Abstract
Lipopolysaccharide (LPS) is a potent natural adjuvant, commonly used to amplify Th1 responses. Here, we report that systemic immunization using LPS generates large numbers of specific Th17 cells in murine small intestinal lamina propria. The priming of these Th17 cells required IL-23p19 production by bone marrow-derived cells. In contrast, IL-23 had no impact on Th1 differentiation or overall numbers of Ag-specific regulatory T cells. Experiments using T-cell adoptive transfers revealed a previously unappreciated mechanism for how Th17 responses are amplified in vivo: stimulation through LPS expanded precommitted Th17 cells rather than causing Th17 differentiation. Second, LPS drove Th17 cell expansion independently of IL-23, demonstrating that this cytokine is not necessary for expansion and possibly functions at an earlier stage in Th17 priming. Our data provide an impetus for using LPS-based peripheral vaccination to augment specific T-cell-mediated immunity in the gut mucosa.
Keywords: T Cells, vaccination, superantigens, cell differentiation
Introduction
Lipopolysaccharide (LPS) is a natural adjuvant synthesized by gram-negative bacteria that enhances the survival, effector capability, and homing of antigen-stimulated T cells (reviewed by McAleer and Vella [1]). Administering LPS within 24 h after antigen increases the number of specific T cells in lymphoid and nonlymphoid tissue [2, 3], as well as their ability to produce IFN-γ [4]. Although IFN-γ is required for protection against intracellular pathogens [5,6,7,8,9,10], the potential for autoimmune inflammation independently of IFN-γ has unveiled the identification of another T-helper cell subset, called Th17, which produces IL-17A, IL-17F, IL-21, and IL-22 [11,12,13,14].
Interleukin-17A (referred to as IL-17) acts on a broad range of cells to promote inflammatory gene induction and neutrophil migration and has been implicated in host defense against Klebsiella pneumoniae, Candida albicans, Salmonella typhimurium, Staphylococcus aureus, and Citrobacter rodentium [14,15,16,17,18]. Disregulated IL-17 production is also associated with chronic inflammation and autoimmunity (reviewed by Iwakura et al. [19]). Although Th17 cells are highly represented within intestinal lamina propria [20], the processes by which they develop and contribute to inflammation in vivo are incompletely understood. The study of IL-23, a heterodimer composed of p19 and p40 subunits, has proven useful in this respect since Th17 responses are significantly reduced in IL-23p19−/− mice [13, 21,22,23,24,25]. The observation that Th17 differentiation in vitro is IL-23 independent suggested it functions as an expansion or maintenance factor for Th17 cells [26,27,28], although a recent study demonstrated that terminal Th17 differentiation is influenced by IL-23 [29].
The protective role of IL-17 during intestinal infections and T-cell-mediated colitis underlies an interest for developing Ag-specific mucosal vaccines [17, 18, 30]. Immunization with LPS elicits Th1 responses in lymphoid tissue, liver, and lung [4, 31, 32]. Although it has been suggested that parenteral vaccination is generally ineffective against mucosal infections [33], the impact of LPS on intestinal T-cell activation has not been well characterized. In vitro studies suggest that LPS can support Th17 responses, in part, as it readily induces IL-6, IL-23p19, IL-12/23p40, and transforming growth factor (TGF)-β [28, 34, 35]. In addition, an in vivo study demonstrated that a minor population of specific IL-17-producing CD4 T cells can be found in liver following LPS immunization [32], although how LPS promotes such a response is unknown.
Here, we studied how the balance between Th17 and Th1 subsets is formed following intraperitoneal (i.p.) immunization with LPS as an adjuvant. While the spleen supported a Th1-biased immune response, LPS generated nearly equivalent numbers of endogenous Th17 and Th1 cells in small intestinal lamina propria. Using a bone marrow chimera approach, we found that expression of IL-23p19 by bone marrow-derived cells was necessary for Th17 formation. To investigate the mechanism of Th17 cell accumulation, adoptive transfer experiments using naïve T cells were performed and revealed that LPS preferentially drives Th1, not Th17, differentiation in vivo. Nevertheless, LPS responses were able to expand out precommitted Th17 cells independently of IL-23, consistent with recent evidence of IL-23 being a differentiation factor rather than a requirement for clonal expansion [29].
Our data demonstrate that vaccines which are known to elicit Th1 responses can also cause the outgrowth of gut mucosal Th17 cells, possibly because of the unique intestinal niche that favors their formation. Because the magnitude of Th17 cell accumulation directly correlated with IL-23 levels, this cytokine is a likely target for selectively controlling Th17 responses. Further, we show that adoptive transfer models only partially recapitulate immune responses in vivo, which consist of diverse T-cell populations resulting from multiple microenvironmental niches that influence their differentiation.
MATERIALS AND METHODS
Mice
C57BL/6, CD45.1, and IL-12/23p40−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). SM1 TCR transgenic mice (CD4+ CD90.1+ Vβ2+ RAG−/−) [36] and TEa TCR transgenic mice (CD4+ CD90.1+ Vα2+ Vβ6+ RAG−/−) [37] were bred by our laboratory. IL-23p19−/− mice [38] were backcrossed to C57BL/6 for 8 generations and maintained by Schering-Plough Biopharma. All mice at The University of Connecticut Health Center were kept under specific pathogen-free conditions and handled in accordance to National Institutes of Health federal guidelines.
Immunization
Reagents were diluted in PBS and injected i.p. in a total volume of 0.2 ml. For superantigen studies, staphylococcal enterotoxin A (SEA; Toxin Tech; Sarasota, FL, USA) was injected at 1 μg per mouse, followed by LPS derived from Salmonella typhimurium (40-60 μg; Sigma-Aldrich; St. Louis, MO, USA) 18 h later.
For adoptive transfer studies, ∼5 × 105 bulk cells from lymph nodes and spleens of SM1 mice were injected i.v. on day −1, corresponding to 0.12–0.2 million SM1 T cells (CD4+ Vβ2+ CD90.1+). The next day, flagellin peptide (FL-pep; residues 427-441; Invitrogen; Carlsbad, CA, USA) was injected at 100 μg per mouse at time 0, followed by LPS (150 μg) at 18 h. On day 10, mice were injected with SEA (1 μg) and FL-pep (100 μg), followed by LPS (65-70 μg) 18 h later. LPS doses were determined from titration studies on individual batches to find the amount providing maximal T-cell survival.
Tissue processing
Spleens were crushed through nylon mesh cell strainers (Falcon/BD Biosciences; San Jose, CA, USA) and treated with ammonium chloride to lyse RBCs. Liver lymphocytes were obtained as described previously [39]. Briefly, following perfusion livers were crushed through cell strainers and partitioned on a 35% Percoll (Sigma-Aldrich) gradient.
Isolation of intestinal lamina propria lymphocytes was based on a previous report [40] with some modifications. The small intestine was excised from mice followed by removal of Peyer’s Patches, fat, and feces. The tissue was then flushed with balanced salt solution (BSS), sliced open longitudinally, cut into ∼1-cm pieces, and washed with Ca/Mg-free BSS. Tissue was stirred twice at 37°C in Ca/Mg-free BSS containing 5 mM EDTA and 0.15 mg/ml dithioerythritol for 10 min, with the supernatant removed after each incubation. Then, tissue was cut into small pieces, washed, and stirred twice at 37°C in BSS containing 1 mM CaCl2, 1 mM MgCl2, 0.3 mg/ml collagenase (Sigma-Aldrich), and 0.1 mg/ml DNase I (Sigma-Aldrich). Supernatants from collagenase-treated tissue were poured over cell strainers and spun down at 1400 rpm. The cells were then fractionated on a 44% and 67% Percoll gradient (Amersham Biosciences; Piscataway, NJ, USA), with lymphocytes partitioning at the interface.
Serum IL-23 level
Blood was taken from tail veins of mice at 1.5, 3, and 6 h following injection of 100 μg LPS. Blood was kept on ice for 30 min followed by centrifugation at 13,000 rpm and 4°C for 20 min, with serum partitioning as the upper fraction. Levels of IL-23 were determined by ELISA (BD Biosciences).
Generation of bone marrow chimeras
Bone marrow cells were flushed from femurs and tibias of C57BL/6 and IL-23p19−/− mice. Red blood cells were lysed with ammonium chloride, and the remaining cells resuspended at 1.4 × 107 cells/ml in balanced salt solution (BSS) supplemented with HEPES, L-glutamine, penicillin, streptomycin, and gentamycin sulfate. For bone marrow reconstitution, congenic CD45.1 C57BL/6 mice were γ-irradiated twice with 550 RAD at a 3-h interval, followed by i.v. injection of 1.4 × 106 bone marrow cells. Bone marrow chimeras were rested for ≥8 weeks before use.
Cell culturing
For in vitro restimulation, one million cells were cultured for 5 h at 37°C in 0.2 ml complete tumor medium (CTM), consisting of MEM with FBS, amino acids, salts, and antibiotics. Cells were cultured with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml; Calbiochem, Gibbstown, NJ, USA) plus ionomycin (1 μg/ml; Invitrogen), SEA (1 μg/ml), and brefeldin A (BFA; 5 μg/ml; Calbiochem), as indicated, and stained intracellularly for cytokine production.
For in vitro Th17 differentiation, 106 splenocytes from SM1 or TEa mice were cultured with 1.5 million antigen-presenting cells (APCs) for 4 days at 37°C and 5% CO2 with murine GM-CSF (20 ng/ml; Pierce Biotechnology, Rockford, IL, USA), soluble anti-mouse CD3e (1 μg/ml; BD Biosciences), murine IL-6 (25 ng/ml; R and D Systems), human TGF-β1 (3 ng/ml; R and D Systems), with or without LPS (1.25 μg/ml). For some experiments, specific peptide was used in place of anti-CD3e at 25 μg/ml [FL-pep for SM1 cultures or Ea-pep (Invitrogen) for TEa cultures]. To obtain APCs, naïve C57BL/6 splenocytes were depleted of CD4+ and CD8+ cells using FITC-conjugated antibodies followed by anti-FITC microbeads (Miltenyi Biotec, Auburn, CA, USA). At the end of the culture period, Th17 cells were adoptively transferred into recipients at 4–5 × 105 cells per mouse. For one experiment, T cells underwent a second round of stimulation in vitro prior to adoptive transfer, and in another experiment, T cells were positively selected for CD90.1 expression using anti-PE microbeads (Miltenyi) prior to transfer (Fig. 6B, left scatterplot, triangles). The day after transfer, mice were injected with specific peptides (100 μg FL-pep for SM1 recipients or 150 μg Ea-pep for TEa recipients), followed by LPS 18 h later.
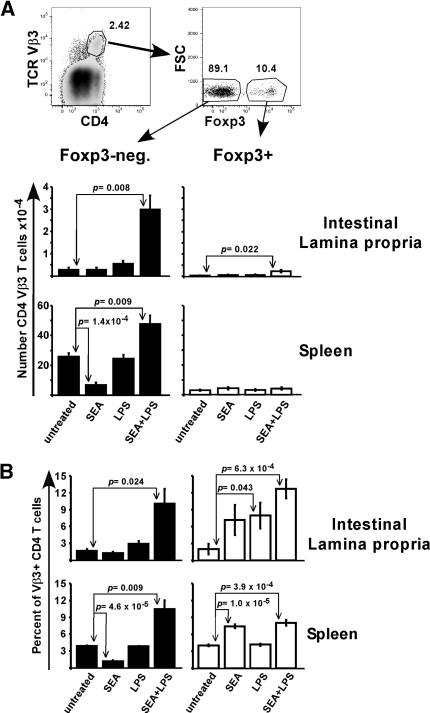
Figure 1.
LPS promotes the accumulation of superantigen-stimulated T cells into intestinal lamina propria. Mice were left untreated or injected with SEA at time 0 and/or LPS at 18 h. On day 7, lymphocytes from the small intestinal lamina propria and spleen were stained for T cell markers and analyzed by flow cytometry. (A) Representative dot plots show the gating strategy. Lymphocytes expressing both CD4 and TCR Vβ3 were divided into Foxp3-negative (closed symbols) and Foxp3+ (open symbols) groups, with the total number for each treatment shown in bar graphs. (B) Percent of Foxp3-negative and Foxp3+ CD4 T cells expressing Vβ3 for each tissue. Data are combined from 5 experiments with n = 5-6 and displayed as mean ± sem. Statistical significance between untreated and other groups was evaluated by two-tailed Student’s t tests, with all P values < 0.05 listed.
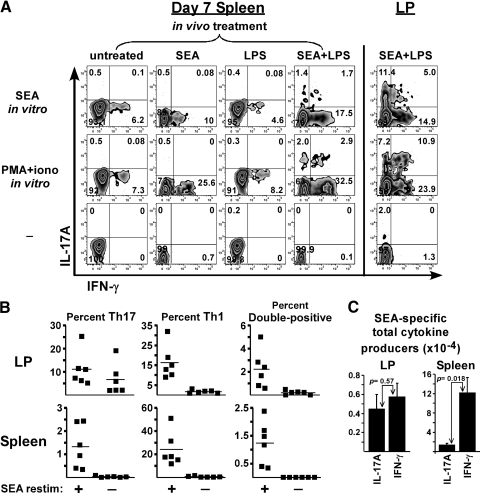
Figure 2.
LPS supports Th17 responses in vivo, and the Th17-Th1 balance is tissue specific. Day 7 cells from the same experiments in Fig. 1 were restimulated in vitro with SEA, PMA plus ionomycin, or neither (−) in the presence of BFA, with production of IL-17A and IFN-γ measured by flow cytometry. (A) Representative dot plots gated on CD4+ Vβ3+ Foxp3-negative cells following immunization. Quadrants were determined from isotype control staining (not shown). (B) Scatterplots show the percent of gated cells staining single-positive for IL-17A (Th17), single-positive for IFN-γ (Th1), or double-positive for individual mice immunized with SEA plus LPS following 5 h culture in the presence or absence of SEA, as indicated. Horizontal bars represent the mean for each group. (C) Bar graphs show total numbers of cytokine-producers among gated cells following a 5-h culture with SEA. To obtain this value, the number of double-positive cells was added to each single-positive population. Data are displayed as mean ± sem with P values listed.
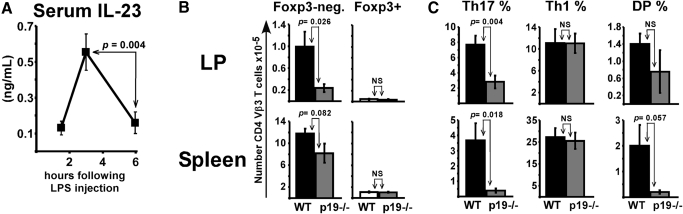
Figure 3.
LPS enhances lamina propria T-cell accumulation and Ag-specific IL-17 production through IL-23p19. (A) WT mice injected with 100 μg LPS were bled at various times. Serum levels of IL-23 were determined by ELISA with data combined from 3 experiments (n=9). (B) WT and p19−/− mice were immunized with SEA at time 0 and LPS at 18 h, with tissues analyzed on day 7 or 8. Number of Foxp3-negative (left) and Foxp3+ (right) T cells expressing CD4 and TCR Vβ3 in tissues. (C) Lymphocytes restimulated with SEA for 5 h were stained for intracellular IL-17A and IFN-γ. Shown are the percentage of gated Foxp3-negative CD4+ Vβ3+ T cells staining single-positive for IL-17 (Th17), single-positive for IFN-γ (Th1), or double-positive (DP). Data are combined from 3 experiments with n = 9 and displayed as mean ± sem with P values given. NS, not statistically significant.
Figure 4.
IL-23p19 production by bone marrow-derived cells is required for LPS to generate Th17 responses. WT (hatched bars) and p19−/− (light gray bars) bone marrow chimeras were immunized with SEA at time 0 and LPS at 18 h. (A) Number of donor bone marrow-derived CD4 Vβ3 T cells in lamina propria and spleen on day 7. (B) Lamina propria lymphocytes cultured for 5 h in the presence or absence (no recall) of SEA were stained for intracellular IL-17A and IFN-γ. Representative dot plots are gated on donor bone marrow-derived CD4 Vβ3 T cells. (C) Percent of gated cells staining single-positive for IL-17A (Th17), single-positive for IFN-γ (Th1), and double-positive following in vitro restimulation with SEA. Data are combined from 3 experiments with n = 6-7 and displayed as mean ± sem, with P values given for statistically significant differences. NS, not significant.
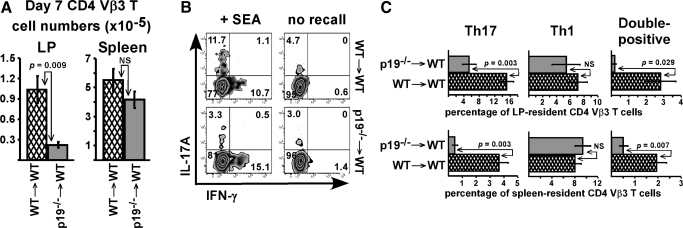
Figure 5.
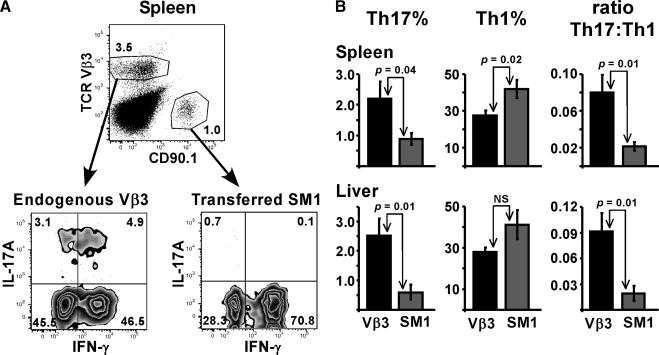
LPS preferentially drives Th1 differentiation in vivo. (A) Naïve SM1 T cells were transferred into naïve WT mice on day −1, followed by immunization with FL-pep on day 0 and LPS 18 h later. On day 10, mice were injected with FL-pep and SEA, followed by LPS on day 11. On day 17, lymphocytes from spleen and liver were restimulated with PMA plus ionomycin for 4 h in vitro. (A) Representative dot plots from spleen gated on CD4 T cells show the potential of endogenous (TCR Vβ3) and transferred TCR transgenic SM1 (CD90.1) T cells to produce IL-17A and IFN-γ. (B) Bar graphs show the percent of gated Vβ3 (black bars) and SM1 (dark gray bars) T cells staining single-positive for IL-17A (Th17) or IFN-γ (Th1), or the ratio of Th17 to Th1 cells, as indicated. Data are displayed as means ± sem with P values < 0.05 listed. Spleen data are combined from 3 experiments with n = 12 and liver data are from 2 experiments with n = 9. NS, not significant.
Figure 6.
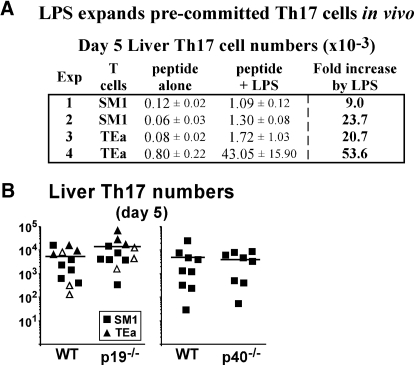
LPS expands pre-committed Th17 cells independently of IL-23. TCR transgenic SM1 or TEa cells were cultured under Th17 conditions, adoptively transferred into mice, and immunized with specific peptide and LPS. On day 5, liver lymphocytes were restimulated in vitro with PMA plus ionomycin for 4 h. (A) Table shows total number of specific T cells staining single-positive for IL-17, displayed as mean ± sem (×10−3). For experiments 1, 2, and 4, n = 3, and n = 2 for experiment 3. (B) Scatterplots show total number of liver-resident Th17 cells in IL-23p19−/− (left) or IL-12/23p40−/− (right) mice compared with WT. Data for each plot are combined from 3-4 independent experiments with horizontal lines representing the mean for individual groups. In one experiment, cultured TEa cells were purified to 92% by CD90.1 expression prior to transfer of 1.3 × 105 cells per mouse (open triangles). Statistical analysis in A between peptide vs. peptide/LPS showed that experiment 1 was P = 0.0015 with an n = 3, experiment 2 was P = 0.000113 with an n =3, experiment 3 had an n = 2, so statistical tests are not valid, but when data from experiments 1, 2, and 3 were combined n = 8 and P = 0.000761. The fourth experiment (n=3) was not statistically significant even though there was an impressive 53-fold difference between the means, and we believe this was due to an outlying data point. For B, there were no statistical differences with P = 0.16 in the left panel and P = 0.75 in the right panel.
Cell staining and flow cytometry
The following mAbs were purchased from eBioscience (San Diego, CA, USA): Allophycocyanin-conjugated CD45.2, IL-17A, rat IgG2a; PE-conjugated CD45.1, CD45.2, Foxp3; FITC-conjugated TCR Vα2; Alexa-700-conjugated IL-10 and rat IgG2a. The following mAbs were purchased from BD Biosciences: biotinylated TCR Vβ3; Pacific Blue-conjugated CD4; PerCP-conjugated CD4, CD90.1, streptavidin; PE-conjugated TCR Vβ3, Vβ6; FITC-conjugated CD4, CD8b.2, CD45.1, TCR Vβ2, Vβ3, IFN-γ, and rat IgG1.
Surface and intracellular staining was performed as described previously [31]. Briefly, cells were resuspended in staining buffer consisting of BSS, 3% FBS, and 0.1% sodium azide. Nonspecific binding was blocked by a solution containing mouse serum, human IgG, and the anti-Fc mAb 2.4G2 [41], followed by incubation with fluorescently conjugated mAbs on ice for 30 min. For intracellular cytokine staining without Foxp3 analysis, surface staining was performed, followed by fixation with 2% paraformaldehyde, permeabilization with 0.25% saponin, and incubation at room temperature with anti-cytokine mAbs. For intracellular staining with Foxp3 analysis, the Foxp3 staining buffer set from eBioscience was used. Flow cytometry was conducted on BD FACSCalibur and LSR II flow cytometers with data analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Two-tailed Student’s t tests were performed with P < 0.05 representing a significant statistical difference. The type of variance was determined by F tests, with F > 0.05, corresponding to equal variance and F < 0.05 corresponding to unequal variance.
RESULTS
LPS supports superantigen-stimulated T-cell accumulation into the small intestinal lamina propria
The commensal pathogen S. aureus secretes immunomodulatory enterotoxins that target T cells expressing specific Vβ chains, leading to food poisoning and shock (reviewed by Marrack and Kappler [42]). Injecting SEA into mice results in the activation and expansion of endogenous CD4 and CD8 T cells expressing TCR Vβ3, followed by their deletion in lymph nodes and spleen [43]. Injecting LPS within 24 h after SEA rescues Vβ3 T cells from deletion by promoting their long-term survival [2]. To determine whether systemic LPS administration impacts intestinal T-cell activation, C57BL/6 (WT) mice were left untreated or injected i.p. with SEA at time 0 and/or LPS at 18 h. On day 7, CD4 Vβ3 T cell levels were assessed in small intestinal lamina propria (LP) and spleen. Since endogenous T-cell populations potentially consist of effectors and suppressive cells, the contribution of each was determined by staining for the regulatory T cell (Treg) marker Foxp3.
Untreated mice contained low numbers of isolatable LP-resident CD4 Vβ3 T cells, ∼1 percent the size of isolated spleen cells. Treatment with SEA alone did not significantly change numbers in the LP even though there was a 3.8-fold reduction in the spleen, as expected (Fig. 1A). Clonal deletion was only observed within the Foxp3-negative fraction and correlated with an increased percentage of Foxp3+ T cells expressing Vβ3 (Fig. 1B). In LP, LPS alone doubled CD4 Vβ3 T cell numbers, while the combination of SEA plus LPS dramatically increased the Foxp3-negative cells by 11.2-fold over untreated (Fig. 1A). In comparison, the same treatment led to a 1.8-fold increase in the spleen. Specific Foxp3+ T cells were also increased in the LP; however, the total numbers of Foxp3-negative cells were much higher (Fig. 1A).
LPS drives Th17 responses in vivo, and the Th17-Th1 balance is tissue specific
To examine T-cell effector potential, lymphocytes were restimulated in vitro for 5 h with SEA or PMA plus ionomycin and stained for intracellular cytokines. Regulatory T cells were excluded from analysis by gating on Foxp3-negative CD4 Vβ3 T cells. In untreated mice, ∼6 percent of cells within this gated population produced IFN-γ in response to SEA in vitro, and in vivo immunization 7 days previously with SEA increased this number to ≥10 percent (Fig. 2A). LPS alone had no effect on T-cell-derived cytokines, while immunization with SEA plus LPS resulted in ∼20 percent of gated cells having IFN-γ potential. Stimulation with PMA plus ionomycin further increased this number to 30 percent. In contrast to IFN-γ, only 2-5% of splenic T cells produced IL-17 (Fig. 2A). Two populations of similar magnitude were observed: IL-17+ IFN-γ-negative (Th17) and IL-17+ IFN-γ+ (double-positive). In LP, the percentages of IFN-γ-single-positive cells (Th1) were similar as the spleen; however, the LP contained a much larger Th17 population averaging 11% of gated cells and a moderately increased double-positive (DP) population. Even though some of the LP-resident Vβ3 T cells produced IL-17 in unstimulated cultures (Fig. 2B), restimulation with SEA increased the mean fluorescence intensity of IL-17 staining by about twofold (data not shown). This Ag-specific IL-17 production required LPS since immunization with SEA alone resulted in ∼2% of LP-resident CD4 Vβ3 T cells having IL-17 potential (data not shown). Analyzing the total numbers of SEA-specific effectors revealed no significant difference between IL-17 and IFN-γ in the LP, while in the spleen IFN-γ producers were present in log-fold greater numbers (Fig. 2C and Table 1). Thus, the splenic Th1-biased response was associated with an unbiased response in LP, where similar numbers of Th17 and Th1 cells were found.
TABLE 1.
Total Number of CD4 Vβ3 Cytokine Producers on Day 7 (×10−3)
| Lamina propria
|
Spleen
|
|||||
|---|---|---|---|---|---|---|
| Restimulated with | Th17 | Th1 | Double-positive | Th17 | Th1 | Double-positive |
| SEA | 3.59 ± 1.26 | 4.84 ± 1.14 | 0.85 ± 0.41 | 7.00 ± 2.56 | 115 ± 31.4 | 6.23 ± 1.78 |
| PMA + ionomycin | 3.55 ± 1.78 | 4.54 ± 2.09 | 1.56 ± 0.96 | 9.87 ± 4.09 | 141 ± 24.2 | 9.21 ± 2.57 |
| none | 1.80 ± 0.9 | 0.49 ± 0.11 | 0.05 ± 0.02 | 0.12 ± 0.04 | 2.65 ± 1.07 | 0 |
Mice were immunized with SEA at time 0 and LPS 18 h later. On day 7, lymphocytes from small intestine lamina propria and spleen were cultured for 5 h with SEA, PMA + ionomycin, or neither, as indicated. The total number of CD4+ Vβ3+ Foxp3- negative T cells producing IL-17A (Th17), IFN-γ (Th1), or both (double-positive) are displayed as Mean ± sem. Data are from same experiments used for Figure 1 with n = 6. SEA, staphylococeal enterotoxin A; PMA, phorbol 12-myristate 13-acetate.
LPS drives Th17 priming through production of IL-23p19 by bone marrow-derived cells
Since IL-23 supports Th17 responses [21] and LPS injection increases serum levels of IL-23 (Fig. 3A), we hypothesized a critical role for this cytokine in the LPS-driven Th17 response. WT and IL-23p19−/− mice were immunized with SEA at time 0 and LPS 18 h later, with T cells analyzed on day 7 or 8. The total number of Foxp3-negative CD4 Vβ3 T cells was 75 percent lower in the LP of p19−/− mice but only 30 percent lower in the spleen, demonstrating a preferential effect on the LP (Fig. 3B). The reduced numbers were not caused by conversion to Tregs since Foxp3+ Vβ3 T cell numbers were similar between WT and p19−/− mice. Following in vitro restimulation with SEA, the percentage of specific T cells producing IL-17 was significantly decreased by 63 percent in the LP and 90% in the spleen of p19−/− mice, although IFN-γ production on a per cell basis was normal (Fig. 3C). When coupled to its effect on overall T cell accumulation (Fig. 3B), IL-23p19-deficiency decreased total numbers of SEA-specific Th17 cells by 15- to 18-fold in spleen and LP, respectively, while Th1 numbers were only decreased by 1.3- to 3.7-fold. Therefore, IL-23p19 contributed preferentially but not exclusively to Th17 cell accumulation.
To determine whether bone marrow-derived cells are a significant source of IL-23p19, congenic CD45.1 WT mice were irradiated and reconstituted with bone marrow from either p19−/− (p19 → WT) or WT (WT → WT) mice, as described in Materials and Methods. Approximately 8 wk following bone marrow reconstitution, the chimeras were immunized with SEA and LPS, with tissues analyzed on day 7. Consistent with results from p19−/− mice (Fig. 3), the total number of donor-derived CD4 Vβ3 T cells was reduced by 4.7-fold in the LP of p19 chimeras, while there was no significant effect in spleen (Fig. 4A). Significantly less production of IL-17 in response to SEA was also observed in p19 chimeras, even though basal IL-17 levels from unstimulated cultures appeared to be normal (Fig. 4, B and C). Importantly, IL-23p19-deficiency did not impact Th1 differentiation (Fig. 4C). Therefore, bone marrow-derived cells were a significant source of the IL-23p19 involved in Th17 cell priming in intestinal LP and spleen, although our experiments do not fully rule out a contribution from nonhematopoeitic cells.
Effects of LPS on endogenous vs. adoptively-transferred transgenic T cells
Our data suggest that in conjunction with T-cell stimulation, LPS either expands out endogenous Th17 cells in vivo, drives Th17 differentiation, or both. To assess T-cell differentiation, naïve TCR transgenic SM1 CD4 T cells were adoptively transferred into WT mice, followed by immunization with the Ag flagellin peptide (FL-pep) and LPS. SM1 T cells express the congenic marker CD90.1 to allow their identification in CD90.2+ hosts. Since the transferred cells were naïve, they would have to differentiate in vivo in order to produce IL-17 or IFN-γ.
Several initial experiments revealed that SM1 T cells did not produce IL-17 after primary responses, but rather differentiated into Th1 (data not shown), prompting us to ask whether the transferred naïve TCR transgenic CD4 SM1 T cells could be pushed into Th17 differentiation through bystander effects. Mice were initially immunized with FL-pep and LPS to increase the number of potential SM1 effector T cells; therefore, any Th17 differentiated SM1 T cells would have an excellent opportunity to acquire bystander stimulation through SEA and LPS. Ten days after the primary FL-pep injection, mice were injected with both FL-pep and SEA, LPS 18 h later, and tissues examined on day 17. The percentage of SM1 T cells in LP was 10 times less than CD4 Vβ3 T cells (data not shown), and thus intracellular cytokine analysis was not feasible. Consistent with this, very few T cells are present in the LP of SM1 × RAG−/− transgenic mice, suggesting they do not efficiently traffic to the intestine and are perhaps unable to receive environmental cues for Th17 differentiation. In spleen and liver, endogenous CD4 Vβ3 T cells were also present in higher numbers than SM1 cells; however, at these sites, the two populations differed by less than an order of magnitude (Fig. 5A top plot, and data not shown). Further, many of the SM1 T cells were capable of IFN-γ production, demonstrating they had been primed in vivo. In comparison, the percentage of endogenous T cells producing IFN-γ was lower than SM1 (Fig. 5). When analyzing IL-17 production in the spleen, only 0.9% of the SM1 T cells were Th17 on average, while 2.2% of CD4 Vβ3 T cells were identified as Th17 (Fig. 5B). A greater difference was observed in liver, where the Th17:Th1 ratio was 4.8-times higher among the endogenous CD4 Vβ3 population (Fig. 5B). While LPS preferentially supported Th1 differentiation of SM1 T cells, it is also likely that adoptive transfer models may not physiologically reflect Th17 steady-state conditions unless the transferred naïve T cells migrate to or through the gut.
LPS expands out precommitted Th17 cells independently of IL-23
Since LPS was not effective at driving naïve Th to Th17 differentiation in vivo (Fig. 5), we hypothesized that it supports Th17 immunity by expanding out predifferentiated T cells. To test this, SM1 T cells were cultured under Th17 conditions and transferred into WT mice, followed by immunization with FL-pep alone or FL-pep plus LPS. On day 5, liver lymphocytes were restimulated in vitro and analyzed for cytokine production. Although LPS enhanced total Th17 cell numbers, it did not significantly alter Th17 percentages (data not shown), but consistent with its role as an expansion and survival factor, it did increase total numbers in liver by ninefold in experiment 1 and 24-fold in experiment 2 (Fig. 6A). To determine whether this was a general effect, experiments were repeated with TCR transgenic TEa CD4 T cells that are specific for Eα peptide (Eα-pep). Similar to results for SM1, LPS expanded out Th17-differentiated TEa cells by 21- and 54-fold in experiments 3 and 4 (Fig. 6A). In the spleen, LPS had a less dramatic effect and increased Th17 numbers by 2.6- to 7.8-fold (data not shown). Similar to the tissue distribution observed during primary responses, LPS also enhanced Th17 cell accumulation in the lung by a similar magnitude as observed in liver (data not shown).
The role of IL-23 in Th17 responses in vivo is not fully characterized. To determine whether IL-23 is required for LPS-driven Th17 cell expansion, Th17 cells were transferred into mice lacking either p19 or p40, and compared with WT. To negate a role for adoptively transferred APCs, T cells were purified prior to their transfer in one experiment (Fig. 6B, open triangles). On day 5, similar numbers of Th17 cells were recovered among WT and IL-23-deficient mice, demonstrating this cytokine was not required for Th17 expansion (Fig. 6B). Thus, we favor the hypothesis that multiple cytokines and costimulatory molecules generated from LPS stimulation contribute to the expansion of Th17 lineage cells, which use IL-23 for their priming in specialized niches in vivo.
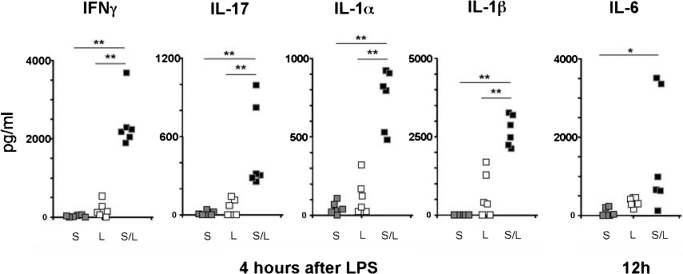
To search for candidate cytokines that may contribute to Th17 cell expansion, mice were immunized with SEA and/or LPS at time 0 and 18 h, with serum collected at 4 and 12 h post-LPS treatment. SEA alone did not significantly increase serum levels of IL-17 (Fig. 7), correlating well with our intracellular cytokine data (Fig. 2). Although LPS alone resulted in some IL-17, the absolute levels were not impressive (≤200pg/ml). However, the combination of SEA plus LPS synergistically increased serum IL-17, demonstrating this treatment generated the most favorable conditions for Th17 cell expansion (Fig. 7). In these dual-treated mice, serum levels of IL-1α and IL-1β were elevated 4 h post-LPS, and IL-6 was significantly increased at 12 h. Thus, IL-1 and IL-6 are likely candidates to contribute to Th17 cell expansion, although the extent to which our data can be extrapolated to non-LPS-based adjuvants is unknown. Interleukin-21, which is another factor that may contribute to Th17 cell outgrowth, was not detected in serum or by intracellular cytokine analysis (data not shown). Importantly, the combination of SEA plus LPS also significantly increased serum levels of the Th1 cytokine IFN-γ (Fig. 7). Overall, the diverse array of cytokines induced through LPS appears to simultaneously support both Th1 and Th17 responses in vivo.
Figure 7.
Staphylococcal aureus enterotoxin A accentuates cytokine production in response to LPS that may assist skewing and maintenance of Th1 and Th17 cells. C57BL/6 mice were injected with SEA (S), LPS (L), or SEA and 18 h later LPS (S/L). Serum was collected at either 4 or 12 h post-LPS and cytokines were measured by sample testing through Quansys Biosciences (Logan, UT, USA). The cytokine signal at 12 h in the S/L group for IFNγ, IL-17, IL-1α, and IL-1β was substantially less than the 4-h results and therefore was not shown, and the opposite was the case for IL-6. The serum cytokine signal from naïve mice was negligible and therefore not shown. Each data point represents the average of duplicate readings from a single mouse serum sample. Total sample number for each treatment group is n = 6, derived from 2 separate experiments. For the purpose of quantitation, data points that were below the lower limit of detection were considered 0 pg/ml. Statistical difference between treatment groups was analyzed using Student’s t test; *, P < 0.05; **, P < 0.01.
DISCUSSION
Interleukin-17-producing T cells contribute to host defense, although uncontrolled responses are also associated with autoimmunity (reviewed by Iwakura [19]). Previous studies showed that LPS is a physiologically relevant Th17 adjuvant by demonstrating TLR4-dependent IL-17 production during infection with K. pneumoniae and Bordetella pertussis [44, 45]. Here, we identify the mechanism of this adjuvant activity and found that, in contrast its effect on Th1 responses, LPS did not promote significant levels of Th17 differentiation but rather expanded out preexisting Th17 cells (Figs. 5, 6A). This extends the in vitro findings of LPS-induced Th17 differentiation via IL-6 in the presence of Tregs [28]. Therefore, naïve T cells primed with LPS in vivo do not necessarily receive the proper signals for Th17 differentiation.
TGF-β is one such limiting factor [27, 46]. In its absence, Th17 cells gradually lose the ability to make IL-17 and convert to a Th1 phenotype [47, 48]. These findings revealed the plasticity of Th17 cells, their close relationship with Th1, and suggest that local production of TGF-β could be an important factor determining their anatomical distribution. Indeed, the largest steady-state population of Th17 cells is found in the intestine where the action of TGF-β on T cells is required to prevent spontaneous inflammatory bowel disease [20, 49,50,51]. Here, we demonstrated that i.p. immunization with Ag and LPS increased the number of endogenous Th17 cells in the intestinal LP, and the balance between Th17 and Th1 subsets was tissue dependent (Fig. 2). While the spleen promoted a Th1-biased response, the number of IL-17-producers nearly equaled that of IFN-γ-producers in the LP, suggesting that tissue-specific signals determine effector cytokine production. Alternatively, Th17-committed cells may preferentially traffic to the intestine following their activation; however, we did not observe this to be the case (data not shown). Further, data from other groups strongly suggest that Th17 differentiation in vivo occurs within the intestine [52,53,54].
Our findings illustrate how the balance among effector and regulatory T-cell subsets is controlled in vivo. In the spleen, immunization with SEA alone caused preferential deletion of Foxp3-negative cells (Fig. 1A), similar to effects of other superantigens [55, 56]. LPS not only rescued the Foxp3-negative cells from deletion but increased their proportion relative to Foxp3+ cells, revealing a way LPS can break tolerance to an Ag. In LP, SEA did not cause Vβ3 T cell deletion (Fig. 1A), suggesting the tissue microenvironment influences survival of Ag-specific T cells. Immunization with SEA plus LPS increased total numbers of both Foxp3-negative and Foxp3+ Vβ3 T cells in LP, so their relative proportions were similar to those observed in spleen (Fig. 1A). Interleukin-23p19 was specifically required for accumulation of Foxp3-negative T cells in LP, but not the spleen (Fig. 3B). This suggests that IL-23 could be an intestinal niche-specific survival factor for effector CD4 T cells. Accordingly, dendritic cells in LP but not other sites were found to constitutively express IL-23 following their uptake of commensal bacteria [57].
Both Th1 and Th17 subsets appear during inflammatory responses [13, 21, 23, 24], with IL-23p19 specifically required for IL-17 production (Fig. 3), consistent with its known role on T cells [58]. In addition, we identified bone marrow-derived cells as an important source of IL-23p19 in vivo (Fig. 4). As IL-23 can enhance T cell proliferation [59], we thought it might be required for Th17 clonal expansion. However, precommitted Th17 cells expanded in IL-23-deficient mice (Fig. 6B). This may be peculiar to the adjuvant LPS, which induces many cytokines that support Th function (Fig. 7). It remains to be clarified whether other adjuvants or TLR ligands require IL-23 for Th17 expansion. The ability of LPS to expand out precommitted Th17 cells independently of IL-23 suggests this cytokine functions prior to clonal expansion (Fig. 6B), or possibly during terminal differentiation [29]. A colitis study found that IL-23 was required for maintaining Th17 cells in RAG-deficient mice [48], consistent with its ability to induce proliferation of memory T cells [59]. Our LPS study shows that factors other than IL-23 can cause the expansion of Th17 cells (Fig. 6). One caveat is that T cells could have been a potential source of IL-23. Nevertheless, IL-23 may control multiple facets of the Th17 response, depending on experimental conditions, analogous to the effects of IL-4 on Th2 differentiation, proliferation, and survival [60,61,62,63,64]. What factors, then, contribute to Th17 cell expansion? Recent work on IL-1 has demonstrated its role in Th17 responses [65,66,67,68]. We found the combination of SEA and LPS treatment to synergistically increase IL-1 levels in serum, correlating with increased IL-17 levels (Fig. 7). Interleukin-6 was also increased, and future studies are necessary to define the roles of individual cytokines in Th17 cell commitment, expansion, and maintenance. Importantly, the highest levels of proinflammatory cytokines were observed following dual stimulation with SEA and LPS (Fig. 7), demonstrating that T cells enhance innate sensitivity to LPS even though they do not appear to be directly stimulated by LPS in vivo [(31), and data not shown].
Freund’s complete adjuvant is commonly used to generate Th17 responses in mice [14, 29, 69], possibly because of the presence of TLR2 ligands, which induce higher levels of IL-23p19 than LPS [35]. Unfortunately, the toxicity of Freund’s complete adjuvant makes it irrelevant for human vaccination. Although whole LPS is also toxic, detoxified derivatives can retain adjuvant activity, making them adjuvant candidates [70,71,72]. In particular, monophosphoryl lipid A stimulates CD4 T-cell responses by preferentially activating the TRIF-dependent pathway [73, 74]. Recently, the LPS-TRIF axis was shown to drive Th1 differentiation and accumulation into liver and lung [32], but its effect on Th17 immunity in small intestinal LP was not examined. This is important considering that Th17 polarization in vitro associated with preferential stimulation of the MyD88, not TRIF, pathway [75]. Perhaps this is due to induction of IL-27 through TRIF, which actively blocks Th17 differentiation [76,77,78,79]. Thus, generating optimal Th17 responses may require neutralizing the negative effect of IL-27. Nevertheless, a vaccine containing monophosphoryl lipid A increased specific Th17 cell numbers and enhanced protection from Mycobacterium tuberculosis challenge [80], demonstrating the utility of LPS-based vaccination for mucosal immunity.
Even though peripheral immunization was able to prime intestinal Th17 cells (Fig. 2), other routes may also be efficacious. Oral vaccination with gut flora-derived DNA induced Th17 cells through TLR9 [53], and intranasal immunization with multiple adjuvants was shown to be an effective route for pulmonary Th17 responses [81]. Interestingly, peripheral LPS immunization was shown to generate α4β7+ and CXCR5+ OT-II cells in mesenteric LN, suggesting that LPS supports differentiation into Th17 and T follicular helper lineages, respectively, by stimulating intestinal DCs [82]. In contrast, Poly I:C inhibited Th17 responses in an EAE model through the induction of type I IFN [79]. Thus, PAMPs can either promote or block Th17 responses depending on the route of delivery and local cytokine balance.
It is important to delineate the cell types responding to adjuvants in vivo. Using an adoptive transfer model, we previously reported that MyD88-deficient CD4 T cells underwent normal clonal expansion, survival, and Th1 differentiation, suggesting LPS stimulates T cells indirectly through APCs [31]. Supporting this, other groups have found that while murine T cells respond to some TLR ligands, they only respond very weakly to LPS or not at all [83, 84]. Although strong evidence suggests that murine Th1 cells are not directly stimulated by LPS, this issue is not resolved for Th17. TLR4 expression has been detected on the CD45RBhigh subset of CD4 T cells in mice, which cause colitis upon transfer into lymphopenic RAG-deficient hosts [85, 86]. In this model MyD88-deficient T cells were found to have a reduced capacity for proliferation and IL-17 production [85, 86], although TLR4-deficient T cells were not examined. In our model, we found no evidence for TLR4 expression by murine intestinal CD4 T cells (data not shown). The issue of TLR4 expression by T cells may be relevant for human studies, however, as HIV-infection was associated with increased TLR4 expression on Th17 cells [87]. Therefore, studies examining the direct action of PAMPs on T cells may ultimately benefit human vaccine studies. It is also important to consider the anatomical distribution of adjuvants following immunization. For example, high-molecular-weight complexes such as LPS appear to get trapped in the subcapsular sinus of lymph nodes [88], which is anatomically distinct from T-cell regions. Thus, direct T-cell responsiveness to pathogen-associated patterns is intrinsically and anatomically regulated.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01-AI42858 and R01-AI52108 to A. T. V, AI070603 and AI077283 to Z. L., and J. P. M was supported by T32-AI07080.
Disclosures
The authors declare no conflicting financial interests.
Footnotes
Abbreviations: APC=antigen-presenting cells, BFA=brefeldin A, BSS= balanced salt solution, CTM=complete tumor medium, LP=small intestine lamina propria; LPS=lipopolysaccharide, PMA=phorbol 12-myristate 13-acetate, SEA=saphylococcal enterotoxin A, TGF=transforming growth factor
References
- McAleer J P, Vella A T. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A T, McCormack J E, Linsley P S, Kappler J W, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Reinhardt R L, Khoruts A, Merica R, Zell T, Jenkins M K. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Pape K A, Khoruts A, Mondino A, Jenkins M K. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J L. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Harrington L E, Hatton R D, Mangan P R, Turner H, Murphy T L, Murphy K M, Weaver C T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Langrish C L, Chen Y, Blumenschein W M, Mattson J, Basham B, Sedgwick J D, McClanahan T, Kastelein R A, Cua D J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang X O, Chang S H, Nurieva R, Wang Y H, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez F H, Kanaly S, Stocking K L, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito J E, Bagby G J, Nelson S, Charrier K, Peschon J J, Kolls J K. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel P L, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos R L, Verhoeven D E, George M D, Wilson R P, Winter S E, Godinez I, Sankaran S, Paixao T A, Gordon M A, Kolls J K, Dandekar S, Bäumler A J. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Ivanov I I, McKenzie B S, Zhou L, Tadokoro C E, Lepelley A, Lafaille J J, Cua D J, Littman D R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Murphy C A, Langrish C L, Chen Y, Blumenschein W, McClanahan T, Kastelein R A, Sedgwick J D, Cua D J. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney A L, De Sauvage F J. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- Happel K I, Dubin P J, Zheng M, Ghilardi N, Lockhart C, Quinton L J, Odden A R, Shellito J E, Bagby G J, Nelson S, Kolls J K. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S A, Pearl J E, Sakamoto K, Gilmartin L, Bell G K, Jelley-Gibbs D M, Ghilardi N, deSauvage F, Cooper A M. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Kleinschek M A, Muller U, Brodie S J, Stenzel W, Kohler G, Blumenschein W M, Straubinger R K, McClanahan T, Kastelein R A, Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom T B, Oukka M, Weiner H L, Kuchroo V K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Mangan P R, Harrington L E, O'Quinn D B, Helms W S, Bullard D C, Elson C O, Hatton R D, Wahl S M, Schoeb T R, Weaver C T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking R J, Atkins C J, Locksley R M, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- McGeachy M J, Chen Y, Tato C M, Laurence A, Joyce-Shaikh B, Blumenschein W M, McClanahan T K, O'Shea J J, Cua D J. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor W, Jr, Kamanaka M, Booth C J, Town T, Nakae S, Iwakura Y, Kolls J K, Flavell R A. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer J P, Zammit D J, Lefrancois L, Rossi R J, Vella A T. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. J Immunol. 2007;179:6524–6535. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- McAleer J P, Rossi R J, Vella A T. Lipopolysaccharide potentiates effector T cell accumulation into nonlymphoid tissues through TRIF. J Immunol. 2009;182:5322–5330. doi: 10.4049/jimmunol.0803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Assoian R K, Fleurdelys B E, Stevenson H C, Miller P J, Madtes D K, Raines E W, Ross R, Sporn M B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger J L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- McSorley S J, Asch S, Costalonga M, Reinhardt R L, Jenkins M K. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Grubin C E, Kovats S, deRoos P, Rudensky A Y. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Cua D J, Sherlock J, Chen Y, Murphy C A, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo A L, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath M F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- Unkeless J C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- McCormack J E, Callahan J E, Kappler J, Marrack P C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- Happel K I, Zheng M, Young E, Quinton L J, Lockhart E, Ramsay A J, Shellito J E, Schurr J R, Bagby G J, Nelson S. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S C, Jarnicki A G, Lavelle E C, Mills K H. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking R J, Flavell R A, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Lexberg M H, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang H D. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Lee Y K, Turner H, Maynard C L, Oliver J R, Chen D, Elson C O, Weaver C T. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell R A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo J P, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess J H, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Hall J A, Bouladoux N, Sun C M, Wohlfert E A, Blank R B, Zhu Q, Grigg M E, Berzofsky J A, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I I, Frutos Rde L, Manel N, Yoshinaga K, Rifkin D B, Sartor R B, Finlay B B, Littman D R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiernik M, do Carmo, Leite-de-Moraes M, Pontoux C, Joret A M, Rocha B, Penit C, Dy M. T cell deletion induced by chronic infection with mouse mammary tumor virus spares a CD25-positive, IL-10-producing T cell population with infectious capacity. J Immunol. 1997;158:4642–4653. [PubMed] [Google Scholar]
- Eroukhmanoff L, Oderup C, Ivars F. T-cell tolerance induced by repeated antigen stimulation: selective loss of Foxp3- conventional CD4 T cells and induction of CD4 T-cell anergy. Eur J Immunol. 2009;39:1078–1087. doi: 10.1002/eji.200838653. [DOI] [PubMed] [Google Scholar]
- Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle P R, Autenrieth I, Neurath M F. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie M H, de Sauvage F J, Gurney A L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans J C, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams J S, Moore K W, Rennick D, de Waal-Malefyt R, Hannum C, Bazan J F, Kastelein R A. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Pfeifer J D, McKenzie D T, Swain S L, Dutton R W. B cell stimulatory factor 1 (interleukin 4) is sufficient for the proliferation and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Exp Med. 1987;166:1464–1470. doi: 10.1084/jem.166.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C S, Heimberger A B, Gold J S, O'Garra A, Murphy K M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I C, Hodge M R, Rooney J W, Glimcher L H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Vella A, Teague T K, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A T, Dow S, Potter T A, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E V, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang S H, Martinez G J, Yang X O, Nurieva R, Kang H S, Ma L, Watowich S S, Jetten A M, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller E T, Zou W. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G X, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- Schenck J R, Hargie M P, Brown M S, Ebert D S, Yoo A L, McIntire F C. The enhancement of antibody formation by Escherichia coli lipopolysaccharide and detoxified derivatives. J Immunol. 1969;102:1411–1422. [PubMed] [Google Scholar]
- McIntire F C, Hargie M P, Schenck J R, Finley R A, Sievert H W, Rietschel E T, Rosenstreich D L. Biologic properties of nontoxic derivatives of a lipopolysaccharide from Escherichia coli K235. J Immunol. 1976;117:674–678. [PubMed] [Google Scholar]
- Takayama K, Ribi E, Cantrell J L. Isolation of a nontoxic lipid A fraction containing tumor regression activity. Cancer Res. 1981;41:2654–2657. [PubMed] [Google Scholar]
- Thompson B S, Chilton P M, Ward J R, Evans J T, Mitchell T C. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. 2005;78:1273–1280. doi: 10.1189/jlb.0305172. [DOI] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton P M, Casella C R, Mitchell T C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- Fedele G, Nasso M, Spensieri F, Palazzo R, Frasca L, Watanabe M, Ausiello C M. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis differently modulate human dendritic cell functions resulting in divergent prevalence of Th17-polarized responses. J Immunol. 2008;181:208–216. doi: 10.4049/jimmunol.181.1.208. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin N M, Danilenko D M, Lucas S, Lee J, de Sauvage F J, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Stumhofer J S, Laurence A, Wilson E H, Huang E, Tato C M, Johnson L M, Villarino A V, Huang Q, Yoshimura A, Sehy D, Saris C J, O'Shea J J, Hennighausen L, Ernst M, Hunter C A. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- Guo B, Chang E Y, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S A, Bell G K, Pearl J E, Fountain J J, Rangel-Moreno J, Cilley G E, Shen F, Eaton S M, Gaffen S L, Swain S L, Locksley R M, Haynes L, Randall T D, Cooper A M. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Zygmunt B M, Rharbaoui F, Groebe L, Guzman C A. Intranasal immunization promotes Th17 immune responses. J Immunol. 2009;183:6933–6938. doi: 10.4049/jimmunol.0901144. [DOI] [PubMed] [Google Scholar]
- Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Gelman A E, Zhang J, Choi Y, Turka L A. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua D J, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Fukata M, Breglio K, Chen A, Vamadevan A S, Goo T, Hsu D, Conduah D, Xu R, Abreu M T. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol. 2008;180:1886–1894. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Kanai T, Fujii T, Nemoto Y, Okamoto R, Tsuchiya K, Totsuka T, Sakamoto N, Akira S, Watanabe M. MyD88-dependent pathway in T cells directly modulates the expansion of colitogenic CD4+ T cells in chronic colitis. J Immunol. 2008;180:5291–5299. doi: 10.4049/jimmunol.180.8.5291. [DOI] [PubMed] [Google Scholar]
- Miller Sanders C, Cruse J M, Lewis R E. Toll-like receptor and chemokine receptor expression in HIV-infected T lymphocyte subsets. ExperMolec Pathol. 2010 doi: 10.1016/j.yexmp.2009.09.006. In press. [DOI] [PubMed] [Google Scholar]
- Gretz J E, Norbury C C, Anderson A O, Proudfoot A E, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]