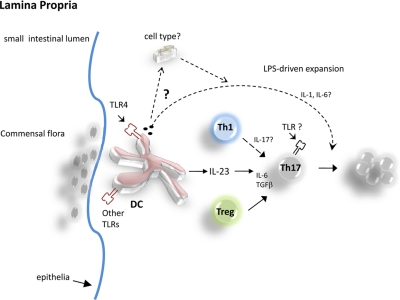
The molecular bases underlying the differentiation and expansion of distinct T cell subsets by TLRs are gradually being revealed. In this issue, McAleer and Vella report that immunization of antigen together with the TLR4 agonists LPS promotes the generation of Th17 cells in the small intestinal LP [1]. Intriguingly, this study suggests that although IL-23 is necessary for Th17 differentiation, it is plays a lesser role in LPS-induced Th17 expansion in the LP. In this editorial, we summarize recent findings regarding the role for TLRs in Th17 generation and maintenance.
Th17 cells are a distinct lineage of T cells programmed by the transcription factors RORα, RORγT, and Stat3. For initial differentiation of these cells from naive T cells to occur, TGF-β and IL-6 are required; these factors in turn induce IL-21, which serves as an autocrine/paracrine factor in Th17 proliferation [2] (Fig. 1). TGF-β and IL-6 also function to induce the expression of IL-23R, which makes the T cells responsive to IL-23 [2]. The Th17 cells also express IL-1R and can be expanded by IL-1 [3]. Fully differentiated Th17 cells grown in the presence of these proinflammatory cytokines express the effector molecules IL-17, IL-17F, IL-21, and IL-22. Although the role of Th17 cells in autoimmunity is well documented, there is growing evidence that the Th17 lineage and other IL-17-producing cells are critical for host defense against bacterial, fungal, and viral infections at mucosal surfaces [4].
Figure 1.
Schematic diagram to show proposed relationships among TLR agonists, DCs, and Th17 cells in the lamina propria. TLR agonists, such as LPS, derived from pathogens, activate DCs in the small intestinal LP to secrete IL-23 and TGF-β, promoting Th17 differentiation. TGF-β in LP further promotes the development of forkhead box p3+ T regulatory (Treg) and Th17 cells. In addition to IL-23 and TGF-β production, LPS supports Th17 expansion, in part, by inducing IL-1 and IL-6 production. It is unknown if Th1 cells that traffic to LP produce IL-17 in the presence of IL-23 and TGF-β (dashed arrows). Furthermore, a role of TLR-MyD88 signals within Th17 cells remains unclear.
TLRs are among the fundamental proteins that sense infectious organisms by recognizing highly conserved PAMPs derived from all known infectious agents. Expressed primarily on innate immune cells, such as DCs and macrophages, each TLR can recognize one or more PAMPs and can form homo- or heterodimers to detect a broader array of microbes. The TLR4 ligand LPS has been shown to induce IL-17 in vitro [5] (Fig. 1). and to promote the production of IL-23 subsequently by macrophages or DCs in the lungs after infection with gram-negative bacteria Klebsiella pneumonia [5] (Fig. 1). Khader et al. [4] have shown that IL-17 and IL-22 are necessary for optimal host defense against several extracellular pathogens, including K. pneumoniae, Candida albicans, and Streptococcus pneumoniae and moreover, that Th17 cells can be elicited in vivo after vaccination with several adjuvants, including Freund’s-based antigen solutions, cholera toxin, and the TLR4 ligand monophosphoryl lipid A/trehalose dicorynomycolate emulsions [4]. Taken together, these findings suggest that certain TLR agonists, such as LPS, can be a useful adjuvant for inducing Th17 responses in vivo.
In this issue, McAleer and Vella report that TLR4 activation augments the number of Th17 cells in the LP by expanding out pre-existing Th17 cells [1]. Several aspects of these studies warrant further comment. First, although the identity of the TLR4-responding target cell was not fully defined, McAleer et al. demonstrated a critical role for IL-23-producing hematopoietic cells, such as DCs. This finding supports previous observations that DCs express IL-23 following their uptake of Gram-negative commensal bacteria, further suggesting that TLR4-activated DCs in the LP play an important role in the LPS-mediated expansion of Th17 cells [4, 5]. TLR4 activation on DCs can also induce the production of various proinflammatory cytokines, such as IL-6 and TNF-α, which are critical for Th17 differentiation and expansion, additionally implicating DCs as mediators in TLR4-induced Th17 expansion.
A second important aspect of these studies concerns the signal pathway (in DCs) for the observed TLR4-promoted Th17 expansion. Unlike any other known TLR, TLR4 can activate two distinct signaling pathways by transducing signals via two different adopter proteins. One pathway transduces signals via MyD88. An alternate channel uses another adopter molecule, known as the TRIF [6]. In their recent studies, McAleer et al. observed significant increases in IL-1, IL-6, and IL-23 serum levels—factors that have been shown to depend on MyD88 signals [6]. However, in contrast to the pro-Th17 cytokine milieu induced by MyD88-dependent signals, TRIF-mediated signals have been shown to negatively impact Th17 cell generation by inducing type I IFNs (IFN-α and IFN-β), which tend to promote IL-27 production. These data suggest that LPS promoted Th17 expansion in the LP by activating the MyD88 pathway in DCs.
These findings by McAleer et al. also suggest that in the absence of IL-23, LPS induced specifically the expansion of pre-committed Th17 cells. This observation raises a question as to what factors may have contributed to Th17 expansion in the absence of IL-23 (Fig. 1). Current investigations about IL-6 and IL-1β have established an important role for them in Th17 development and maintenance. The studies reported herein reveal that LPS plus antigen synergistically augmented IL-1α, IL-1β, and IL-6 levels in serum, thus indicating an important role for these factors in augmenting Th17 cell numbers. Alternatively, the increased number of Th17 cells in the LP might be explained by the preferential recruitment of CCR6-expressing Th17 cells to the LP. In support of this assertion, activation of TLR4 has been shown to induce the expression of CCL20 (CCR6 ligand) in the lamina propria of the intestine [7]. The chemokine expression profile on LPS-induced LP Th17 cells merits additional investigation. The elevated number of Th17 cells could also be explained by enhanced Th17 cell survival. For example, LPS has been shown to induce the production of specific cytokines, such as GM-CSF, which may have increased Th17 cell numbers by augmenting IL-6-dependent Th17 cell survival [4].
The current studies highlight that LPS administered via i.p. injection promoted Th17 expansion exclusively in the LP, whereas no changes in the Th1-to-Th17 ratios were observed in the spleen. These observations raise the question as to whether systemic LPS influenced Th17 cells at other mucosal sites. Although the current studies did not investigate Th17 generation in other tissues, such as the skin, lung, or genital tract, other studies have emphasized that different TLR agonists as well as the route of administration can positively or negatively impact Th17 generation in an organ-specific manner. For example, oral vaccination or intranasal immunization with the TLR9 agonists (unmethylated microbial DNA) has been shown to promote preferential expansion of Th17 cells in the lungs. In contrast, the TLR3 agonist polyinosinic:polycytidylic acid inhibits Th17 responses.
Recent studies by us and other research groups show that CD4 and CD8 T cells express functional TLRs [8,9,10]. The TLR expression profile varies on different T cell subsets and depending on which TLR is activated, results in distinct responses by each subset. For example, the ligation of TLR3 or TLR1/2 on murine CD8 T cells has been shown to enhance the production of various effector molecules, including IFN-γ, IL-2, and granzyme B [8]. TLR3, TLR4, or TLR9 engagement, directly on CD4 Th cells, has also been shown to enhance T cell responses by modulating (sustaining) cytokine IL-2R expression [9], and TLR1/2-stimulated CD8 T cells have been shown to increase cytolytic activity [8]. Studies by Tak and colleagues [11] and Sobek et al. [12] found that TLR9 or TLR2 agonists in the synovium of patients with rheumatoid arthritis costimulated T cells, resulting in enhanced cytolytic function and IFN-γ production. Furthermore, recent studies by Martin and colleagues [13] have demonstrated that IL-17-producing γδ T cells express a functional TLR1/TLR2 heterodimer and that TLR1/2 engagement enhanced IL-17 production. How or whether TLR engagement directly on Th17 cells influences various parameters of Th17 cell function (i.e., cytokine secretion and cytokine receptor expression) is an issue that remains to be explored.
Intriguingly, TLR9 stimulation, directly on CD4 Th cells, has been shown to enhance T cell survival by modulating the expression levels of antiapoptotic proteins (i.e., Bcl-2, bcl-xl, A1). TLR2 ligation on IL-17-producing γδ T cells has also been shown to promote their survival and expansion in vitro [13]. Furthermore, two recent studies by Fukata et al. [14] and Tomita et al. [15] have underscored an import role for MyD88 signals in T cells. Each study demonstrated that MyD88-deficient CD4+CD45Rbhigh T cells expressed a broad array of TLRs, including TLR4, and were impaired in their ability to induce chronic colitis in recombination activating gene 1−/− recipients. Furthermore, LP–derived MyD88−/− CD4 T cells lowered IL-2 and IL-17 levels significantly. Similarly, mice receiving MyD88−/− CD4 T cells produced less IL-6 and IL-23 than controls receiving wild-type cells. Although McAleer et al. did not address directly a direct role for TLR-mediated activation of the MyD88 pathway in Th17 cells, the authors did indicate that their data support the expansion of Th17 cells via TLR4 activation on APCs. Nonetheless, emerging studies suggest an important role for TLR-MyD88 signals within T cells. The significance of these signals in Th17 cells is unknown but warrants further investigation.
In summary, recent progress in the study of Th17 differentiation and expansion underscores the pleotropic effects that TLR agonists exert on adaptive immune responses. Gaining a greater understanding of the interplay between these signals is critical for designing effective clinical strategies to enhance host defense against infection agents and to regulate autoimmune responses.
ACKNOWLEDGMENTS
E. Davila is supported by National Cancer Institute 1R01CA140917-01, 1P20 RR021970, and the Louisiana Cancer Research Consortium; and J. Kolls is supported by the following National Institutes of Health grants: R01-HL079142, P50-HL084932, and R01-AA016688.
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 21
Abbreviations: DC=dendritic cell, LP=lamina propria, PAMP=pathogen-associated molecular pattern, RORα/γ=retinoic acid receptor-related orphan receptor α/γ, TRIF=Toll/IL-1R domain-containing adaptor-inducing IFN-β
References
- McAleer J P, Liu B, Li Z, Ngoi S M, Dai J, Oft M, Vella AT. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J Leukoc Biol. 2010;88:21. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C T, Hatton R D, Mangan P R, Harrington L E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang S H, Martinez G J, Yang X O, Nurieva R, Kang H S, Ma L, Watowich S S, Jetten A M, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S A, Gaffen S L, Kolls J K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel K I, Zheng M, Young E, Quinton L J, Lockhart E, Ramsay A J, Shellito J E, Schurr J R, Bagby G J, Nelson S, Kolls J K. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Shang L, Fukata M, Thirunarayanan N, Martin A P, Arnaboldi P, Maussang D, Berin C, Unkeless J C, Mayer L, Abreu M T, Lira S A. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008;22:3628–3637. doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg G S, Xu D, Liew F Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Asprodites N, Keene A H, Rodriguez P, Brown K D, Davila E. TLR9 engagement on CD4 T lymphocytes represses γ-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. 2008;111:2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijden I M, Wilbrink B, Tchetverikov I, Schrijver I A, Schouls L M, Hazenberg M P, Breedveld F C, Tak P P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sobek V, Birkner N, Falk I, Würch A, Kirschning C J, Wagner H, Wallich R, Lamers M C, Simon M M. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6:R433–R446. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua D J, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Fukata M, Breglio K, Chen A, Vamadevan A S, Goo T, Hsu D, Conduah D, Xu R, Abreu M T. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol. 2008;180:1886–1894. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Kanai T, Fujii T, Nemoto Y, Okamoto R, Tsuchiya K, Totsuka T, Sakamoto N, Akira S, Watanabe M. MyD88-dependent pathway in T cells directly modulates the expansion of colitogenic CD4+ T cells in chronic colitis. J Immunol. 2008;180:5291–5299. doi: 10.4049/jimmunol.180.8.5291. [DOI] [PubMed] [Google Scholar]