Abstract
Objective
To determine the respective trends in mortality of Zambian mother-infant pairs based on maternal infection with HIV-1 and human herpesvirus type 8 (HHV-8).
Methods
A prospective cohort study was done on Zambian mother-infant pairs, stratified by maternal serologic status and followed from 6 weeks postdelivery for 48 months. Statistical analysis of the differences in the calculated mortality rates among the four groups was done using Stata 7.0. Kaplan-Meier analysis and Cox proportional hazard models were used to measure subject survival time.
Results
Between September 1998 and March 2002, a total of 1,425 mother-infant pairs were enrolled. The crude mortality rate among children born to dually infected mothers was ~9 times higher (245.90 deaths per 1,000 live births) when compared with the death ratio of children born to seronegative mothers (24.63 deaths per 1,000 live births). The incidence rate for death was 0.34/1,000 in infants of co-infected mothers in comparison with 0.32/1,000 among HIV-1–infected mothers, 0.0336/1,000 among uninfected mothers, and 0.0403/1,000 among HHV-8–infected mothers (χ2 = 154.56; P < 0.01). Infants of co-infected mothers had a comparable risk of death in comparison with infants infected with HIV-1 alone {hazard ratio, 9.91 [95% confidence interval (95% CI), 5.08–19.37] for co-infected versus 9.26 [95% CI, 4.75–18.07] for HIV-1–infected alone}. Infants of mothers infected only with HHV-8 also had comparable survival in comparison with uninfected infants (hazard ratio, 1.21; 95% CI, 0.56–2.61).
Conclusion
Infants born to mothers dually infected with both HIV-1 and HHV-8 have comparable survival with infants exposed to HIV-1 alone. Infants born to mothers infected only with HHV-8 have comparable survival with uninfected infants.
Introduction
AIDS- and HIV-1–associated mortality among both adults and children declined significantly in the developed world following the introduction of highly active antiretroviral therapy (1, 4). This has not been the case, however, in those areas of the developing world where highly active antiretroviral therapy is presently not available. Whereas the magnitude of the AIDS epidemic in sub-Saharan Africa has been amply described and there are now data documenting an associated decrease in life expectancy in this region (5), there is a paucity of specific data about AIDS-related mortality (6) especially among HIV-1–infected mother and child pairs. What little data there is has been obtained primarily from retrospective studies (6–8), which, not surprisingly, have documented an increase in mortality among children born to HIV-1–infected mothers. Similarly, there is as yet little prospective data (9) about the effect of the AIDS pandemic on mortality in this same patient population. Whereas the majority of these deaths are infection related (6), there is no information on the role that other viruses may have had in contributing to mortality in these groups.
Zambia, located in south-central Africa, has been severely affected by the AIDS pandemic (10). Approximately 30% of the obstetric patients at the University Teaching Hospital in Lusaka are HIV-1 infected, and prior reports have noted that 20% to 39% of the infants born to these women are infected perinatally (11, 12). Zambia has also experienced an AIDS-related epidemic of Kaposi’s sarcoma among both adults and children. The seroprevalence of human herpesvirus type 8 (HHV-8) or the Kaposi’s sarcoma–associated herpesvirus, the necessary casual agent of Kaposi’s sarcoma (13–17), in this same obstetric population in Lusaka approaches 40% (18). HHV-8 studies with African children have found seroprevalence ranging from 13% to 58%, depending on the region surveyed (19–21). HHV-8 has also been associated with primary effusion lymphoma and multicentric Castlemen’s disease (22), but the natural history of HHV-8 infection and how it might contribute to the pathogenesis of HIV-1 infection in co-infected patients are not known. It is also not known how or to what extent HHV-8 alone contributes to mortality among African adults and children.
As part of a continuing prospective cohort study investigating the epidemiology and natural history of HHV-8 and HIV-1 infection among Zambian mothers and their infants in Lusaka, Zambia, in this article, we describe the mortality patterns in this same population. To our knowledge, this is the first such large-scale, prospective study of its kind. The availability of such data will be crucial to present efforts to prioritize heath care efforts in Zambia and other resource-limited countries.
Materials and Methods
Study Design
A prospective cohort study investigating the epidemiology of HIV-1 and HHV-8 infection among Zambian mothers and their infants was initiated in September of 1998 at the University Teaching Hospital, University of Zambia School of Medicine, Lusaka, Zambia. The study protocol was reviewed and approved by the Institutional Review Boards at the University Teaching Hospital, the University of Miami School of Medicine, and the University of Nebraska. Written informed consent was obtained from all mothers who were interested in participating before entry into the study. After the consent process, blood was drawn from the mothers for HIV-1 and HHV-8 testing. The mothers were classified into four subgroups based on their HHV-8 and HIV-1 serologic status: group 1, HHV-8 and HIV-1 positive; group 2, HHV-8 positive only; group 3, HIV-1 positive only; and group 4, seronegative for both agents (used as a control for this study). Their state of physical health was assessed at each of the follow-up visits. The following information was recorded at each follow-up visit: interval medical history, vital signs and symptoms, complete physical examination, and use of new medication with emphasis on whether these were antibiotics or antimalarial drugs. When patients did not return for follow-up visits, our research staff made home visits to ensure follow-up or inquire about possible illness or death. Death reports were usually taken from family or friends, and in some cases, hospital records were used if deaths occurred at the University Teaching Hospital in Lusaka.
Patient Population
Pregnant Zambian women in the earliest stages of labor were approached for recruitment for the study at the time of presentation to the labor ward at the University Teaching Hospital. Women who were in active labor or who had >5-cm cervical dilation at the time of presentation to the labor ward or who did not live in Lusaka or had visible signs and/or symptoms of AIDS, tuberculosis, or cancer were excluded from the screening process. In cases of multiple gestation, only the firstborn infant was enrolled into the study. Mother-infant pairs who returned for the first follow-up visit became part of the active HIV-1/HHV-8 cohort, which was followed up for this study and described in detail below.
Sample Collection and HIV-1 Testing
Blood samples from the mothers drawn before delivery were used to determine their serologic status. Blood specimens were collected by venipuncture in acid citrate dextrose tubes and processed within 6 h of being drawn. Plasma was screened for HIV-1 antibodies using two rapid assays: Capillus (Cambridge Biotech) and Determine (Abbott Laboratories). Both assays were done according to the manufacturers’ instructions. An immunofluorescence assay was then used to confirm the status of any sera that were positive by one or both of the rapid tests. The HIV-1 immunofluorescence assay was done using a chronically HIV-1–infected T-cell line, HUT-78/ARV (kindly provided by Dr. Cecilia Cheng-Mayer). Uninfected HUT-78 cells were used for background control. Patients were considered HIV-1 infected only if they tested positive by one or both of the rapid assays and the confirmatory immunofluorescence assay. Any indeterminate results were resolved by subsequent testing of specimen collected at a subsequent follow-up visit.
Immunofluorescence Assay for HHV-8
Maternal plasma was tested for HHV-8 antibodies by indirect immunofluorescence assay. BC-3 cells, a HHV-8–positive and EBV-negative B-cell lymphoma cell line, were used for the immunofluorescence assay. The immunofluorescence assay was done using the procedure described by Lennette et al. (23) with minor modifications. Briefly, 5 × 105 cells/mL were stimulated with 12-O-tetradecanoylphorbol-13-acetate (20 ng/mL; Sigma) for 72 h in culture medium (90% RMPI 1640, 10% FCS, 100 units/mL penicillin G, and 100 µg/mL streptomycin). Two lab workers tested all samples at 1:40 dilution. To exclude false-positive results due to background staining, all positive sera were retested with BJAB cells (a Kaposi’s sarcoma–associated herpesvirus negative B lymphoma cell line). Only sera found to be negative with uninfected cells were considered positive.
Statistical Analysis
The data analyzed included information collected during the 48 months of follow-up. Statistical analysis was done using Stata 8.0. Both descriptive and inferential analyses were used (24, 25). Between-group comparisons were carried out by calculating standardized mortality ratios using the mortality rate of infants of mothers not infected with either virus as the standard. Group-specific standardized mortality ratios were calculated as follows: observed deaths/ expected deaths. Differences in mortality among the four groups were assessed by χ2 analysis and determination of relative risk of death. Respective differences in survival were assessed by Kaplan-Meier survival analysis and Cox proportional hazard models. A log-rank test was used to compare the equality of survival time among infants born to dually infected mothers, infants born to uninfected mothers, and infants born to mothers infected with HIV-1 and HHV-8 alone.
Results
Patient Enrollment
Mother-infant pairs were recruited at the University Teaching Hospital over a 42-month period from September 1998 until March 2002. Three thousand one hundred sixty-one were initially recruited after providing informed consent. Of these, 1,425 (45.1%) returned for the first follow-up visit as per the protocol schedule and formed the basis of the cohort, which was subsequently followed to obtain data for this analysis. We have HIV and HHV-8 serology on 1,424 of the 1,425 recruited for the cohort. We had previously reported that of the 3,150 screened mothers, 438 were dually infected with both HIV-1 and HHV-8, 818 were positive for HHV-8 alone, and 519 were positive for HIV-1 alone (18). One thousand three hundred seventy-five (1,375) tested negative for both HIV-1 and HHV-8. The seroprevalences of HIV-1 and HHV-8 in the group as a whole were 30.38% and 39.87%, respectively. Forty-five percent (1,428) of these mothers returned for the first research visit and constituted the cohort followed based on the mothers’ willingness to continue in the study. Each of the mother-infant pairs was assigned to one of four groups based on the mother’s serologic status: group 1 (mother HIV-1+, HHV-8+), 244 mother-infant pairs; group 2 (mother HIV-1−, HHV-8+), 503 mother-infant pairs; group 3 (mother HIV-1+, HHV-8−), 271 mother-infant pairs; and group 4 (mother HIV-1-, HHV-8−), 406 mother-infant pairs. There were some small differences in the sociodemographic maternal and infant delivery variables between the 1,737 mother-infant pairs who did not return for the first follow-up visit and the 1,424 mother-infant pairs that compose the final cohort. Participants were slightly older than nonparticipants, 24.96 versus 23.67 years (P < 0.01; Table 1). Study nonparticipant infants had slightly lower birth-weight (P < 0.01) and Apgar scores at 5 minutes (P < 0.01) than the infants of women who decided to participate in the study. Participants were also more likely to be co-infected with HIV-1/HHV-8 and infected solely with HIV-1 and HHV-8 in comparison with nonparticipants (P < 0.01). Data for mortality were analyzed from the first date of recruitment, September 14, 1998, through 48 months of follow-up.
Table 1.
Socicodemographic and clinical differences between participants and nonparticipants and their infants in the final cohort
| Variable | Participants, mean (SD) or % (n = 1,424) | Nonparticipants, mean (SD) or % (n = 1,737) | P* |
|---|---|---|---|
| Maternal age (y) | 24.96 (6.09) | 23.67 (5.87) | <0.01 |
| Marital status (% married) | 90.10 | 88.72 | 0.21 |
| Infant sex (% female) | 48.12 | 47.54 | 0.75 |
| Birth-weight (g) | 2,972.52 (503.55) | 2,916.48 (538.18) | <0.01 |
| Apgar 5 min | 8.93 (0.44) | 8.75 (1.10) | <0.01 |
| Maternal HIV-1+/HHV-8+ (%) | 17.06 | 13.80 | |
| Maternal HIV-1+ (%) | 35.39 | 22.72 | |
| Maternal HHV-8+ (%) | 18.89 | 11.88 | |
| Maternal HIV-1−/HHV-8− (%) | 28.65 | 51.59 | <0.01 |
χ2 and t tests were used to test for significance.
Infant Mortality and Follow-up of Cohort
There were a total of 190 deaths, 151 among the infants and 37 among the mothers during the 48 months of the study period (Table 2). The majority of the infant deaths (122 of 151, 80.79%) occurred among those who were born to HIV-1–infected mothers (60 in group 1 and 62 in group 3, with group-specific death rates of 245.90 and 228.78 per 1,000 live births, respectively; Table 3). Of the infants who died (n = 151), the mean age of death was 15.07 ± 11.55 months and the median age of death was 12.00 months.
Table 2.
Number of deaths among mothers and infants according to mother’s serologic status
| Serologic status of mother | No. infant deaths* |
No. deaths in mother † |
Total |
|---|---|---|---|
| HIV-1 and HHV-8 infected | 60 | 18 | 76 |
| HHV-8 infected | 19 | 3 | 22 |
| HIV-1 infected | 62 | 18 | 80 |
| Noninfected | 10 | 0 | 10 |
| Total | 151 | 37 | 188 |
χ2 = 146.53, P < 0.01 (for group serostatus for infants).
χ2 = 51.42, P < 0.01 (for group serostatus for mothers).
Table 3.
Standardized infant mortality ratios
| Serologic status of mother | No. deaths | Group-specific deaths per 1,000 | Standardized mortality ratio |
|---|---|---|---|
| HIV-1+/HHV-8+ (n = 244) | 60 | 245.90 | 9.98 |
| HHV-8+ (n = 503) | 19 | 37.77 | 1.53 |
| HIV-1+ (n = 271) | 62 | 228.78 | 9.29 |
| Noninfected (n = 406)* | 10 | 24.63 | 1.00 |
| Total (n = 1,424) | 151 | 106.04 | 4.31 |
The noninfected group serves as the reference group.
The crude child mortality rate for the entire cohort was 106.04 deaths per 1,000 live births. The group-specific child mortality rates ranged between 24.63 deaths per 1,000 births for group 4 and 245.90 deaths per 1,000 births for group 1. Children of uninfected mothers (group 4) had the fewest deaths and were used to calculate the standardized child mortality ratios. Children of HHV-8 and HIV-1 co-infected mothers were ~10 times more likely to die when compared with the children born to the uninfected mothers [hazard ratio (HR), 9.91; 95% confidence interval (95% CI), 5.08–19.37; Table 4], whereas those whose mothers were infected with HHV-8 only had no greater mortality risk (HR, 1.21; 95% CI, 0.56–2.61; Table 5). Adjusting for maternal age, 5-minute Apgar score, maternal death, and infant birth-weight, the HRs did not change significantly, indicating that potential confounding factors did not change these associations [HR(adj), 10.58 (95% CI, 5.23–21.41), for risk of death for children of co-infected mothers in comparison with children of uninfected mothers, and 10.01 (95% CI, 4.96–20.20), for infants of HIV-1+ mothers; Table 6]. The adjusted HR for infants from mothers infected with HHV-8 alone in comparison with uninfected infants was 1.38 (95% CI, 0.63–3.06). Infants from dually infected mothers (group 1) were 8.18 times more likely to die when compared with infants whose mothers were infected with HHV-8 alone [HR, 8.18 (95% CI, 4.88–13.71); HR(adj), 7.65 (95% CI, 4.53–12.91)]. There was no increased risk of death among the coaffected infants (group 1) when compared with those infants perinatally exposed to HIV-1 alone [group 1 versus group 3; HR, 1.07 (95% CI, 0.75–1.53); HR(adj), 1.06 (0.74–1.51)].
Table 4.
Differences between participants and those lost to follow-up at 48 mo
| Variable | Participants, mean (SD) or % (n = 878) | Nonparticipants, mean (SD) or % (n = 368) | P* |
|---|---|---|---|
| Maternal age (y) | 25.38 (6.32) | 24.67 (6.10) | 0.06 |
| Marital status (% married) | 90.76 | 89.86 | 0.63 |
| Infant sex (% female) | 49.46 | 47.15 | 0.46 |
| Birth-weight (g) | 3,000.13 (489.25) | 2,992.65 (521.03) | 0.61 |
| Apgar 5 min | 8.93 (0.45) | 8.92 (0.46) | 0.69 |
| Maternal HIV-1+/HHV8+ (%) | 13.59 | 13.90 | |
| Maternal HIV-1+ (%) | 45.38 | 35.88 | |
| Maternal HHV-8+ (%) | 16.06 | 14.40 | |
| Maternal HIV-1−/HHV-8 (%) | 34.17 | 26.63 | <0.01 |
NOTE: Data exclude those mothers who died or had infants who died before 48 mo of age.
χ2 and t tests were used to test for significance.
Table 5.
Infant survival: Cox proportional hazards model
| Serostatus | HR (95% CI) |
|---|---|
| Uninfected | 1.00 |
| HIV-1+/HHV-8+ | 9.91 (5.08–19.37) |
| HIV-1−/HHV-8+ | 1.21 (0.56–2.61) |
| HIV-1+/HHV-8− | 9.26 (4.75–18.07) |
Table 6.
Infant survival: Cox proportional hazards model
| Serostatus | Adjusted* HR (95% CI) |
|---|---|
| Uninfected | 1.00 |
| HIV-1+/HHV-8+ | 10.58 (5.23–21.41) |
| HIV-1−/HHV-8+ | 1.38 (0.63–3.06) |
| HIV-1+/HHV-8− | 10.01 (4.96–20.20) |
Adjusted for maternal age, 5 min Apgar, infant birth-weight, and maternal death.
We did not find any differences between those lost to follow-up at 48 months and those still in the cohort with regard to maternal age, infant birth-weight, infant Apgar score at 5 minutes, or infant sex. We did see important differences in HIV-1 and HHV-8 seropositivity between those lost to follow-up and those still in the cohort at 48 months. Participants were more likely to be infected with HIV-1 alone in comparison with nonparticipants (45.38% versus 35.88%), but there were no significant differences in the prevalence of co-infection. Participants were followed for a mean of 26.60 ± 18.13 months (range, 0–48 months; Table 4).
Maternal Mortality
A total of 37 death events occurred among the mothers: 16 among co-infected mothers, 3 among HHV-8 infected mothers, and 18 among those infected only with HIV-1. Mothers who were co-infected with HHV-8 and HIV-1 did not have a lower survival than mothers infected with HIV-1 alone (HR, 0.95; 95% CI, 0.49–1.87). As the number of events was small, we were unable to assess the difference in survival between uninfected mothers and those infected with HHV-8.
Infant Mortality and Survival Data
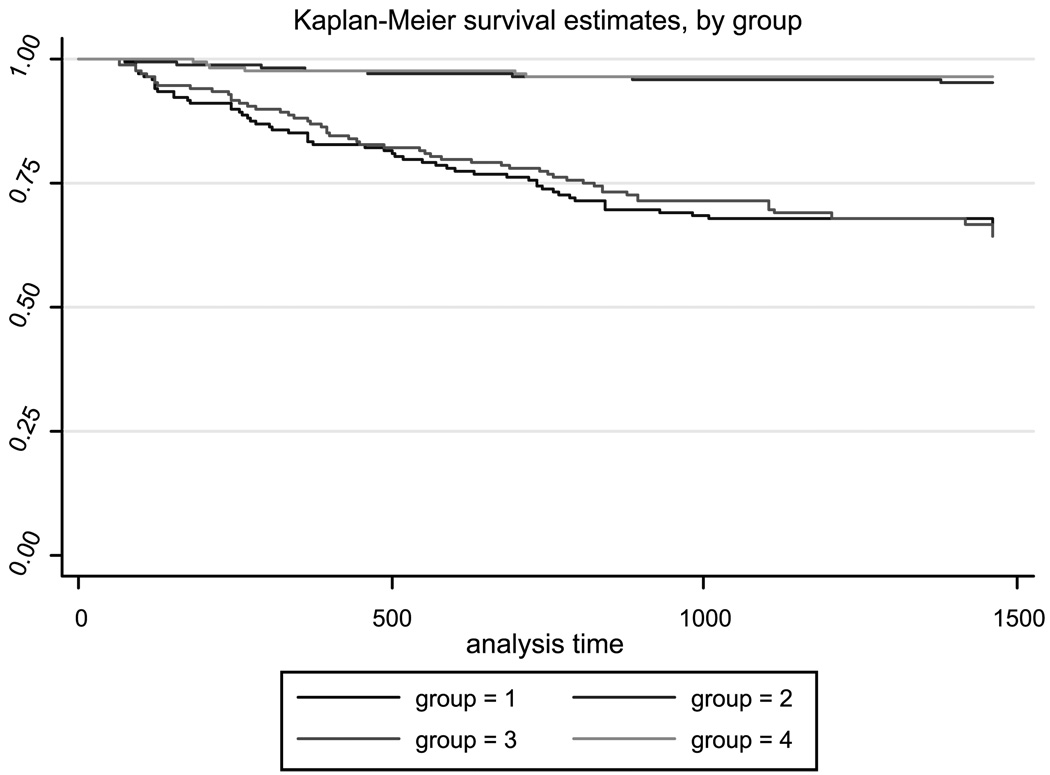
Survival data were obtained from the 1,424 mother and child pairs followed up during the 48-month study period. The mean time of death was 14.40 (±11.09) months for group 1, 15.07 (±11.70) months for group 2, 16.47 (±12.46) months for group 3, and 10.36 (±7.16) months for group 4. These trends were confirmed by Kaplan-Meier survivorship analysis. When the four separate groups of infants were plotted separately, children that were born to the HIV-1 and HHV-8 co-infected mothers showed the poorest survival with an incidence rate of death being 0.34/1,000 and those infected with HIV-1 having an incidence rate of death of 0.32/1,000. Uninfected children had an incidence rate of death of 0.0336/1,000, as did those infected only with HHV-8. The log-rank test for equality of survival functions indicates statistically significant differences between groups (X2 = 154.56, P < 0.01; Fig. 1).
Fig. 1.
Infant Kaplan-Meier survival curves based on the mother’s serologic status.
Discussion
Kaposi’s sarcoma, previously the most common neoplasm associated with AIDS, is responsible for extensive morbidity and increased mortality in this patient population (26). Although endemic in sub-Saharan Africa, its incidence and diversity in terms of the segments of the population affected have both markedly increased since the advent of the global HIV-1 pandemic (17, 22, 26, 27). This is especially true in Zambia where Kaposi’s sarcoma rates have increased among men, women, and children (13–16). With the increasing prevalence of HIV, Zambia has reported a 10-fold increase in the incidence of pediatric Kaposi’s sarcoma. Before 1986, Kaposi’s sarcoma accounted for 6.02% of all childhood cancers; between 1987 and 1992, it accounted for 19.34% of all tumors in children in Zambia, making it the second most common childhood tumor. Fifty-nine percent of reported cases occurred in children under 4 years of age, the peak incidence being between 1 and 2 years of age (15, 17).
The necessary causal agent of Kaposi’s sarcoma, HHV-8, is also endemic in sub-Saharan Africa (22, 28, 29). Most infections occur in early childhood, with a linear increase in prevalence during adulthood. Whereas it seems to be transmissible sexually between homosexual and heterosexual partners (30–36), its exact modes of horizontal and vertical transmissions have not been delineated (37–40).
Our study was designed to describe the epidemiology and natural history of HHV-8 infection among Zambian mothers and their infants. We also were interested in studying the potential effect of HHV-8 and HIV-1 co-infection in these same mother-infant pairs. The latter objective formed the basis for this mortality analysis. Retrospective studies from Zambia, Uganda, and Cote d’Ivoire have found a mortality rate under 5 years of age of 151 to 163 deaths per 1,000 births among HIV-exposed children (6–8), clearly exceeding the targeted goal of 98 to 132 deaths per 1,000 births set by the United Nations during 1991 (10). A retrospective study of 193 vertically infected children from a socioeconomically disadvan-taged neighborhood in Cape Town, South Africa found that 34% of these children died before their 5th birthday (7). Prospective data from a perinatally exposed cohort of infants in Durban, South Africa documented a similar mortality rate, with 35.4% of the infants having died after 48 months of follow-up (6). Whereas the Durban study was free of the bias of earlier, retrospective studies of only symptomatic children, the number of infants followed in this study was relatively small (181, of which 48 were infected, 93 uninfected, and 40 remained indeterminate). The exact causes of death among those 25 infants who died were not specified, although diarrhea, severe thrush, marasmus, and pneumonia were among the most common causes of morbidity among those 17 children dying of AIDS from the Durban study. The mean age at death in this latter group was 10.1 months (range, 1–48 months), with 83% of these deaths occurring before the age of 10 months. Additional prospective data from Rwanda (9) documented an estimated risk of death among HIV-1–infected children of 45% at 2 years and 62% at 5 years. The latter was 21 times the rate observed among uninfected children.
During the 48 months of follow-up in this study, we documented an overall infant mortality rate of 106.04 deaths per 1,000 live births. This figure was higher than the 2001 estimates of infant deaths in Zambia of 90.89 deaths per 1,000 live births (38). Not surprisingly, the majority of deaths occurred among infants perinatally exposed to HIV-1. A similar mortality rate, however, occurred among infants born to mothers infected with both viral agents (group 1). Additionally, infants of mothers infected with HHV-8 had a similar rate of death in comparison with uninfected infants (group 4).
Our survival analysis identified a clear distinction in the survival curves between the groups of infants infected with HIV-1 in comparison with those that were uninfected, and no differences between those that were co-infected with HIV-1 and HHV-8 and those infected with HIV-1 alone. Although the HIV-1 and HHV-8 serologic status of all of the children in our cohort was not established in this study but only maternal infection, preliminary data from our study (data not shown) suggest that whereas HIV-1 was vertically transmitted from mothers to their infants, HHV-8 could be acquired by these infants both by horizontal as well as perinatal transmission (18, 39). For the infants of co-infected mothers, HIV-1 infection probably accounted for the greater part of the infant deaths in this study because the relative frequency of HHV-8 infection by 12 months was less than that of HIV-1 infection (18, 40).
The major limitation of our study was the annual loss to follow-up rate of 10% to 15% at the 12th, 24th, and the 36th month visits. The difficulties encountered with retention in this population highlight the need to combine observational studies with interventional trials, when possible, in such resource-limited areas. Despite this occurrence, differential bias seemed to be minimized as evidenced by the lack of any significant sociodemo-graphic difference between the study participants and those lost to follow-up. The significant differences in HIV-1 and HHV-8 serostatus among those lost to follow-up at 48 months and those still in the cohort could be related to unverified deaths in these groups. It is possible that the infant death rate may have been even higher among the infants of co-infected mothers, and that the HRs reported here may actually have been even higher if we were able to completely track all participants.
The fact that we were unable to verify death through death reports was another limitation of our study, as was our inability to ascertain the HIV-1 and HHV-8 infant serostatus.
The result of this prospective, controlled observational study, which, to our knowledge, is the largest of its kind to date, suggests that there is appreciable infant mortality associated with HIV-1 infection in Zambia. However, we also found that infants born to mothers dually infected with both HIV-1 and HHV-8 have comparable survival with infants perinatally exposed to HIV-1 alone, and that exposure to HHV-8 does not increase risk of death in comparison with unexposed infants.
Acknowledgments
Grant support: Public Health Service grants TW98-002 to CM; CA75903, CA76958, the NCRR COBRE grant RR15635 and the Fogarty International TW01429 to CW.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.de Martino M, Tovo PA, Balducci M, Galli L, Gabiano C. Reduction in mortality with availability of antiretroviral therapy for children with Perinatal HIV-1 Infection. JAMA. 2000;284:190–197. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 2.The global HIV and AIDS epidemic, 2001. MMWR. 2001;50:434–439. [PubMed]
- 3.Justice AC, Ladefeld CS, Asch SM, Gifoford AL, Whalen CC, Covisky KE. Justification for a new cohort study of people aging with and without HIV infection. J Clin Epidemiol. 2001;54:S3–S8. doi: 10.1016/s0895-4356(01)00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Dunford A, Carter YH. Routine care of people living with HIV and AIDS. Should interested general practitioners take the lead. Br J Gen Pract. 2001;51:399–403. [PMC free article] [PubMed] [Google Scholar]
- 5.Mathers CD, Sadana R, Salomon JA, Murray CJL, Lopez AD. Healthy life expectancy in 191 countries. Lancet. 2001;357:1685–1691. doi: 10.1016/S0140-6736(00)04824-8. [DOI] [PubMed] [Google Scholar]
- 6.Bobat R, Coovadia H, Moodley D, Coustsoudis A. Mortality in a cohort of children born to HIV-1 infected mothers from Durban, South Africa. S Afr Med J. 1999;89:646–648. [PubMed] [Google Scholar]
- 7.Hussey GD, Reijnhart RM, Sebens AM, Burgess J, Schaaf S, Potgieter S. Survival of children in Cape Town known to be vertically infected with HIV-1. S Afr Med J. 1998;88:554–558. [PubMed] [Google Scholar]
- 8.Agjololo-Johnson G, De Kock K, Ekpini, et al. Prospective comparison of mother to child transmission of HIV-1 and HIV-2 in Abijan, Ivory Coast. JAMA. 1994;272:462–466. [PubMed] [Google Scholar]
- 9.Spira R, Lepage P, Msellati P, et al. the Mother-to-Child HIV-1 Transmission Study Group. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Pediatrics. 1999;104:1–9. doi: 10.1542/peds.104.5.e56. [DOI] [PubMed] [Google Scholar]
- 10.Preble E. Impact of HIV/AIDS on African children. Soc Sci Med. 1990;31:671–680. doi: 10.1016/0277-9536(90)90249-r. [DOI] [PubMed] [Google Scholar]
- 11.Stringer J, Sinkala M, Goldenberg, et al. A pilot study of nevirapine administered uponp resentation in labor without HIV testing [abstract 300]. Presented at the 3rd Conference on Global Strategies for the Prevention of HIV Transmission from Mothers to Infants; Kampala, Uganda. 2001. [Google Scholar]
- 12.Hira S, Kamanga J, Bhat GJ, et al. Perinatal transmission of HIV-1 in Lusaka, Zambia. Br Med J. 1989;299:1250–1252. doi: 10.1136/bmj.299.6710.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayley AC. Occurrence, clinical behaviour and management of Kaposi’s sarcoma in Zambia. Cancer Surv. 1991;10:53–71. [PubMed] [Google Scholar]
- 14.Patil P, Elem B, Zumla A. Pattern of adult malignancies in Zambia (1980–1989) in light of the human immunodeficiency virus type 1 epidemic. J Trop Med Hyg. 1995;98:281–284. [PubMed] [Google Scholar]
- 15.Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73:100–105. doi: 10.1136/adc.73.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patil PS, Elem B, Gwavava NJ, Urban MI. The pattern of paediatric malignancy in Zambia (1980–1989): a hospital-based histopathological study. J Trop Med Hyg. 1992;95:124–127. [PubMed] [Google Scholar]
- 17.Kasolo FC, Mpabalwani E, Gompels UA. Infection with AIDS-related herpesviruses in human immunodeficiency virus-negative infants and endemic childhood Kaposi’s sarcoma in Africa. J GenVirol. 1997;78:847–855. doi: 10.1099/0022-1317-78-4-847. [DOI] [PubMed] [Google Scholar]
- 18.Brayfield B, Phiri S, Kankasa C, et al. Postnatal human herpesvirus-8 and human immunodeficiency virus-1 infection in mothers and infants from Zambia. J Infect Dis. 2003;187:559–568. doi: 10.1086/367985. [DOI] [PubMed] [Google Scholar]
- 19.Dedicoat M, Newton R, Alkharsha KR, et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–1075. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 20.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–1785. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 21.Mbulaiteye SM, Biggar RJ, Pfeiffer RM, et al. Water socioeconomic factors and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38:474–479. doi: 10.1097/01.qai.0000132495.89162.c0. [DOI] [PubMed] [Google Scholar]
- 22.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus. In: Knipe DM, Howley PM, editors. Fields virology. 4th ed. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 2802–2834. [Google Scholar]
- 23.Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858–861. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- 24.Friis RH, Sellers TA. Epidemiology for public health practice. Aspen Publishers; 1999. pp. 99–102. [Google Scholar]
- 25.Schlesselman J. Case control studies. Oxford University Press; 1982. pp. 135–143. [Google Scholar]
- 26.Safai B, Dias BM. Kaposi’s sarcoma and cloacogenic carcinoma associated with AIDS. In: Broder S, Merigan TC, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore: Williams and Wilkins; 1994. pp. 401–414. [Google Scholar]
- 27.Santarelli R, De Marco R, Masala MV, et al. Direct correlation between human herpesvirus-8 seroprevalence and classic Kaposi’s sarcoma incidence in Northen Sardinia. J Med Virol. 2001;65:368–372. doi: 10.1002/jmv.2043. [DOI] [PubMed] [Google Scholar]
- 28.Schatz O, Monini P, Bugarini R, et al. Kaposi’s sarcoma-associated herpesvirus serology in Europe and Uganda: multicentric study with multiple and novel assays. J Med Virol. 2001;65:123–132. [PubMed] [Google Scholar]
- 29.Olsen SJ, Chang Y, Moore PS, Biggar RJ, Melbye M. Increasing Kaposi’s sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi’s sarcoma endemic region, Zambia in 1985. AIDS. 1998;12:1921–1925. doi: 10.1097/00002030-199814000-00024. [DOI] [PubMed] [Google Scholar]
- 30.He J, Bhat G, Kankasa C, et al. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi’s sarcoma (KS) and mother-child pairs with KS. J Infect Dis. 1998;178:1787–1790. doi: 10.1086/314512. [DOI] [PubMed] [Google Scholar]
- 31.Dukers NH, Renwick N, Prins M, et al. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000;151:213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 32.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 33.Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seroposi-tivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–548. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Casper C, Wald A, Pauk J, Tabet SR, Corey L, Celum CL. Correlates of prevalent and incident Kaposi’s sarcoma-associated herpesvirus infection in men who have sex with men. J Infect Dis. 2002;185:990–993. doi: 10.1086/339605. [DOI] [PubMed] [Google Scholar]
- 35.Challine D, Roudot-Thoraval F, Sarah T, et al. Seroprevalence of human herpes virus 8 antibody in populations at high or low risk of transfusion, graft, or sexual transmission of viruses. Transfusion. 2001;41:1120–1125. doi: 10.1046/j.1537-2995.2001.41091120.x. [DOI] [PubMed] [Google Scholar]
- 36.Sosa C, Klaskala W, Chandran B, et al. Human herpesvirus 8 as a potential sexually transmitted agent in Honduras. J Infect Dis. 1998;178:547–551. doi: 10.1086/517471. [DOI] [PubMed] [Google Scholar]
- 37.Cook RD, Hodgson TA, Waugh AC, et al. Mixed patterns of transmission of human herpesvirus-8 (Kaposi’s sarcoma-associated herpesvirus) in Malawian families. J Gen Virol. 2002;83:1613–1619. doi: 10.1099/0022-1317-83-7-1613. [DOI] [PubMed] [Google Scholar]
- 38.Bourboulia D, Whitby D, Boshoff C, et al. Serologic evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus infection. JAMA. 1998;280:31–32. doi: 10.1001/jama.280.1.31-a. [DOI] [PubMed] [Google Scholar]
- 39.Sitas F, Newton R, Boshoff C. Increasing probability of mother-to-child transmission of HHV-8 with increasing maternal antibody titer for HHV-8. N Engl J Med. 1999;340:1923. doi: 10.1056/NEJM199906173402414. [DOI] [PubMed] [Google Scholar]
- 40.Mantina H, Kankasa C, Klaskala W, et al. Vertical transmission of Kaposi’s sarcoma-associated herpesvirus. Int J Cancer. 2001;94:749–752. doi: 10.1002/ijc.1529. [DOI] [PubMed] [Google Scholar]