Abstract
The Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP) brought together 10 cohorts to conduct a prospective study of the association between vitamin D status, measured as serum concentrations of 25-hydroxyvitamin D (25(OH)D), and the development of 7 rarer cancer sites: endometrial, esophageal, gastric, kidney, non-Hodgkin lymphoma, ovarian, and pancreatic cancers. The cohorts come from 3 continents, with participants from a wide range of latitude who are racially diverse. Across each cancer site, there was no evidence of a protective association between higher concentrations of 25-hydroxyvitamin D (>75 nmol/L) and cancer outcome. An increased risk at very high levels (≥100 nmol/L) was noted for pancreatic cancer, confirming previous reports. The articles included in this issue detail the overall design and governance of the project, correlates of vitamin D status, and results from the cancer site-specific investigations. The Vitamin D Pooling Project realizes a major goal of consortium efforts, namely, to rigorously test hypotheses for rarer cancer outcomes that may not be adequately addressed in any one prospective cohort study. The results of this study have application for the planning and conduct of intervention trials, especially in determining potential risks.
Keywords: case-control studies, neoplasms, prospective studies, vitamin D
Adequate vitamin D concentrations are necessary for bone health and prevention of rickets. The widespread prevalence of low concentrations of vitamin D has triggered great clinical, research, and public health interest in determining the amount of vitamin D required for optimal health. The interest in vitamin D as a cancer preventive agent arises from its biologic role in proliferation and apoptosis along with the presence of vitamin D in most tissues.
The main source of circulating vitamin D is conversion of 7-dehydrocholesterol in the skin to cholecalciferol (D3) upon exposure to ultraviolet B radiation (1). Dietary sources, even with fortification of food, remain a minor contributor to vitamin D status. With the recognition of widespread prevalence of deficient or insufficient vitamin D concentrations, some have advocated increasing vitamin D through supplements. In May 2009, a committee was appointed by the Food and Nutrition Board, Institute of Medicine of the National Academies, to evaluate and update the dietary reference intake for vitamin D, as well as calcium (2). The Committee is charged with evaluating evidence of both the adequacy of current dietary vitamin D intake recommendations, including optimal dose and range of intake, and the potential harms from excess intake.
In 2008, the evidence pertaining to vitamin D and its association with cancer was reviewed by the International Agency for Research on Cancer (3). As part of that review, updated meta-analyses were conducted regarding the associations between serum levels of 25-hydroxyvitamin D (25(OH)D) and colorectal, breast, and prostate cancers. The results showed a statistically significant decrease in risk of colorectal cancer per 1-ng/mL increase in serum 25(OH)D concentration among prospective studies (relative risk = 0.984, 95% confidence interval (CI): 0.976, 0.991). For prospective breast cancer studies, results were heterogeneous, and the decreased risk observed was not statistically significant (relative risk = 0.994, 95% CI: 0.964, 1.024). No evidence for an association between 25(OH)D and prostate cancer risk was observed (3). Published data for other cancer sites were too sparse to conduct meta-analyses. Since that review by the International Agency for Research on Cancer, an additional prospective study of 25(OH)D concentrations and pancreatic cancer was published (4). Similar to the prior publication, an increased risk of pancreatic cancer was observed among individuals with the highest levels, but there was no dose-response association (4).
This issue contains a series of articles from the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP), a collaborative effort involving 10 cohorts that are members of the National Cancer Institute Cohort Consortium. The VDPP was undertaken to address the gap in knowledge of the association between vitamin D and cancer, in particular the rarer cancers sites (3, 5). The VDPP, using a central laboratory and standards provided by the National Institute of Standards and Technology, examined the associations between serum or plasma 25(OH)D concentrations, the main circulating form of vitamin D, and the development of 7 types of rarer cancer: endometrial, esophageal, gastric, kidney, non-Hodgkin lymphoma, ovarian, and pancreatic cancers (6–11). The project was a nested case-control study with the vast majority of samples assayed specifically for this project. The reports include an overall design paper, describing the governance of the consortium and detailing the design and statistical approaches used in the investigation, as well as a paper detailing the factors correlated with vitamin D status. The results for each cancer site are reported in separate papers except for gastric and esophageal cancers, which were combined as upper gastrointestinal cancers.
The Cohort Consortium (http://epi.grants.cancer.gov/Consortia/cohort.html) was established in 2000 to foster large collaborative studies to investigate gene-gene and gene-environment interactions with cancer. The first 2 Consortium projects focused on genetic studies of breast and prostate cancer. The VDPP realizes a major goal and advantage of the Cohort Consortium network, namely, the study of rare cancer outcomes that no one cohort alone may be able to examine. The VDPP brought together prospective cohort studies with stored blood samples, diverse in ethnicity and geographic distribution, to address the question of whether vitamin D concentrations are associated with the development of rarer cancer sites (Table 1). The cancer sites investigated were chosen because prior ecologic, preclinical studies or observational studies suggested possible associations with vitamin D. In addition, the consortium prospective approach has advantages for cancer sites that present at advanced stage at diagnosis and have high case-fatality rates, such as esophageal, pancreatic, and ovarian cancers. The considerable variation within and across cohorts in racial groups, latitude of residence, and vitamin D intake provided the opportunity to examine associations across a wide range of clinically relevant concentrations of vitamin D, measured as circulating 25(OH)D.
Table 1.
Participating Cohorts in the Vitamin D Pooling Project of Rarer Cancers and Number of Cases per Cancer Site
| Median Follow-up Time, years (25th, 75th Percentile of VDPP Cases) | No. of Cancer Cases Contributed by Cohort |
|||||||
| Cohort and Location | Population | Endometrial | Kidney | Lymphoma | Ovarian | Pancreatic | Upper GI (Esophageal and Gastric) | |
| ATBC Study, Finland | Smokers | 8.7 (4.9, 12.7) | 0 | 286 | 208 | 0 | 313 | 416 |
| CPS-II, United States—national | General | 2.3 (1.3, 3.6) | 51 | 58 | 135 | 27 | 65 | 40 |
| CLUE, United States—Washington County, Maryland | General | 10.1 (5.3, 14.7) | 192 | 102 | 236 | 102 | 123 | 88 |
| HPFS, United States—national | Health professionals | 4.4 (2.6, 6.7) | 0 | 0 | 133 | 0 | 0 | 0 |
| MEC, United States—Hawaii and California | General | 2.1 (1.1, 3.3) | 39 | 64 | 96 | 18 | 109 | 82 |
| NYU-WHS, United States—New York | Mammography screening | 10.8 (6.0, 14.6) | 139 | 35 | 73 | 94 | 73 | 27 |
| NHS, United States—national | Registered nurses | 7.0 (4.0, 9.5) | 163 | 0 | 145 | 127 | 0 | 0 |
| PLCO (32, 33), United States—national | General | 4.5 (2.2, 6.8) | 147 | 161 | 286 | 74 | 183 | 99 |
| SMHS, China | General | 1.7 (0.9, 2.7) | 0 | 32 | 8 | 0 | 27 | 131 |
| SWHS, China | General | 4.7 (2.4, 6.6) | 99 | 37 | 33 | 74 | 59 | 182 |
| Total cancer cases | 830 | 775 | 1,353 | 516 | 952 | 1,065 | ||
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; CPS-II, Cancer Prevention Study II Nutrition Cohort; GI, gastrointestinal; HPFS, Health Professionals Follow-up Study; MEC, Multiethnic Cohort Study; NHS, Nurses’ Health Study; NYU-WHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; VDPP, Cohort Consortium Vitamin D Pooling Project of Rarer Cancers.
The overall design, description of the cohorts, and statistical methodology are outlined in the methods paper (12). A nested case-control approach was used, with samples assayed in a central laboratory. A unique feature of the VDPP was the availability of the first serum standards for assays of 25(OH)D provided by the National Institute of Standards and Technology. The hypothesis being tested was that higher concentrations of vitamin D would be associated with a lower risk of developing the cancers being investigated.
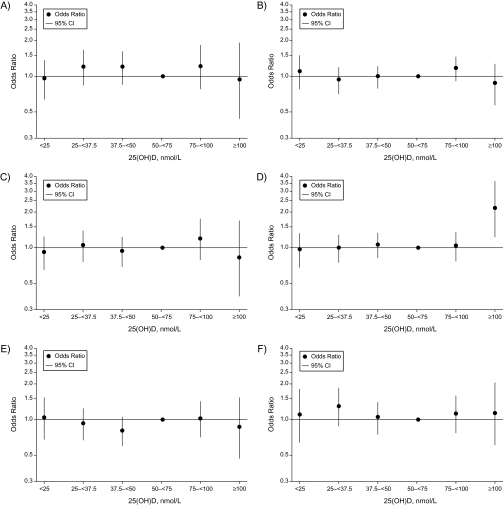
The results of the VDPP do not suggest a benefit from higher concentrations of vitamin D, nor do they suggest an increased risk from lower concentrations with respect to the cancer sites studied (Figure 1). The observations from the study of pancreatic cancer were consistent with prior reports of an excess risk associated with concentrations of 25(OH)D greater than 100 nmol/L (adjusted odds ratio = 2.12, 95% CI: 1.23, 3.64) (11). Because the previously reported studies of 25(OH)D and pancreatic cancer also participated in the VDPP, analyses were conducted excluding these cohorts. A similar point estimate of risk was observed in association with concentrations exceeding 100 nmol/L, although the estimate was no longer statistically significant (odds ratio = 2.23, 95% CI: 0.82, 6.08). Even in this large collaborative study, the numbers of cases and controls were limited at the extreme high end of the distribution, emphasizing the challenge of studying both rare cancers and the associations with the extremes of exposures. These results, though not conclusive, raise concern about recommendations for use of high-dose supplementation with vitamin D that may result in high serum concentrations of vitamin D. The observation of a decreased risk of upper gastrointestinal cancer with low concentrations of 25(OH)D among Asians was also consistent with previously published studies among Asian populations that observed a lower risk of cancer among individuals in the low range of vitamin D (7, 13, 14). Although data are sparse at the extremes of 25(OH)D concentrations and in population subgroups, the consistency with other reports in different populations makes it likely that these results are not by chance. However, these results should be confirmed in other collaborative prospective cohort projects.
Figure 1.
Odds ratios and 95% confidence intervals for cancer risk by site across categories of circulating levels of 25-hydroxyvitamin D (nmol/L), Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Odds ratios were derived from conditional logistic regression models. Reference category: 50–<75 nmol/L 25(OH)D. A, kidney cancer adjusted for education, body mass index, height, smoking status at blood draw, history of high blood pressure at blood draw, history of diabetes at blood draw, and alcohol use at blood draw. B, non-Hodgkin lymphoma adjusted for height (≤165, >165–171, >171–177.781, >177.781 cm). C, upper gastrointestinal (combined esophageal and gastric) cancers adjusted for alcohol, smoking, education, and history of gastric surgery. D, pancreatic cancer adjusted for body mass index (<18.5, 18.5–<25.0, 25.0–<30.0, 30.0–<35.0, ≥35.0 kg/m2 (WHO categories), missing), smoking (never, former quit ≥15 years ago, former quit 1–<15 years ago, former quit <1 year or currently smoking <20 cigarettes per day, and former quit <1 year or currently smoking ≥20 cigarettes per day), and diabetes (yes, no, missing). The highest category of vitamin D and association with pancreatic cancer is statistically significant (95% confidence interval: 1.23, 3.64). E, endometrial cancer adjusted for education (less than high school, completed high school, vocational school, some college, college graduate, graduate studies, missing), menopausal status (pre-, peri-, post-, missing), age at menarche (<13, ≥13 years of age, missing), parity (0, 1, 2, 3, ≥4, missing), oral contraceptive use (never, ever, missing), hormone replacement therapy (never, ever, missing), smoking (never, former, current, missing), history of high blood pressure (yes, no, missing), history of diabetes (yes, no, missing), and body mass index (<25, 25–<30, ≥30 kg/m2, missing). F, ovarian cancer adjusted for duration of oral contraceptive use and number of pregnancies. CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; WHO, World Health Organization.
As part of the project, an analysis of correlates of 25(OH)D was conducted to both guide analyses of site-specific papers and to take advantage of the wide spectrum of populations represented in the VDPP (15). Consistent with other reports, individuals with higher 25(OH)D levels tended to be male and to be lean, to engage in vigorous physical activity, to have a greater dietary intake of vitamin D, and to have greater use of multivitamin and calcium supplements.
The current recommended daily intake according to the Food and Nutrition Board of the Institute of Medicine, National Academies, is age dependent and ranges from 200 to 600 IU, with the highest dose recommendations for elderly women. The tolerable upper limit of intake, defined as the amount that is likely to pose no overall risk of adverse effects, varies from 1,000 IU in infants to 2,000 IU in adults (16). These recommendations are currently under review by the Food and Nutrition Board (Institute of Medicine) and may be altered. Finding the optimal dose of vitamin D is important, as there appears to be risk at both extremes of the distribution of vitamin D concentrations. Higher mortality rates occur at the extreme low concentrations, as well as at the high end of the distribution (3, 17). The VDPP suggests a possible increased risk for pancreatic cancer at higher vitamin D concentrations.
As the safety of high-dose supplementation for prolonged periods is uncertain and reports of harm have surfaced at the high end of 25(OH)D concentrations, caution should be exercised in using high-dose supplementation in both clinical practice and research settings. If high doses are to be used, serum 25(OH)D concentrations should be monitored. Clinically, high-dose supplementation may be recommended when measured vitamin D concentrations are very low. Research studies may also use doses at the higher end of the tolerable safe upper limit in order to maximize the ability to detect effects. A search of the Clinical Trials Registry maintained by the National Institutes of Health (http://clinicaltrials.gov/), with vitamin D as the key term and limitation to interventional studies, yielded 360 open studies. Further search with key terms of “vitamin D and prevention” and “vitamin D as an intervention” yielded 59 open studies. The populations being studied included pregnant women, children, and adults. Among those studies that listed the dose of vitamin D, 28 studies had at least 1 intervention arm with a vitamin D dose of 2,000 IU per day or higher. For example, a weekly dose of 20,000 IU of vitamin D (for an average daily dose exceeding 2,500 IU) is being investigated in a 5-year intervention trial among individuals with impaired glucose tolerance. A study with recruitment beginning in January 2010 per the study website is the Vitamin D and Omega-3 Trial (referred to as “VITAL”), which plans to recruit 20,000 individuals to test vitamin D supplementation at a dose of 2,000 IU per day (http://www.vitalstudy.org/). Participants are asked to limit their supplement intake to no more than 800 IU per day for a potential supplementation dose of 2,800 IU per day. As noted previously, the safety of these doses, especially with prolonged supplementation of 1 year or more, is uncertain. Given the current information on risks at extreme levels, trial participants should have regular monitoring of blood concentrations.
The predicted vitamin D levels have been suggested as a surrogate for serum measures of 25(OH)D, but these may not be sufficiently reliable for safety monitoring (3). The Women's Health Initiative observed a statistically significant but very modest correlation between reported diet and supplement intake and measured 25(OH)D concentrations (r = 0.19; P < 0.001) (18). Indeed, only 3% of women in the upper fifth of the distribution (cutpoint, 67.6 nmol/L) reported intakes greater than 1,000 IU per day. Among controls in the VDPP, a similarly low correlation between total vitamin D intake and serum levels was observed (r = 0.26; P < 0.0001). This relatively poor prediction of serum concentrations from reported diet and supplement intake emphasizes the need for clinical monitoring in practice and on research studies. The need for monitoring may be particularly critical for research participants with baseline pretrial 25(OH)D concentrations in the nondeficient range. In the VDPP study, increments of intake of 1,000 IU were associated with 18 nmol/L higher 25(OH)D. Therefore, an individual in the adequate range of vitamin D concentrations (e.g., >75 nmol/L) who is taking more than 2,000 IU per day of total vitamin D intake may have vitamin D concentrations in the range associated with increased risk of pancreatic cancer (11, 15).
The report from the International Agency for Research on Cancer examining the association between vitamin D status and cancer risk calls for randomized trials of vitamin D for cancer prevention, stating that observational studies are unlikely to “disentangle the complex relationships between vitamin D and known cancer risk factors” (3, p. 1). The report also points to contradictory results between observational studies and randomized trials as further evidence for the need to conduct more trials rather than additional observational studies (3). However, observational studies examine a broad range of exposures and can evaluate multiple health outcomes and potential harms, including rare outcomes. Clinical trials are unlikely to be large enough or to be conducted long enough to detect rare adverse events. In the relatively short history of cancer chemoprevention, unwarranted harms have occurred in intervention trials with doses of supplements previously considered safe (19–21). Cancer prevention trials require large sample sizes because cancer outcomes are rare, even for the more common cancer sites. As a consequence, many individuals are exposed, but relatively few can derive the actual benefit of a cancer prevented, if the intervention does indeed decrease the risk of cancer. Thus, under the principle of “first do no harm” as well as the wise expenditure of research dollars, it is critical to have compelling evidence of potential benefit for a proposed preventive intervention that far outweighs harms, before embarking on large-scale trials. Observational studies may provide such evidence, especially when outcomes are rare. The results of the VDPP study should be included in the overall evaluation of potential risks and benefits of vitamin D supplementation proposed for future trials or being used in ongoing prevention trials.
Acknowledgments
Author affiliation: The Prevention and Research Center, The Weinberg Center for Women's Health and Medicine, Mercy Medical Center, Baltimore, Maryland.
This work was supported by the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute (NCI) (Bethesda, Maryland), and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, NCI. The New York University Women's Health Study was supported by the NCI (grant R01 CA098661). The Health Professionals Follow-up Study and the Nurses’ Health Study were supported by the NCI (grants P01 CA055075, P01 CA87969, R01 CA49449, and R01 CA082838). The Multiethnic Cohort Study was supported by the NCI (grants R37 CA54281, P01 CA33619, R01 CA063464, and N01-PC35137). The Shanghai Men's Health Study was supported by the NCI (grant R01 CA82729). The Shanghai Women's Health Study was supported by the NCI (grants R37 CA70867 and N02-CP-11010-66). The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial was supported by contracts from the NCI to the University of Colorado, Denver, Colorado (grant N01-CN-25514); Georgetown University, Washington, DC (grant N01-CN-25522); and the Pacific Health Research Institute, Honolulu, Hawaii (grant N01-CN-25515); the Henry Ford Health System, Detroit, Michigan (grant N01-CN-25512); the University of Minnesota, Minneapolis, Minnesota (grant N01-CN-25513); Washington University, St. Louis, Missouri (grant NO1-CN-25516); the University of Pittsburgh, Pittsburgh, Pennsylvania (grant N01-CN-25511); the University of Utah, Salt Lake City, Utah (grant N01-CN-25524); the Marshfield Clinic Research Foundation, Marshfield, Wisconsin (grant N01-CN-25518); the University of Alabama, Birmingham, Alabama (grant NO1-CN-75022); Westat, Inc., Rockville, Maryland (grant N01-CN-25476); and the University of California, Los Angeles, California (grant NO1-CN-25404). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the NCI and the US Public Health Service (grants N01-CN-45165, N01-RC-45035, and N01-RC-37004). CLUE was supported by the National Institute on Aging (grant U01 AG018033) and the National Cancer Institute (grants R01 CA105069 and K07 CA73790). The participation of CLUE investigators was also supported by an NCI contract awarded to Mercy Medical Center through the University of Hawaii (Honolulu, Hawaii). The Cancer Prevention Study II Nutrition Cohort was supported by the American Cancer Society (Atlanta, Georgia).
The authors thank Dr. Karen Phinney of the National Institute of Standards and Technology for providing the vitamin D in human serum (SRM 972) used in this work.
Members of the VDPP Steering Committee: Christian C. Abnet, Demetrius Albanes, D. Michal Freedman, Lisa Gallicchio, Susan E. Hankinson, Patricia Hartge, Virginia Hartmuller, Chinonye Harvey, Kathy J. Helzlsouer (Chair), Ronald L. Horst, Laurence N. Kolonel, Francine Laden, Marjorie L. McCullough, Mark P. Purdue, Xiao-Ou Shu, Kirk Snyder, Rachael Z. Stolzenberg-Solomon, Stephanie J. Weinstein (Chair, Data Coordinating Center), Kai Yu, Anne Zeleniuch-Jacquotte, and Wei Zheng.
This report is based at least in part on information provided by the Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene.
Dr. Ronald L. Horst is the President and Chief Executive Officer of Heartland Assays, Inc. (Ames, Iowa).
Glossary
Abbreviations
- CI
confidence interval
- 25(OH)D
25-hydroxyvitamin D
- VDPP
Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
References
- 1.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10(2):110–117. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Nutrition Board of the Institute of Medicine of the National Academies. Washington, DC: National Academy of Sciences; 2009. Dietary reference intakes for vitamin D and calcium. ( http://www.iom.edu/Activities/Nutrition/DRIVitDCalcium.aspx). (Accessed October 15, 2009) [Google Scholar]
- 3.International Agency for Research on Cancer. IARC Working Group Reports. Vol. 5. Lyon, France: International Agency for Research on Cancer; 2008. Vitamin D and cancer. [Google Scholar]
- 4.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the Prostate, Lung, Colorectal, and Ovarian Screening Trial. Cancer Res. 2009;69(4):1439–1447. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proceedings of the conference Vitamin D and Cancer: Current Dilemmas and Future Needs, Bethesda, Maryland, USA, May 7–8, 2007. Nutr Rev. 2007;65(8 pt 2):S71–S137. [PubMed] [Google Scholar]
- 6.Zeleniuch-Jacquotte A, Gallicchio L, Hartmuller V, et al. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):36–46. doi: 10.1093/aje/kwq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abnet CC, Chen Y, Chow WH, et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):94–106. doi: 10.1093/aje/kwq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallicchio L, Moore LE, Stevens VL, et al. Circulating 25-hydroxyvitamin D and risk of kidney cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):47–57. doi: 10.1093/aje/kwq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purdue MP, Freedman DM, Gapstur SM, et al. Circulating 25-hydroxyvitamin D and risk of non-Hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–69. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, Danforth KN, Tworoger SS, et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):70–80. doi: 10.1093/aje/kwq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D levels and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81–93. doi: 10.1093/aje/kwq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallicchio L, Helzlsouer KJ, Chow W-H, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abnet CC, Chen W, Dawsey SM, et al. Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1889–1893. doi: 10.1158/1055-9965.EPI-07-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Dawsey SM, Qiao YL, et al. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97(1):123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(2):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: The National Academy Press; 1997. [PubMed] [Google Scholar]
- 17.Melamed ML, Michos ED, Post W, et al. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100(22):1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albanes D, Heinonen OP, Huttunen JK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62(6 suppl):1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 20.Albanes D, Heinonen OP, Taylor PR, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 21.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]