injections of ketamine, as needed. Muscle biopsies were collected by first cleaning a small region in the flank of the animal near the hind flipper, with alternating wipes of isopropyl alcohol and betadine, followed by a subcutaneous injection of 2–3 ml of lidocaine (Henry Schein, Melville, NY, USA). A small (<1.5 cm) incision was made using a sterile scalpel, and a muscle biopsy (ca. 50 mg) was collected with a sterile biopsy punch needle (Henry Schein). Biopsies were rinsed with ice-cold PBS, placed in cryogenic vials, frozen by immersion in liquid nitrogen and stored at −80°C until analyzed. Blood samples were centrifuged for 15 min at 3000 g at 4°C, plasma was transferred to cryo-vials, frozen by immersion in liquid nitrogen and stored at −80°C. Red blood cells (RBCs) were packed by adding two volumes of 0.9% saline solution and centrifuged for 10 min at 3000 g. Buffy coat was discarded and the RBC pellet was lysed by vortexing in four volumes of HPLC-grade water for 1 minute. Lysed RBCs were centrifuged and the supernatant collected, frozen by immersion in liquid nitrogen and stored at −80°C. Frozen tissue samples were homogenized in two volumes of 50 mmol l−1 potassium phosphate buffer containing 1 mmol l−1 EDTA, 1% Triton X-100, 1% PMSF and 1% protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Total protein content in tissue samples was measured by using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
Western blot
Thirty μg of total protein were resolved in 4–15% Tris-HCl SDS gradient gels. Proteins were electroblotted using the Bio-Rad trans-blot, semi-dry cell onto 0.45 μm nitrocellulose membranes. Membranes were blocked with 3% bovine serum albumin in PBS containing 0.05% Tween 20, and incubated overnight with primary antibodies against NADPH oxidase 4 (Nox4), angiotensin receptor type 1 (AT1), catalase (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CuZn-superoxide dismutase (CuZnSOD), Mn-superoxide dismutase (MnSOD), glutathione peroxidase (GPx) or actin (Assay Designs, Ann Arbor, MI, USA) diluted 1:500 to 1:5000. Membranes were washed, incubated with HRP-conjugated secondary antibodies (Pierce, Rockford, IL, USA), re-washed and developed by using the Immun-Star Western C kit (Bio-Rad). Blots were visualized using a Chemi-Doc XRS system (Bio-Rad) and quantified by using Quantity One software (Bio-Rad).
Inflammatory markers and oxidative damage
Plasma TNF-α levels were measured using a commercially available EIA kit (Cayman Chemical). Plasma C-reactive protein (hs-CRP) concentration was quantified by using a Tina-quant high-sensitivity assay (Roche Diagnostics, Indianapolis, IN, USA). Circulating levels of 8-isoprostanes (8-iso-PGF2α) were quantified by GC-MS, as described previously (Morrow and Roberts, 1999). Muscle lipid peroxidation was evaluated by measuring the content of thiobarbituric acid reactive substances (TBARS) using a commercially available kit (Cayman Chemical, Ann Arbor, MI, USA). The relative concentration of 4-hydroxynonenal (4-HNE) in muscle samples as well as plasma and muscle levels of total nitrotyrosine (NT) were measured by slot blot. Thirty micrograms of total protein (for tissue samples) or 25 μl of plasma were loaded onto 0.45 μm nitrocellulose membranes using the Bio-Dot SF microfiltration apparatus (Bio-Rad). Membranes were blocked, probed with antibodies against 4-HNE (Calbiochem, San Diego, CA, USA) and NT (Cayman Chemical) and developed as described above. Protein carbonyl content in muscle samples was evaluated by using an Oxyblot™ protein oxidation detection kit (Millipore, Temecula, CA, USA). Fifteen micrograms of total protein were treated and loaded onto membranes by using the Bio-Dot SF. Membranes were blocked, probed and developed as described above.
Enzyme activities
Plasma renin activity (PRA) was measured using a commercially available RIA kit (DiaSorin, Stillwater, MN, USA), previously validated for its use with northern elephant seal plasma (Ortiz et al., 2000; Ortiz et al., 2003b; Ortiz et al., 2006). Total SOD and GPx activities were measured in tissue extracts and RBC lysates with commercially available kits (Cayman Chemical). Catalase activity was measured by monitoring the removal of 10 mmol l−1 H2O2 at 240 nm, as described previously (Vázquez-Medina et al., 2006). NADPH oxidase activity was measured by using the lucigenin-enhanced chemiluminescence (CL) assay (Griendling et al., 1994). Homogenates were incubated in the dark with 100 μmol l−1 NAPDH and 5 μmol l−1 lucigenin in PBS. Relative CL was measured for 10 minutes in a luminometer (Berthold, Oak Ridge, TN, USA). Basal activity was measured in the absence of NADPH. A buffer blank was subtracted from each reading. NADPH oxidase activity was expressed as RLU mg protein−1.
Statistics
Means were compared between early and late fasting groups by two-sample t-tests with Bonferroni adjustment. Means (±s.e.m.) were considered significantly different at P<0.05. Statistical analyses were performed with the SYSTAT 11.0 software (SPSS, Richmond, CA, USA).
RESULTS
Fasting induces loss of body mass
Loss of body mass in elephant seals was calculated in order to evaluate the effects of prolonged fasting on the overall body composition of elephant seals. The average body mass of elephant seals at weaning was 124±6 kg and at sampling 92±5 kg. Elephant seals lost 26±2% (P<0.001) of their body mass during the course of fasting (ca. 8 weeks). By contrast, muscle total protein content did not change with fasting (early: 4.2±0.5 mg ml−1 vs late: 4.5±0.6 mg ml−1). These results suggest that, despite losing nearly one-quarter of their body mass, elephant seals possess robust physiological mechanisms to tolerate this potentially detrimental condition.
Fasting activates RAS
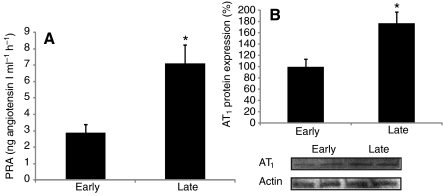
PRA and AT1 protein expression were measured to evaluate the effects of prolonged fasting on RAS activation in elephant seals. Fasting increased PRA nearly 2.5 fold (early: 2.8±0.5 vs late: 7.1±1.1 ng Ang I ml−1 h−1, P<0.01) and muscle AT1 protein expression by 77% (early: 100±13 vs late: 177±19, P<0.05) (Fig. 1), indicating that RAS is activated as a response to prolonged fasting in elephant seals.
Fig. 1.
Prolonged fasting activates the renin–angiotensin system in northern elephant seal pups. Mean (±s.e.m.) (A) plasma renin activity (PRA) and (B) muscle AT1 protein content (expressed as the percentage change from ‘early’) between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods. *P<0.05.
Fasting increases Nox4 protein expression and NADPH oxidase activity
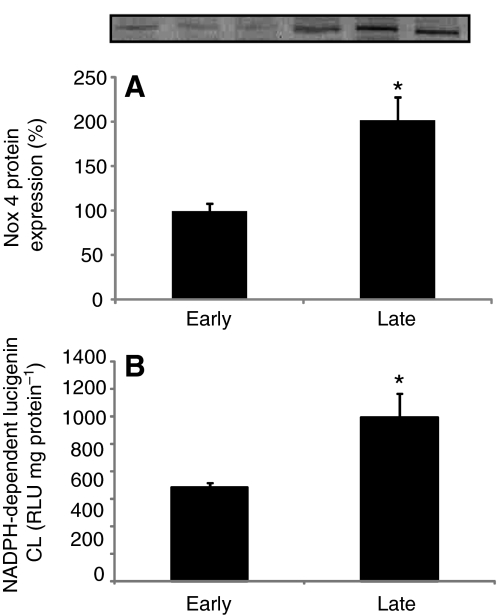
Nox4 muscle protein expression and NADPH oxidase activity were measured to evaluate whether prolonged fasting stimulates ROS-producing proteins in elephant seals. Fasting induced a twofold increase in Nox4 muscle protein expression (early: 100±8 vs late: 201±26, P<0.01) and a 74% increase in NADPH oxidase activity (early: 686±31 vs late: 1194±172RLU mgprotein−1, P<0.05) (Fig. 2). These results demonstrate that fasting stimulates the pro-oxidant system, which can potentially contribute to increased ROS production and oxidative damage.
Fig. 2.
Nox4 protein expression and NADPH oxidase activity increase with fasting in northern elephant seal pups. Mean (±s.e.m.) (A) muscle Nox4 protein content (expressed as the percentage change from ‘early’) and (B) muscle NADPH oxidase activity between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods. *P<0.05.
Neither oxidative damage nor inflammation increases with fasting
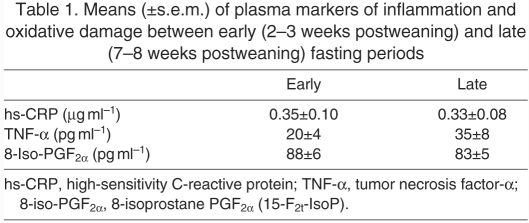
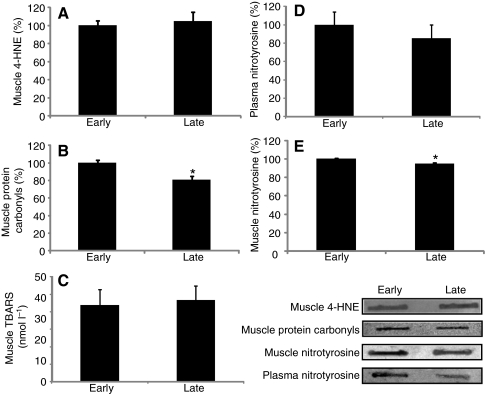
Circulating levels of 8-iso-PGF2α, NT, TNF-α and hs-CRP along with muscle levels of 4-HNE, TBARS, NT and protein carbonyls were measured to evaluate the effects of prolonged fasting on local and systemic indices of oxidative damage and inflammation in elephant seals. None of this suite of markers of oxidative damage and inflammation in plasma or muscle increased over the course of fasting (Table 1, Fig. 3). Moreover, muscle protein carbonyls (early: 100±3 vs late: 81±4) and NT levels (early: 100±1 vs late: 95±1) decreased (P<0.05) with fasting (Fig. 3). These results show that, despite the fasting-induced activation of RAS and Nox4, neither systemic nor local oxidative damage or inflammation increased with fasting, suggesting that these animals have evolved robust physiological mechanisms to avoid oxidative stress and inflammation during this potentially detrimental condition.
Table 1.
Means (±s.e.m.) of plasma markers of inflammation and oxidative damage between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods
Fig. 3.
Neither systemic nor muscle levels of oxidative damage increase with fasting in northern elephant seal pups. Mean (±s.e.m.) expressed as the percentage change from ‘early’ of (A) muscle 4-hydroxynonenal (4-HNE), (B) muscle protein carbonyls, (C) muscle thiobarbituric acid reactive substances (TBARS), (D) plasma nitrotyrosine and (E) muscle nitrotyrosine between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods. *P<0.05.
Fasting increases antioxidant enzymes
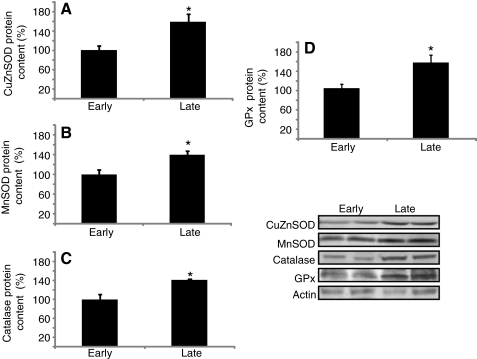
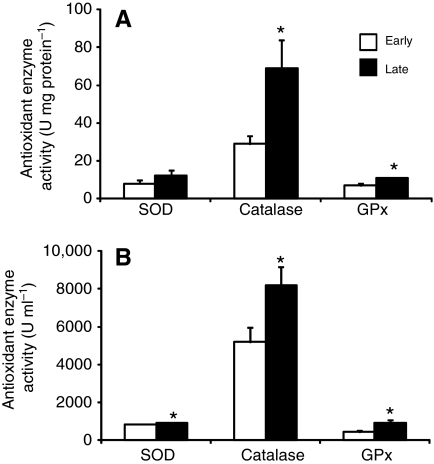
In order to explore the mechanisms elephant seals have evolved to cope with fasting-induced RAS and Nox4 activation, we compared muscle protein content of the antioxidant enzymes CuZnSOD, MnSOD, GPx and catalase, as well muscle and RBC SOD, catalase and GPx activities, between early and late fasted seals. CuZnSOD (early: 100±9 vs late: 158±17), MnSOD (early: 100±9 vs late: 140±8), GPx (early: 100±7 vs late: 151±14) and catalase (early: 100±10 vs late: 142±2) protein expression increased 40–60% (P<0.05) with fasting (Fig. 4). Similarly, the activities of muscle catalase (early: 29±4 vs late: 69±15 U mg protein−1) and GPx (early: 6.9±1.4 vs late: 10.7±0.5 U mg protein−1), and RBC SOD (early: 839±16 vs late: 920±24 U mL−1), catalase (early: 5192±753 vs late: 8185±973 U ml−1) and GPx (early: 430±90 vs late: 935±149 U ml−1) increased (P<0.05) with fasting (Fig. 5). These results indicate that fasting stimulates the antioxidant enzymatic system in elephant seal pups, which likely contributes to the suppression of oxidative stress and inflammation, despite the increases in RAS and Nox4.
Fig. 4.
Expression levels of antioxidant enzymes increase with fasting in northern elephant seals. Mean (±s.e.m.) content expressed as the percentage change from ‘early’ of (A) copper-zinc superoxide dismutase (CuZnSOD), (B) manganese superoxide dismutase (MnSOD), (C) catalase and (D) glutathione peroxidases (GPx) between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods. *P<0.05.
Fig. 5.
Activities of antioxidant enzymes increase with fasting in northern elephant seal pups. Mean (±s.e.m.) activities of total superoxide dismutase (SOD), catalase and glutathione peroxidases (GPx) in (A) muscle and (B) red blood cells (RBCs) between early (2–3 weeks postweaning) and late (7–8 weeks postweaning) fasting periods. *P<0.05.
DISCUSSION
Prolonged food deprivation is a potentially detrimental behavior, which stimulates ROS production and oxidative damage that, when chronically elevated, can contribute to a host of complications, including cardiovascular, renal and hepatic diseases and multi-organ failure (Mancini et al., 1996; Kimi and Iwao, 2000; McFarlane et al., 2001; Cooper et al., 2007). The present study demonstrates that some mammals are uniquely adapted to counteract the detrimental effects of extreme behaviors or environmental conditions such as prolonged food and water deprivation. Our results show that, while prolonged fasting increases the expression of the ROS-producing protein Nox4, along with NADPH oxidase activity, an increase in antioxidant enzymes is sufficient to avoid the oxidative damage and inflammation commonly associated with fasting in terrestrial mammals.
Prolonged food and water deprivation can be detrimental by promoting oxidative stress and inflammation through activation of RAS. While northern elephant seals have adapted to tolerate such a situation, this behavior is associated with a number of chronic metabolic adjustments that include the activation of RAS (Ortiz et al., 2000; Ortiz et al., 2006). Elevated RAS increases the expression/activity of ROS-producing proteins in rat and human (Rajagopalan et al., 1996; Cai and Harrison, 2000; Landmesser et al., 2002; Lambeth, 2004; Bedard and Krause, 2007) and, thus, has been associated with increased concentrations of tissue and systemic markers of oxidative damage and inflammation (Mancini et al., 1996; Kimi and Iwao, 2000; McFarlane et al., 2001; Cooper et al., 2007). Elevated RAS has been shown to increase Nox4 protein expression in vitro and in vivo in rat and human (Wingler et al., 2001; Lapante et al., 2005). Other than these examples, data on the effects of elevated RAS on ROS-generating proteins during prolonged fasting are scarce. The present study demonstrates that prolonged fasting increases the expression of the ROS-producing protein Nox4, along with NADPH oxidase activity, in the presence of increased PRA and AT1, suggesting that elevated RAS might contribute to the generation of ROS through Nox4. Alternatively, the previously observed increases in cortisol in fasting pups (Ortiz et al., 2001; Ortiz et al., 2003a; Ortiz et al., 2003b) might have contributed to the increase in Nox4 and NADPH oxidase activity as elevated glucocorticoids increase ROS production and oxidative damage as well (Mitsui et al., 2002; Caro et al., 2007).
More interestingly, despite the increases in Nox4 and RAS, neither local nor systemic oxidative damage or inflammation indices increase with fasting in elephant seals, indicating that these animals have evolved robust physiological mechanisms to alleviate the potentially detrimental consequences of this extreme condition. Further elucidation of these mechanisms can provide significant advancement of our understanding of diseases related to oxidative stress and RAS.
The lack of increases in local and systemic indices of oxidative stress and inflammation despite the observed increases in Nox4 and RAS can be explained, at least in part, by the observed increases in antioxidant enzymes. It has been suggested that diving seals possess an enhanced antioxidant system that contributes to the protection against ROS produced in response to recurrent episodes of dive-induced ischemia/reperfusion (Elsner et al., 1998; Hermes-Lima and Zenteno-Savín, 2002; Zenteno-Savín et al., 2002; Johnson et al., 2004; Johnson et al., 2005). Higher activities of the antioxidant enzymes SOD, catalase, GPx, glutathione S-transferase and glutathione disulfide reductase, as well as higher GSH content, have been found in seal tissues than in tissues of terrestrial mammals (Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007). In the present study, protein expression of CuZnSOD, MnSOD, catalase and GPx, as well as their activity levels, increased with fasting, which likely contributed to the avoidance of oxidative damage and inflammation. These data suggest that fasting seals are responsive to increased antioxidant capacity, which contributes to their ability to avoid the detrimental effects of pro-oxidants. Furthermore, the end of the fasting period in northern elephant seal pups immediately precedes the beginning of their diving lifestyle (Le Boeuf et al., 1972). Therefore, it is possible that the accumulation of antioxidants during this period prepares elephant seals to cope with the potential ROS overproduction associated with diving-induced ischemia/reperfusion. The increase in NADPH oxidase activity likely stimulated the upregulation of antioxidant enzymes because ROS production can be one of the main factors that regulate antioxidant enzyme expression and activity (Warner et al., 1996; Franco et al., 1999; Pahl and Baeuerle, 2005).
Fasting seals utilize their fat stores as the primary source of energy, resulting in extensive lipid mobilization and increased concentrations of circulating non-esterified fatty acids (NEFA) (Ortiz et al., 1978; Castellini et al., 1987; Rea and Costa, 1992; Houser and Costa, 2001; Ortiz et al., 2003b). In humans, elevated NEFA concentrations are associated with a higher content of circulating 8-iso-PGF2α (Stojiljkovic et al., 2001; Stojiljkovic et al., 2002), a widely used marker of systemic lipid peroxidation (Morrow and Roberts, 1999; Morrow and Roberts, 2000). In the rat, elevated RAS increases circulating levels of 8-iso-PGF2α (Aizawa et al., 2002). In the present study, circulating concentrations of 8-iso-PGF2α remained unchanged with fasting. Moreover, in contrast to the previously reported increases in protein oxidation/nitration induced by fasting and increased RAS activation in terrestrial mammals (Di Simplicio et al., 1997; Sorensen et al., 2006; Cooper et al., 2007), muscle and plasma NT levels remained unchanged, and muscle protein carbonyls decreased, suggesting that fasting seals possess robust cellular mechanisms to protect lean tissue from the potentially damaging effects of oxidation associated with elevated RAS. The decrease in protein carbonyls is likely attributable to the fact that protein catabolism contributes to less than 4% of their average daily metabolic rate and that protein turnover decreases over the fasting period (Adams and Costa, 1993; Houser and Costa, 2001).
In summary, our results show that, despite the fasting-induced increases in RAS and Nox4, neither local nor systemic oxidative damage and inflammation indices were increased. The observed increases in antioxidant enzymes likely contributed to the suppression of oxidative damage and inflammation. Thus, the increased antioxidant response likely alleviated the potentially deleterious effects of fasting-derived ROS production. Elucidating the mechanism by which animals evolved to be uniquely and naturally adapted to extreme environmental and behavioral conditions will prove to be fruitful as we strive to gain a better understanding of how oxidative stress and inflammation promote a number of pathophysiological conditions.
ACKNOWLEDGEMENTS
We thank Dr J. Morrow (posthumously) for the measurements of 8-iso-PGF2α and Dr D. P. Costa (UCSC) for providing laboratory space. C. Champagne, J. Phillips, A. Norris and B. Kelso helped with sampling. J. Viscarra, D. Conte and J. Phillips provided assistance with laboratory analysis. J.P.V.M. is supported by a UC MEXUS-CONACYT Doctoral Fellowship and a fellowship from the Mexican Ministry of Education (SEP). Research funded by NIH NHLBI R01-HL091767. Deposited in PMC for release after 12 months.
Footnotes
- AT1
- angiotensin receptor type 1
- GPx
- glutathione peroxidase
- GSH
- glutathione
- 4-HNE
- 4-hydroxynonenal
- hs-CRP
- high-sensitivity C-reactive protein
- NT
- nitrotyrosine
- RAS
- renin–angiotensin system
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- TBARS
- thiobarbituric acid reactive substances
REFERENCES
- Adams S. H., Costa D. P. (1993). Water conservation and protein metabolism in northern elephant seal pups during the postweaning fast. J. Comp. Physiol. B 166, 367-373 [DOI] [PubMed] [Google Scholar]
- Ahmet I., Wan R., Mattson M. P., Lakatta E. G., Talan M. (2005). Cardioprotection by intermittent fasting in rats. Circulation 112, 3115-3121 [DOI] [PubMed] [Google Scholar]
- Aizawa T., Ishizaka N., Usui S., Ohashi N., Ohno M., Nagai R. (2002). Angiotensin II and catecholamines increase plasma levels of 8-Epi-Prostaglandin F2α with different pressor dependencies in rats. Hypertension 39, 149-154 [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. (2007). The Nox family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245-313 [DOI] [PubMed] [Google Scholar]
- Cai H., Harrison D. G. (2000). Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840-844 [DOI] [PubMed] [Google Scholar]
- Caro P., Gómez J., Sanz A., Portero-Otín M., Pamplona R., Barja G. (2007). Effect of graded corticosterone treatment on aging-related markers of oxidative stress in rat liver mitochondria. Biogerontology 8, 1-11 [DOI] [PubMed] [Google Scholar]
- Castellini M. A., Costa D. P., Huntley A. C. (1987). Fatty acid metabolism in fasting northern elephant seal pups. J. Comp. Physiol. B 157, 445-449 [DOI] [PubMed] [Google Scholar]
- Chandrasekar B., Nelson J. F., Colston J. T., Freeman G. L. (2001). Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 280, H2094-H2102 [DOI] [PubMed] [Google Scholar]
- Cooper S. A., Whaley-Connell A., Habibi J., Wei Y., Lastra G., Manrique C., Stas S., Sowers J. R. (2007). Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 293, H2009-H2023 [DOI] [PubMed] [Google Scholar]
- Crescimanno M., Armata M. G., Rausa L., Gueli M. C., Nicotra C., D'akessandro N. (1989). Cardiac peroxisomal enzymes and starvation. Free Radic. Res. 7, 67-72 [DOI] [PubMed] [Google Scholar]
- Crocker D. E., Webb P. M., Costa D. P., Le Boeuf B. J. (1998). Protein catabolism and renal function in lactating northern elephant seals. Physiol. Zool. 71, 485-491 [DOI] [PubMed] [Google Scholar]
- De Souza Rocha G., Fonseca A. S., Rodrigues M. P., Dantas F. J. S., Caldeira-de-Araujo A., Santos R. (2008). Comet assay to determine DNA damage induced by food deprivation in rats. Acta Biol. Hung. 59, 315-325 [DOI] [PubMed] [Google Scholar]
- Di Simplicio P., Rossi R., Falcinelli S., Ceserani R., Formento M. L. (1997). Antioxidant status in various tissues of the mouse after fasting and swimming stress. Eur. J. Appl. Physiol. 76, 302-307 [DOI] [PubMed] [Google Scholar]
- Elsner R., Oyasaeter S., Almaas R., Saugstad O. D. (1998). Diving seals, ischemia-reperfusion and oxygen radicals. Comp. Biochem. Physiol. A Physiol. 119, 975-980 [DOI] [PubMed] [Google Scholar]
- Franco A. A., Odoma R. S., Rando T. A. (1999). Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic. Biol. Med. 27, 1122-1132 [DOI] [PubMed] [Google Scholar]
- Grattagliano I., Vendemiale G., Caraceni P., Domenicali M., Nardo B., Cavallari A., Trevisani F., Bernardi M., Altomare E. (2000). Starvation impairs antioxidant defense in fatty livers of rats fed a choline-deficient diet. J. Nutr. 130, 2131-2136 [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. (1994). Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74, 1141-1148 [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M., Zenteno-Savín T. (2002). Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 133, 537-556 [DOI] [PubMed] [Google Scholar]
- Houser D. S., Costa D. P. (2001). Protein catabolism in suckling and fasting northern elephant seal pups (Mirounga angustirostris). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 171, 635-642 [DOI] [PubMed] [Google Scholar]
- Johnson P., Elsner R., Zenteno-Savín T. (2004). Hypoxia-inducible factor in ringed seal (Phoca hispida) tissues. Free Radic. Res. 38, 847-854 [DOI] [PubMed] [Google Scholar]
- Johnson P., Elsner R., Zenteno-Savín T. (2005). Hypoxia-inducible factor 1 proteomics and diving adaptations in ringed seal. Free Radic. Biol. Med. 39, 205-212 [DOI] [PubMed] [Google Scholar]
- Kimi S., Iwao H. (2000). Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 52, 11-34 [PubMed] [Google Scholar]
- Kondoh M., Kamadaa K., Kuronagaa M., Higashimotoa M., Takiguchia M., Watanabeb Y., Sato M. (2003). Antioxidant property of metallothionein in fasted mice. Toxicol. Lett. 143, 301-306 [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. (2004). Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181-189 [DOI] [PubMed] [Google Scholar]
- Landmesser U., Cai H., Dikalov S., McCann L., Hwang J., Jo H., Holland S. M., Harrison D. G. (2002). Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40, 511-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapante M. A., Wu R., Moreau P., de Champlain J. (2005). Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic. Biol. Med. 38, 589-596 [DOI] [PubMed] [Google Scholar]
- Le Boeuf B. J., Whiting R. J., Gantt R. F. (1972). Perinatal behavior of northern elephant seal females and their young. Behaviour 43, 121-156 [DOI] [PubMed] [Google Scholar]
- Mancini J. G. B., Henry G. C., Macaya C., O'Neill B. J., Pucillo A. L., Carere R. G., Wargovich T. J., Mudra H., Lüscher T. F., Klibaner M. I., et al. (1996). Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation 94, 258-265 [DOI] [PubMed] [Google Scholar]
- Martensson J. (1986). The effect of fasting on leukocyte and plasma glutathione and sulfur amino acid concentrations. Metabolism 35, 118-121 [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Wan R. (2005). Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 16, 129-137 [DOI] [PubMed] [Google Scholar]
- McFarlane S. I., Banerji M., Sowers J. R. (2001). Insulin resistance and cardiovascular disease. J. Clin. Endocrinol. Metab. 86, 713-718 [DOI] [PubMed] [Google Scholar]
- Mitsui T., Umaki Y., Nagasawa M., Akaike M., Aki K., Azuma H., Ozaki S., Odomi M., Matsumoto T. (2002). Mitochondrial damage in patients with long-term corticosteroid therapy: development of oculoskeletal symptoms similar to mitochondrial disease. Acta Neuropathol. 104, 260-266 [DOI] [PubMed] [Google Scholar]
- Morales A. E., Pérez-Jiménez A., Hidalgo M. C., Abellán E., Cardenete G. (2004). Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 139, 153-161 [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Roberts L. J., II (1999). Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 300, 3-12 [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Roberts L. J., II (2000). Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 28, 505-513 [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 5, 25-44 [DOI] [PubMed] [Google Scholar]
- Ortiz C. L., Costa D., Le Boeuf B. J. (1978). Water and energy flux in elephant seal pups fasting under natural conditions. Physiol. Zool. 51, 166-178 [Google Scholar]
- Ortiz R. M., Wade C. E., Ortiz C. L. (2000). Prolonged fasting increases the response of the renin-angiotensin-aldosterone system, but not vasopressin levels, in postweaned northern elephant seal pups. Gen. Comp. Endocrinol. 119, 217-223 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Wade C. E., Ortiz C. L. (2001). Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am. J. Physiol. Regul. Integr. Comp. Physiol. 208, R790-R795 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Wade C. E., Ortiz C. L., Talamantes F. (2003a). Acutely elevated vasopressin increases circulating cortisol and aldosterone in fasting northern elephant seal pups (Mirounga angustirostris). J. Exp. Biol. 206, 2795-2820 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Noren D. P., Ortiz C. L., Talamantes F. (2003b). GH and gherlin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris). J. Endocrinol. 178, 533-539 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Crocker D. E., Houser D. S., Webb P. M. (2006). Angiotensin II and aldosterone increase with fasting in breeding adult male northern elephant seals (Mirounga angustirostris). Phys. Biochem. Zool. 79, 1106-1112 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Graciano M. L., Seth D., Awayda M. S., Navar G. L. (2007). Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am. J. Physiol. Renal Physiol. 293, F139-F147 [DOI] [PubMed] [Google Scholar]
- Pahl H. L., Baeuerle P. A. (2005). Oxygen and the control of gene expression. BioEssays 16, 497-502 [DOI] [PubMed] [Google Scholar]
- Pascual P., Pedrajas J. R., Toribio F., López-Barea J., Peinado J. (2003). Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem. Biol. Interact. 6, 191-199 [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Kurz S., Münzel T., Tarpey M., Freeman B. A., Griendling K. K., Harrison D. G. (1996). Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. J. Clin. Invest. 97, 1916-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea L. D., Costa D. P. (1992). Changes in standard metabolism during long-term fasting in northern elephant seal pups (Mirounga angustirostris). Physiol. Zool. 65, 731-734 [Google Scholar]
- Sapolsky R. M., Romero M., Munck A. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55-89 [DOI] [PubMed] [Google Scholar]
- Sorensen M., Sanz A., Gómez J., Pamplona R., Portero-Otín M., Gredilla R., Barja G. (2006). Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic. Res. 40, 339-347 [DOI] [PubMed] [Google Scholar]
- Stojiljkovic M. P., Zhang D., Lopes H. F., Lee C. G., Goodfriend T. L., Egan B. M. (2001). Hemodynamic effects of lipids in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1674-R1679 [DOI] [PubMed] [Google Scholar]
- Stojiljkovic M. P., Lopes H. F., Zhang D., Morrow J. D., Goodfriend T. L., Egan B. M. (2002). Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J. Hypertens. 20, 1215-1221 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R. (2006). Antioxidant enzymes in ringed seal tissues: potential protection against dive-derived ischemia/reperfusion. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 142, 198-204 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R. (2007). Glutathione protection against dive-associated ischemia/reperfusion in ringed seal tissues. J. Exp. Mar. Biol. Ecol. 345, 110-118 [Google Scholar]
- Wang C. T., Chin S. Y., Navar L. G. (2000). Impairment of pressure-natriuresis and renal autoregulation in ang II-infused hypertensive rats. Am. J. Physiol. Renal Physiol. 279, F319-F325 [DOI] [PubMed] [Google Scholar]
- Warner B. B., Stuart L., Gebb S., Wispe J. R. (1996). Redox regulation of manganese superoxide dismutase. Am. J. Physiol. Lung Cell. Mol. Physiol. 271, L150-L158 [DOI] [PubMed] [Google Scholar]
- Wingler K., Wünsch S., Kreutz R., Rothermund L., Paul M., Schmidt H. H. (2001). Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic. Biol. Med. 31, 1456-1464 [DOI] [PubMed] [Google Scholar]
- Wu G., Brouckaert P., Olivecrona T. (2004). Rapid downregulation of adipose tissue lipoprotein lipase activity on food deprivation: evidence that TNF-alpha is involved. Am. J. Physiol. Endocrinol. Metab. 286, E711-E717 [DOI] [PubMed] [Google Scholar]
- Zenteno-Savín T., Clayton-Hernández E., Elsner R. (2002). Diving seals: are they a model for coping with oxidative stress? Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 133, 527-536 [DOI] [PubMed] [Google Scholar]