Abstract
Results from epidemiologic studies examining pancreatic cancer risk and vitamin D intake or 25-hydroxyvitamin D (25(OH)D) concentrations (the best indicator of vitamin D derived from diet and sun) have been inconsistent. Therefore, the authors conducted a pooled nested case-control study of participants from 8 cohorts within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP) (1974–2006) to evaluate whether prediagnostic circulating 25(OH)D concentrations were associated with the development of pancreatic cancer. In total, 952 incident pancreatic adenocarcinoma cases occurred among participants (median follow-up, 6.5 years). Controls (n = 1,333) were matched to each case by cohort, age, sex, race/ethnicity, date of blood draw, and follow-up time. Conditional logistic regression analysis was used to calculate smoking-, body mass index-, and diabetes-adjusted odds ratios and 95% confidence intervals for pancreatic cancer. Clinically relevant 25(OH)D cutpoints were compared with a referent category of 50–<75 nmol/L. No significant associations were observed for participants with lower 25(OH)D status. However, a high 25(OH)D concentration (≥100 nmol/L) was associated with a statistically significant 2-fold increase in pancreatic cancer risk overall (odds ratio = 2.12, 95% confidence interval: 1.23, 3.64). Given this result, recommendations to increase vitamin D concentrations in healthy persons for the prevention of cancer should be carefully considered.
Keywords: case-control studies, cohort studies, pancreatic neoplasms, prospective studies, vitamin D
Sun exposure has been associated with lower death rates for pancreatic cancer in several ecologic studies (1–7). Solar ultraviolet B radiation (280–320 nm) induces cutaneous production of precursors of vitamin D and is the primary contributor to vitamin D status in most populations (1, 2, 4, 6). Dietary sources of vitamin D include cholecalciferol (vitamin D3), which occurs naturally in some animal foods (e.g., fatty saltwater fish, liver, and eggs). Supplements and fortified foods such as milk, orange juice, and margarine contain vitamin D3 produced by the irradiation of 7-dehydrocholesterol extracted from lanolin or vitamin D2 (ergocalciferol) obtained from plants (8–10). 25-Hydroxyvitamin D (25(OH)D) is the main circulating form of vitamin D in humans and is considered the best assessment of vitamin D status derived from both sun exposure and diet.
Animal and cell culture studies support vitamin D having a function in the pancreas. Expression of the enzyme 25-hydroxyvitamin D3-1α-hydroxylase, which converts 25(OH)D to 1,25-dihydroxyvitamin D, the bioactive form of the vitamin, has been detected in normal and adenocarcinomatous tissue and normal pancreatic duct cells (11–13). 25-Hydroxyvitamin D3 hinders pancreatic cancer cell line growth (12, 13). 1,25-Dihydroxyvitamin D analogs in vitro stimulate differentiation, promote apoptosis, impede pancreatic cancer cell proliferation (14–16), and in vivo inhibit pancreatic tumor growth (16, 17). There is evidence that vitamin D regulates insulin synthesis, binding, and response (18–20). Experimental and epidemiologic studies support that higher insulin and glucose levels and diabetes contribute to pancreatic cancer development (21–24).
However, epidemiologic studies examining dietary vitamin D, a predicted 25(OH)D status score, or measured 25(OH)D concentrations and pancreatic cancer have shown conflicting results. Two prospective studies demonstrated reduced risk for pancreatic cancer with higher total vitamin D intake (25) and predicted vitamin D status score computed from 6 determinants of 25(OH)D concentrations (dietary and supplementary vitamin D, skin pigmentation, adiposity, geographic residence, and leisure activity) (26). Those studies’ limitation was that vitamin D status was not measured in participants. Conversely, a nested case-control study carried out in the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study population, a cohort of male Finnish smokers, showed an unexpected and statistically significant 3-fold increased risk of pancreatic cancer with higher vitamin D levels (27). The results of the ATBC study may not be comparable to nonsmokers, women, or populations residing at latitudes lower than Finland with higher vitamin D status (27). A subsequent nested case-control study carried out in a US population, the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial cohort, did not confirm the strong positive association between 25(OH)D and pancreatic cancer overall (28). However, the latter study did show an increased risk among participants living at northern latitudes that was similar to the ATBC findings (27).
Given the inconsistent results from the studies examining vitamin D concentrations and pancreatic cancer, we conducted a pooled nested case-control study of participants from 8 cohorts in the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP) to test whether prediagnostic circulating 25(OH)D concentrations were associated with subsequent risk of incident pancreatic cancer. Because the VDPP pooled data on cohorts from multiple geographic regions, latitudes, seasons, and ethnic populations, the present investigation had more cases and a wider range of vitamin D concentrations than the previous studies.
MATERIALS AND METHODS
Study design and population
This pooled nested case-control study included data from the following cohort studies: the ATBC Study (29), CLUE (30), the Cancer Prevention Study II Nutrition Cohort (CPS-II) (31), the New York University Women's Health Study (NYU-WHS) (32), the Multiethnic Cohort Study (MEC) (33), the PLCO (34), and the Shanghai Women's and Men's Health Studies (SWHS and SMHS) (35, 36) (Table 1). The final analytic data set included 952 cases and 1,333 controls from these 8 cohorts.
Table 1.
Characteristics of Cohorts Included in an Investigation of Circulating 25-Hydroxyvitamin D Concentrations and Pancreatic Cancer, by Cohort, Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, 1974–2006
| Cohort | No. of Cases | No. of Controls | Median Time From Blood Collection to Cancer Diagnosis, years (IQRa) | Median Circulating 25-Hydroxyvitamin D Concentration, nmol/L (Range) |
|
| Cases | Controls | ||||
| ATBC | 313 | 513 | 9.4 (5.5–13.0) | 43.6 (2.1–150.9) | 43.0 (2.6–126.9) |
| CLUE | 123 | 123 | 10.6 (5.7–15.2) | 57.8 (9.0–141.1) | 56.4 (5.7–111.1) |
| CPS-II | 65 | 65 | 2.2 (1.3–3.2) | 55.8 (14.1–115.3) | 60.2 (16.6–112.3) |
| MEC | 109 | 109 | 2.1 (1.3–3.5) | 49.5 (2.0–137.6) | 47.7 (5.2–115.9) |
| NYU-WHS | 73 | 73 | 13.5 (8.6–17.0) | 47.2 (16.1–156.0) | 46.8 (6.5–127.2) |
| PLCO | 183 | 364 | 5.6 (2.8–7.7) | 64.7 (13.2–135.5) | 65.1 (16.3–126.0) |
| SMHS | 27 | 27 | 1.5 (0.9–2.7) | 38.8 (13.3–82.0) | 40.3 (9.2–85.4) |
| SWHS | 59 | 59 | 5.5 (2.1–6.7) | 33.4 (8.7–63.5) | 30.4 (14.5–79.5) |
| Total | 952 | 1,333 | 6.5 (2.8–10.9) | 49.3 (2.0–156.0) | 50.8 (2.6–127.2) |
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CPS-II, Cancer Prevention Study II; IQR, interquartile range; MEC, Multiethnic Cohort Study; NYU-WHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
25th–75th percentiles.
Cases included incident primary pancreatic adenocarcinomas (International Classification of Diseases for Oncology, Third Edition, codes C250–C259 or C25.0–C25.3 and C25.7–C25.9). Endocrine pancreatic tumors (International Classification of Diseases for Oncology, Third Edition, code C25.4; histology types 8150, 8151, 8153, 8155, and 8240) were excluded, because the etiology of these cancers is thought to be different. Case ascertainment varied between studies but included linking participants to cancer registries, self- and next-of-kin reports, and use of national death indices.
Controls were selected with a control:case ratio of 1:1 (ATBC, CLUE, CPS-II, NYU-WHS, MEC, SMHS, and SWHS) or 2:1 (ATBC and PLCO) and were alive and free from pancreatic cancer on the date on which the matched case was diagnosed. Data from cases and controls from the previously published ATBC (27) and PLCO (28) nested case-control studies were included in the present analysis. Controls were matched to cases on age and date of blood draw, sex, and race/ethnicity. For some cohorts, controls were additionally matched on other factors, such as fasting status at blood draw, time of blood draw, and, for women, menopausal status.
Data on lifestyle, demographic factors, and possible confounders were collected. Detailed descriptions of data collection methods have been published previously for the individual studies (29–36). From each cohort, information was obtained on history of cigarette smoking, sex, age, race/ethnicity, body mass index (weight (kg)/height (m)2), family history of pancreatic cancer, diet, alcohol consumption, self-reported pancreatitis, and diabetes history.
Informed consent was obtained in each of the individual studies. Each study was approved by its local institutional review board.
Measurement of circulating 25(OH)D
Samples from CLUE, CPS-II, NYU-WHS, MEC, SMHS, and SWHS and a subset of samples from the ATBC Study were sent to Heartland Assays, Inc. (Ames, Iowa) and assayed for 25(OH)D status using the DiaSorin LIAISON 25 OH Vitamin D TOTAL Assay (DiaSorin, Inc., Stillwater, Minnesota) (37). The ATBC and PLCO samples had 25(OH)D measured using the same method in the laboratory of Dr. Reinhold Vieth (Mount Sinai Hospital, Toronto, Ontario, Canada) or at Heartland Assays, Inc., at an earlier time (2005 and 2007), respectively (27, 28). Matched case and control samples were masked to case status and were assayed consecutively within batches. The US National Institute of Standards and Technology (NIST) provided 2 masked serum quality control standards for vitamin D to include within batches: NIST level 1 (∼60 nmol/L) and NIST level 2 (∼35 nmol/L). Quality control samples were also provided by investigators from the individual cohorts. For the VDPP samples, the interbatch and intrabatch coefficient of variation percentages for NIST level 1 samples were 12.7% and 9.3%, respectively, and for NIST level 2 samples, they were 13.6% and 11.0%, respectively. The median inter- and intrabatch coefficient of variation percentages for the cohort quality control samples were 13.2% (range, 4.8%–17.0%) and 9.9% (range, 3.8%–16.4%), respectively. Using a nested components of variance analysis, with logarithmically transformed quality control measurements across all batches (38), the overall (intra- and interbatch) coefficient of variation percentages were 16.5% and 4.7% for the previously assayed 25(OH)D concentrations in the ATBC and PLCO studies, respectively (27, 28).
Statistical analysis
The distributions of selected characteristics of the cases and controls were compared using conditional logistic regression. Dietary nutrients and foods highly correlated with energy intake were energy-adjusted using the residual method described by Willett and Stampfer (39). Variables examined in analyses and/or as potential confounders in risk models were age; smoking status at the time of blood draw and most recently (never smoker, former smoker who had quit ≥15 years previously, former smoker who had quit 1–14 years previously, former smoker who had quit <1 year previously or current smoker of <20 cigarettes/day, and former smoker who had quit <1 year previously or current smoker of ≥20 cigarettes/day); education; hormone use (among women); height; weight; body mass index (<25, 25–<30, or ≥30, or missing data); history of diabetes (yes, no, or missing data); family history of pancreatic cancer; intake of vitamin D-containing foods (fish, milk); nutrients from foods (energy, carbohydrate, fat, saturated fat, calcium, vitamin D, folate, and retinol); nutrients from supplements (folic acid, vitamin D, and calcium); total nutrients (folate, vitamin D, and calcium); consumption of red or processed meat; use of multivitamins (yes, no, or missing data); alcohol consumption; physical activity; season of blood draw (winter, summer); and latitude at study entry. Seasons were defined as winter (December–May) and summer (June–November).
Clinically defined 25(OH)D cutpoints were used for the main analyses: <25, 25–<37.5, 37.5–<50, 50–<75, 75–<100, and ≥100 nmol/L (40, 41). Conditional logistic regression was used to calculate odds ratios and 95% confidence intervals for pancreatic cancer by 25(OH)D level, with 50–<75 nmol/L used as the referent category. Multivariable models were developed by individually adding covariates to the model with forward and backward selection. Covariates were included if they were associated with both the disease and the risk factor and changed the point estimate of risk by 10% or more. The final multivariable models included body mass index and smoking (as categorized above). Interactions were evaluated in stratified analyses, by sex, race/ethnicity, season, latitude, smoking status (never, former, or current; never vs. ever), body mass index (<25 vs. ≥25), and use of vitamin D supplements or multivitamins; statistical significance was tested using a multiplicative risk model. Conditional logistic regression was used to calculate odds ratios stratified by sex and race/ethnicity, while unconditional logistic regression, adjusted for matching factors, was used for the other stratifications.
A 2-stage meta-analysis approach as described by Gallicchio et al. (42) was conducted. Results are presented for high (≥100 nmol/L) or low (<25 nmol/L) 25(OH)D concentrations as compared with the reference category (50–<75 nmol/L). The heterogeneity of cohort-specific estimates was assessed using the Q statistic, and data are presented as forest plots. To investigate whether 1 single study unduly influenced the pooled estimates, we conducted sensitivity analyses in which pooled risk estimates were assessed and compared after systematically excluding each study in turn. Sensitivity analyses were also conducted after excluding both the ATBC and PLCO cohorts, for which reports had been previously published (27, 28). To evaluate the potential influence of preclinical disease on risk, we performed lag analyses that excluded cases that occurred during either the first 2 years of follow-up or the first 5 years of follow-up.
All statistical analyses were performed using SAS software, versions 9.1.3 and 9.2 (SAS Institute, Inc., Cary, North Carolina) and R software, version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria (http://www.r-project.org/)). Statistical tests were 2-tailed.
RESULTS
The characteristics of participants in each cohort are shown in Table 1. Cases were diagnosed with pancreatic cancer after a median of 6.5 years of follow-up (range, 0–30.6 years). The median age at pancreatic cancer diagnosis was 69.5 years. The range of 25(OH)D concentrations varied across the cohorts, with the SMHS and SWHS participants having the lowest concentrations and the PLCO participants having the highest concentrations. All cases had a higher upper range of 25(OH)D concentrations than did the controls, except for those in the Shanghai cohorts. The ranges of 25(OH)D concentrations were 2.0–156.0 nmol/L in cases and 2.6–127.2 nmol/L in controls.
Characteristics of cases and controls are shown in Table 2. The ATBC participants were excluded from the analysis of smoking status in Table 2, because all participants in that study were current smokers at baseline. Compared with the controls, cases more often reported being a current smoker (P < 0.0001), had a greater saturated fat intake, and consumed less fish, carbohydrate, and folate. The proportions of cases and controls with vitamin D concentrations less than 37.5 nmol/L or less than 25 nmol/L were not statistically different.
Table 2.
Selected Characteristics of Cases and Controls in an Investigation of Circulating 25-Hydroxyvitamin D Concentrations and Pancreatic Cancer, Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, 1974–2006
| Characteristic | Cases (n = 952) |
Controls (n = 1,333) |
P Valueb | ||||
| No. | % | Median (IQRa) | No. | % | Median (IQR) | ||
| Age, years | 62 (56–68) | 62 (57–67) | Matched | ||||
| Male sex | 602 | 63.2 | 919 | 68.9 | Matched | ||
| Serum 25(OH)D concentration, nmol/L | 49.3 (34.1–66.9) | 50.8 (35.7–67.5) | 0.18 | ||||
| <37.5 | 279 | 19.3 | 366 | 27.5 | 0.53 | ||
| <25 | 115 | 12.1 | 141 | 10.6 | 0.79 | ||
| Latitude at study entry, degrees North | |||||||
| Total | 40.7 (38.6–60.0) | 42.4 (39.5–60.0) | 0.39 | ||||
| >42 (low sun exposure) | 424 | 44.5 | 694 | 52.1 | 0.39 | ||
| 35–42 (moderate sun exposure) | 311 | 32.7 | 404 | 30.3 | |||
| <35 (high sun exposure) | 217 | 22.8 | 235 | 17.6 | |||
| Race/ethnicity | |||||||
| White | 729 | 76.6 | 1,095 | 82.1 | Matched | ||
| Black | 51 | 5.4 | 56 | 4.2 | |||
| Asian | 125 | 13.1 | 133 | 10.0 | |||
| Other | 34 | 3.6 | 34 | 2.6 | |||
| Missing data | 13 | 1.4 | 15 | 1.1 | |||
| Cigarette smoking historyc | |||||||
| Never smoker | 297 | 46.5 | 418 | 51.0 | <0.0001 | ||
| Former smoker | 209 | 32.7 | 313 | 38.2 | |||
| Current smoker | 119 | 18.6 | 74 | 9.0 | |||
| Missing data | 14 | 2.2 | 15 | 1.8 | |||
| Height, cm | |||||||
| Male | 175 (170–180) | 175 (170–179) | 0.50 | ||||
| Female | 160 (156–165.1) | 160 (157–165.1) | 0.57 | ||||
| Body mass indexd | 25.7 (23.4–28.4) | 26.0 (23.5–28.5) | 0.61 | ||||
| <25.0 | 373 | 39.2 | 503 | 37.7 | 0.56 | ||
| 25.0–<30 | 372 | 39.1 | 563 | 42.2 | |||
| ≥30 | 149 | 15.7 | 207 | 15.5 | |||
| Missing data | 58 | 6.1 | 60 | 4.5 | |||
| History of diabetes mellitus | |||||||
| Yes | 78 | 8.2 | 102 | 7.7 | 0.50 | ||
| Missing data | 23 | 2.4 | 21 | 1.6 | |||
| Family history of pancreatic cancer | |||||||
| Yes | 33 | 3.5 | 27 | 2.0 | 0.09 | ||
| Missing data | 272 | 28.6 | 348 | 71.9 | |||
| Education | |||||||
| Less than high school | 256 | 26.9 | 327 | 24.5 | 0.07 | ||
| High school graduation | 166 | 17.4 | 197 | 14.8 | |||
| Post-high school, vocational training | 147 | 15.4 | 280 | 21 | |||
| Some college | 179 | 18.8 | 253 | 19 | |||
| College graduation | 102 | 10.7 | 126 | 9.5 | |||
| Postgraduate study | 73 | 7.7 | 133 | 10 | |||
| Missing data | 29 | 3.0 | 17 | 1.3 | |||
| Recent multivitamin use | |||||||
| Yes | 156 | 16.4 | 203 | 15.2 | 0.44 | ||
| Missing data | 390 | 41 | 596 | 44.7 | |||
| Daily dietary intake | |||||||
| Vitamin D-rich foods | |||||||
| Fish, ge | 23.3 (11.7–40.1) | 24.8 (13–43.5) | 0.03 | ||||
| Milk, ge | 224 (60.0–485.3) | 248.8 (101.7–549.6) | 0.93 | ||||
| Red meat (energy-adjusted), ge | 52.8 (36.3–72.8) | 51.9 (35.5–72.4) | 0.22 | ||||
| Processed meat (energy-adjusted), gf | 26.9 (12.5–45.5) | 26.6 (11.4–45.7) | 0.06 | ||||
| Alcohol, gg | 2.1 (0–13.7) | 2.4 (0–14) | 0.21 | ||||
| Nutrients | |||||||
| Energy, kcale | 1,979.3 (1,497.3–2,602) | 2,107.8 (1,524–2,733.7) | 0.50 | ||||
| Total fat (energy-adjusted), ge | 81.7 (65.3–93.5) | 79.9 (64.9–93.4) | 0.09 | ||||
| Saturated fat (energy-adjusted), ge | 29.3 (20.8–38.5) | 28.5 (21.1–37.8) | 0.03 | ||||
| Carbohydrate (energy-adjusted), ge | 260.8 (231.2–297.9) | 268.2 (235.3–299.4) | 0.01 | ||||
| Retinol (energy-adjusted), IUh | 1,928.6 (1,036.0–3,265.4) | 2,098.8 (1,239.3–3,422.4) | 0.94 | ||||
| Folate (energy-adjusted), μge | 298 (248.2–376.9) | 309 (252.8–377.4) | 0.05 | ||||
| Vitamin D | |||||||
| Food (energy-adjusted), IUe | 153.6 (102.1–223.2) | 165.6 (110.4–233.0) | 0.24 | ||||
| Total vitamin D, IUi | 218.4 (142–475.9) | 232.9 (152.3–491.5) | 0.46 | ||||
| Calcium | |||||||
| Food (energy-adjusted), mge | 906.9 (711.3–1,104.8) | 940.6 (731.3–1,164.8) | 0.31 | ||||
| Total, mgj | 1,089.7 (727.2–1,490) | 1,159.6 (791.3–1,572.8) | 0.12 | ||||
| Quartile of physical activity | |||||||
| Low/none | 310 | 32.6 | 435 | 32.6 | 0.12 | ||
| Light | 285 | 29.9 | 418 | 31.4 | |||
| Moderate | 113 | 11.9 | 143 | 10.7 | |||
| Vigorous | 125 | 13.1 | 219 | 16.4 | |||
| Missing data | 119 | 12.5 | 118 | 8.9 | |||
Abbreviations: IQR, interquartile range; 25(OH)D, 25-hydroxyvitamin D.
25th–75th percentiles.
Wald P value from conditional logistic regression.
Cases and controls from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study were excluded because they were all current smokers at baseline.
Weight (kg)/height (m)2; World Health Organization categories were used.
Complete data on dietary intake were available for 855 cases and 1,200 controls; 97 cases and 133 controls had missing data. Numbers differ slightly from the total for some exposures because of missing data across categories.
Complete data on dietary intake were available for 769 cases and 1,114 controls; 183 cases and 219 controls had missing data.
Complete data on alcohol consumption were available for 842 cases and 1,190 controls; 110 cases and 143 controls had missing data.
Complete data on retinol intake were available for 682 cases and 861 controls; 270 cases and 472 controls had missing data.
Complete data on total vitamin D intake were available for 575 cases and 915 controls; 377 cases and 418 controls had missing data.
Complete data on total calcium intake were available for 725 cases and 1,067 controls; 227 cases and 266 controls had missing data.
Compared with 25(OH)D concentrations of 50.0–<75.0 nmol/L, concentrations of 100 nmol/L or higher were associated with increased pancreatic cancer risk (Table 3) (in the fully adjusted model, odds ratio (OR) = 2.12, 95% confidence interval (CI): 1.23, 3.64). No statistically significant associations were observed for concentrations of <25.0 nmol/L or 25.0–<37.5 nmol/L, overall or in stratified models. The increased risk in the highest category of 25(OH)D persisted after exclusion of cases that occurred within the first 2 years after blood draw (leaving 772 cases and 1,115 controls; OR = 2.20, 95% CI: 1.22, 3.96) and cases diagnosed within the first 5 years after blood draw (leaving 558 cases and 840 controls; OR = 2.26, 95% CI: 1.13, 4.52). Analyses restricted to US cohorts showed similar risk in the highest 25(OH)D category (OR = 2.98, 95% CI: 1.48, 6.02). Similar odds ratios were observed for analyses that excluded each cohort in turn: excluding ATBC, 2.95 (95% CI: 1.47, 5.93); excluding CLUE, 1.75 (95% CI: 0.97, 3.16); excluding CPS-II, 2.32 (95% CI: 1.32, 4.06); excluding MEC, 2.20 (95% CI: 1.25, 3.86); excluding NYU-WHS, 2.20 (95% CI: 1.26, 3.81); excluding PLCO, 1.60 (95% CI: 0.83, 3.08), and excluding SMHS/SWHS, 2.12 (95% CI: 1.23, 3.65). The odds ratio for pancreatic cancer in the highest 25(OH)D category was 2.23 (95% CI: 0.82, 6.08) after exclusion of the ATBC and PLCO cohorts for which results had been previously published (n = 496 cases and n = 886 controls in the analysis) (27, 28).
Table 3.
Odds Ratios for the Association Between Circulating 25-Hydroxyvitamin D Concentrations and Risk of Pancreatic Cancera, Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, 1974–2006
| Circulating 25-Hydroxyvitamin D Concentrationb, nmol/L |
||||||||||||
| Model | <25 (115/141)c |
25–<37.5 (164/225) |
37.5–<50.0 (208/286) |
50.0–<75.0 (306/458) |
75.0–<100.0 (120/190) |
≥100 (39/30) |
||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Cruded | 0.98 | 0.70, 1.35 | 0.97 | 0.74, 1.26 | 1.06 | 0.84, 1.34 | 1.00 | Referent | 1.01 | 0.77, 1.33 | 2.05 | 1.21, 3.48 |
| Multivariate-adjustede,f | 0.95 | 0.68, 1.32 | 0.98 | 0.75, 1.28 | 1.04 | 0.82, 1.33 | 1.00 | Referent | 1.02 | 0.77, 1.35 | 2.12 | 1.23, 3.64 |
| Multivariate-adjustede,g,h | 1.00 | Referent | 1.04 | 0.74, 1.44 | 1.10 | 0.79, 1.55 | 1.06 | 0.76, 1.48 | 1.08 | 0.73, 1.59 | 2.24 | 1.22, 4.12 |
Abbreviations: CI, confidence interval; OR, odds ratio.
The analysis included a total of 952 cases and 1,333 controls.
Categories were based on a priori cutpoints.
Number of cases/number of controls.
Adjusted for matching variables (age, race/ethnicity, sex, cohort, and date of blood draw) using conditional logistic regression.
Additionally adjusted for body mass index (weight (kg)/height (m)2) in World Health Organization categories (<18.5, 18.5–<25.0, 25.0–30.0, 30.0–<35.0, or ≥35.0, or missing data), smoking (never smoker, former smoker who had quit ≥15 years previously, former smoker who had quit 1–14 years previously, former smoker who had quit <1 year previously or current smoker of <20 cigarettes/day, and former smoker who had quit <1 year previously or current smoker of ≥20 cigarettes/day), and diabetes status (yes, no, or missing data).
Reference category: 50.0–<75.0 nmol/L.
Reference category: <25 nmol/L.
P for trend = 0.14.
Associations did not vary significantly by sex, season, race/ethnicity, latitude, smoking status (Table 4), body mass index, or use of vitamin D supplements or multivitamins (P-interaction > 0.15). However, the increased risk associated with high 25(OH)D concentrations was more apparent in males than in females, was seen only in blood samples drawn during the summer and in Caucasians but not Asians, was statistically significant only for latitudes of 35°N–42°N (though it was suggestive for latitudes ≥42°N), and was not affected by smoking status. Although the number of Asians was not large (125 cases, 133 controls), the pattern of the association appeared different in this subgroup and suggested an elevated risk at lower serum concentrations of 25(OH)D (odds ratios were 1.30, 1.65, and 1.54 for progressively lower concentrations in comparison with the referent category). In adjusted models, no strata showed statistically significant trends across the 25(OH) categories, although among men, P-trend was 0.09 and among former smokers, P-trend was 0.14. Among the small number of African Americans (51 cases, 56 controls) and based on their race/ethnicity-specific 25(OH)D distribution, the adjusted odds ratio for the highest third of 25(OH)D levels (≥42.2 nmol/L) as compared with the lowest third (<28.2 nmol/L) was 1.77 (95% CI: 0.49, 6.42). There was no statistically significant interaction by use of vitamin D supplements or multivitamins. Among nonusers of multivitamins (406 cases, 534 controls), as compared with the reference group, the odds ratios were 0.83, 1.12, 1.10, 1.01, and 4.19 (95% CI: 1.73, 10.16) from the lowest 25(OH)D concentrations to the highest, respectively.
Table 4.
Odds Ratios for the Association Between Circulating 25-Hydroxyvitamin D Concentrations and Risk of Pancreatic Cancer, by Sex, Season, Race/Ethnicity, Latitude, and Smoking Status, Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, 1974–2006
| Total No. # |
Circulating 25-Hydroxyvitamin D Concentrationa, nmol/L |
||||||||||||||||||||||||
| <25 |
25–<37.5 |
37.5–<50.0 |
50.0–<75.0b |
75.0–<100.0 |
≥100 |
P-Interactionc | |||||||||||||||||||
| CA | CO | No. of CA | No. of CO | OR | 95% CI | No. of CA | No. of CO | OR | 95% CI | No. of CA | No. of CO | OR | 95% CI | No. of CA | No. of CO | No. of CA | No. of CO | OR | 95% CI | No. of CA | No. of CO | OR | 95% CI | ||
| Sex | |||||||||||||||||||||||||
| Male | 602 | 919 | 72 | 94 | 91 | 154 | 134 | 199 | 191 | 306 | 83 | 142 | 31 | 24 | |||||||||||
| Cruded | 0.92 | 0.60, 1.40 | 0.84 | 0.60, 1.18 | 1.05 | 0.79, 1.40 | 0.94 | 0.68, 1.31 | 2.25 | 1.23, 4.12 | |||||||||||||||
| M-Ae | 0.87 | 0.56, 1.34 | 0.81 | 0.57, 1.14 | 1.05 | 0.79, 1.41 | 0.95 | 0.68, 1.32 | 2.33 | 1.24, 4.36 | 0.27 | ||||||||||||||
| Female | 350 | 414 | 43 | 47 | 73 | 71 | 74 | 87 | 115 | 152 | 37 | 48 | 8 | 9 | |||||||||||
| Cruded | 1.10 | 0.66, 1.85 | 1.23 | 0.79, 1.91 | 1.07 | 0.71, 1.61 | 1.18 | 0.72, 1.93 | 1.40 | 0.45, 4.29 | |||||||||||||||
| M-Ae | 1.06 | 0.61, 1.84 | 1.27 | 0.80, 2.02 | 0.98 | 0.64, 1.51 | 1.15 | 0.69, 1.91 | 1.46 | 0.47, 4.61 | |||||||||||||||
| Season of blood drawf | |||||||||||||||||||||||||
| Winter | 489 | 698 | 89 | 103 | 98 | 153 | 110 | 165 | 145 | 218 | 41 | 49 | 6 | 10 | |||||||||||
| Crudeg | 1.26 | 0.86, 1.83 | 0.92 | 0.65, 1.30 | 1.01 | 0.73, 1.40 | 1.35 | 0.84, 2.18 | 0.92 | 0.32, 2.62 | |||||||||||||||
| M-Ae,g | 1.22 | 0.83, 1.79 | 0.92 | 0.65, 1.31 | 1.00 | 0.72, 1.40 | 1.37 | 0.85, 2.23 | 0.90 | 0.31, 2.60 | 0.21 | ||||||||||||||
| Summer | 463 | 635 | 26 | 38 | 66 | 72 | 98 | 121 | 161 | 240 | 79 | 141 | 33 | 23 | |||||||||||
| Crudeg | 0.89 | 0.51, 1.57 | 1.27 | 0.84, 1.91 | 1.19 | 0.84, 1.68 | 0.91 | 0.64, 1.29 | 2.36 | 1.32, 4.23 | |||||||||||||||
| M-Ae,g | 0.82 | 0.46, 1.45 | 1.21 | 0.80, 1.85 | 1.19 | 0.84, 1.69 | 0.93 | 0.65, 1.33 | 2.29 | 1.26, 4.15 | |||||||||||||||
| Race/ethnicity | |||||||||||||||||||||||||
| Caucasian | 729 | 1,095 | 76 | 101 | 108 | 160 | 157 | 231 | 251 | 402 | 100 | 174 | 37 | 27 | |||||||||||
| Cruded | 0.97 | 0.65, 1.43 | 1.02 | 0.75, 1.39 | 1.09 | 0.84, 1.41 | 0.96 | 0.72, 1.28 | 2.46 | 1.40, 4.33 | |||||||||||||||
| M-Ae | 0.93 | 0.63, 1.40 | 1.00 | 0.73, 1.37 | 1.06 | 0.81, 1.39 | 0.94 | 0.70, 1.28 | 2.43 | 1.36, 4.35 | |||||||||||||||
| Asian | 125 | 133 | 20 | 21 | 38 | 31 | 29 | 31 | 28 | 33 | 10 | 17 | |||||||||||||
| Cruded | 1.23 | 0.49, 3.07 | 1.46 | 0.67, 3.19 | 1.13 | 0.55, 2.31 | 0.81 | 0.30, 2.20 | |||||||||||||||||
| M-Ae | 1.54 | 0.56, 4.20 | 1.65 | 0.69, 3.92 | 1.30 | 0.58, 2.90 | 1.08 | 0.37, 3.17 | 0.20 | ||||||||||||||||
| Latitude, degrees North | |||||||||||||||||||||||||
| <35 | 217 | 235 | 32 | 30 | 51 | 50 | 48 | 51 | 59 | 71 | 22 | 25 | 5 | 8 | |||||||||||
| Crudeg | 1.19 | 0.61, 2.31 | 1.15 | 0.65, 2.03 | 1.06 | 0.62, 1.84 | 1.18 | 0.59, 2.35 | 0.98 | 0.28, 3.36 | |||||||||||||||
| M-Ae,g | 1.19 | 0.60, 2.36 | 1.18 | 0.66, 2.12 | 1.08 | 0.61, 1.90 | 1.31 | 0.63, 2.69 | 0.99 | 0.28, 3.52 | 0.21 | ||||||||||||||
| 35–42 | 311 | 404 | 24 | 26 | 46 | 49 | 62 | 74 | 119 | 170 | 42 | 76 | 18 | 9 | |||||||||||
| Crudeg | 1.15 | 0.61, 2.16 | 1.27 | 0.78, 2.07 | 1.19 | 0.78, 1.82 | 0.85 | 0.54, 1.35 | 2.89 | 1.23, 6.77 | |||||||||||||||
| M-Ae,g | 0.97 | 0.50, 1.88 | 1.19 | 0.72, 1.96 | 1.13 | 0.73, 1.74 | 0.86 | 0.54, 1.38 | 2.79 | 1.16, 6.71 | |||||||||||||||
| >42 | 424 | 694 | 59 | 85 | 67 | 126 | 98 | 161 | 128 | 217 | 56 | 89 | 16 | 16 | |||||||||||
| Crudeg | 1.16 | 0.77, 1.77 | 0.89 | 0.61, 1.31 | 1.04 | 0.74, 1.46 | 1.12 | 0.75, 1.69 | 1.76 | 0.85, 3.67 | |||||||||||||||
| M-Ae,g | 1.16 | 0.76, 1.76 | 0.90 | 0.61, 1.33 | 1.04 | 0.74, 1.46 | 1.16 | 0.76, 1.75 | 1.77 | 0.84, 3.70 | |||||||||||||||
| Smoking status at blood draw | |||||||||||||||||||||||||
| Never smoker | 297 | 418 | 31 | 39 | 59 | 62 | 56 | 77 | 105 | 172 | 36 | 60 | 10 | 8 | |||||||||||
| Crudeg | 1.03 | 0.57, 1.86 | 1.29 | 0.81, 2.07 | 1.09 | 0.70, 1.70 | 1.13 | 0.69, 1.85 | 2.45 | 0.91, 6.59 | |||||||||||||||
| M-Ae,g | 1.07 | 0.59, 1.95 | 1.44 | 0.88, 2.33 | 1.16 | 0.74, 1.83 | 1.16 | 0.70, 1.93 | 2.54 | 0.93, 6.92 | 061 | ||||||||||||||
| Former smoker | 201 | 310 | 9 | 14 | 20 | 33 | 39 | 56 | 81 | 121 | 40 | 75 | 12 | 11 | |||||||||||
| Crudeg | 0.72 | 0.27, 1.91 | 0.72 | 0.37, 1.41 | 0.91 | 0.54, 1.53 | 0.91 | 0.55, 1.50 | 1.75 | 0.71, 4.28 | |||||||||||||||
| M-Ae,g | 0.65 | 0.24, 1.75 | 0.70 | 0.35, 1.38 | 0.89 | 0.52, 1.51 | 0.96 | 0.57, 1.59 | 1.75 | 0.70, 2.35 | |||||||||||||||
| Current smoker or recent quitter | 440 | 590 | 73 | 88 | 85 | 124 | 106 | 151 | 116 | 161 | 43 | 53 | 17 | 13 | |||||||||||
| Crudeg | 1.20 | 0.79, 1.81 | 1.00 | 0.68, 1.46 | 1.03 | 0.72, 1.48 | 1.12 | 0.69, 1.83 | 1.83 | 0.83, 4.01 | |||||||||||||||
| M-Ae,g | 1.19 | 0.78, 1.79 | 0.99 | 0.68, 1.46 | 1.03 | 0.72, 1.47 | 1.12 | 0.68, 1.83 | 1.79 | 0.81, 3.95 | |||||||||||||||
Abbreviations: CA, cases; CI, confidence interval; CO, controls; M-A, multivariate-adjusted; OR, odds ratio.
Categories were based on a priori cutpoints.
Reference category (OR = 1).
Global P value for interaction.
Adjusted for the matching variables (age, race/ethnicity, sex, cohort, and date of blood draw). Conditional logistic regression was used for analyses stratified by sex and race/ethnicity.
Additionally adjusted for body mass index (weight (kg)/height (m)2) in World Health Organization categories (<18.5, 18.5–<25.0, 25.0–30.0, 30.0–<35.0, or ≥35.0, or missing data), smoking (never smoker, former smoker who had quit ≥15 years previously, former smoker who had quit 1–14 years previously, former smoker who had quit <1 year previously or current smoker of <20 cigarettes/day, and former smoker who had quit <1 year previously or current smoker of ≥20 cigarettes/day), and diabetes status (yes, no, or missing data).
Season of blood draw was defined as summer (June–November) versus winter (December–May).
In unconditional logistic regression models for analyses stratified by season, latitude, and smoking, results were additionally adjusted for matching factors. Numbers of cases and controls may differ slightly from overall numbers in analyses stratified by race/ethnicity and smoking because of missing data.
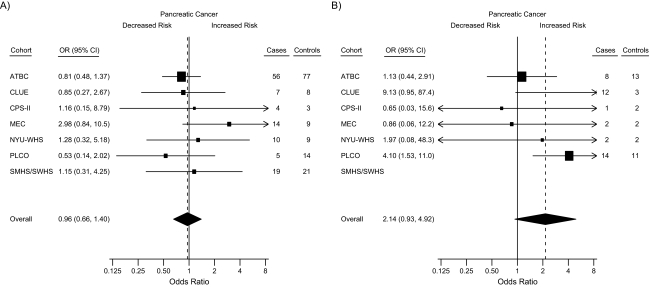
There was no significant heterogeneity among cohorts (Figure 1; Q statistic: P > 0.30). The odds ratios from the meta-analysis for high and low 25(OH)D status, as compared with the referent group, were 2.14 (95% CI: 0.93, 4.92) and 0.96 (95% CI: 0.66, 1.40), respectively. Although the magnitudes of the risks were similar, the pooled odds ratio (Table 3) was more precise (i.e., a narrower confidence interval) than the odds ratio from the meta-analysis (Figure 1).
Figure 1.
Forest plots for meta-analysis of the association between circulating 25-hydroxyvitamin D (25(OH)D) concentrations and risk of pancreatic cancer within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, 1974–2006. Odds ratios (squares) and 95% confidence intervals (bars) were derived from conditional logistic regression models adjusted for body mass index (weight (kg)/height (m)2) in World Health Organization categories (<18.5, 18.5–<25.0, 25.0–30.0, 30.0–<35.0, or ≥35.0, or missing data), smoking (never smoker, former smoker who had quit ≥15 years previously, former smoker who had quit 1–14 years previously, former smoker who had quit <1 year previously or current smoker of <20 cigarettes/day, and former smoker who had quit <1 year previously or current smoker of ≥20 cigarettes/day), and diabetes status (yes, no, or missing data). The size of each square is inversely proportional to the variance of the log odds ratio estimate in each cohort. The pooled estimates (diamonds) were derived from a meta-analysis using random-effects modeling. For each cohort, the plots show estimates for subjects with circulating 25(OH)D concentrations of A) <25 nmol/L and B) ≥100 nmol/L in comparison with the reference group (50–<75 nmol/L). ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, CI, confidence interval; CPS-II, Cancer Prevention Study II Nutrition Cohort; MEC, Multiethnic Cohort Study; NYU-WHS, New York University Women's Health Study; OR, odds ratio; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SM/WHS, Shanghai Men's Health Study and Shanghai Women's Health Study. SM/WHS data were excluded from the analysis of high 25(OH)D concentrations because of unstable estimates due to small numbers.
DISCUSSION
The findings from this pooled nested case-control study of data from 8 cohorts do not support the hypothesis that higher circulating vitamin D concentrations reduce the risk of pancreatic cancer. Indeed, a statistically significant 2-fold increased risk of pancreatic cancer was observed among subjects with circulating 25(OH)D concentrations greater than or equal to 100 nmol/L as compared with those with 25(OH)D concentrations of 50–<75 nmol/L. The positive association persisted when each cohort was excluded from the analysis in turn. The association was independent of race/ethnicity, sex, smoking history, obesity, and diabetes. Positive associations were observed in men, women, smokers, never smokers, and nonusers of multivitamin supplements. Although there were no statistically significant interactions, positive associations appeared more pronounced in Caucasians, participants residing in areas at latitudes above 35°N, and those who had their blood collected during the summer months. No significant associations were observed among participants with lower 25(OH)D status.
These pooled results are consistent with previously published findings from both the ATBC (27) and PLCO (28) cohorts, as all showed some evidence of positive associations, and effects appeared stronger among persons residing at high latitudes. In the previous nested case-control study conducted in the ATBC cohort, a population of male Finnish smokers, prediagnostic serum 25(OH)D concentrations greater than 65.5 nmol/L were associated with a nearly 3-fold increased risk of pancreatic cancer compared with concentrations of 32.0 nmol/L or less (OR = 2.92, 95% CI: 1.56, 5.48; P-trend = 0.001) (27). Although the previous PLCO nested case-control study showed an overall non-statistically significant 45% increased risk of pancreatic cancer for the highest fifth of 25(OH)D concentrations as compared with the lowest fifth, statistically significant 4-fold increased risks were observed with increasing 25(OH)D concentrations (>78.4 nmol/L vs. <49.3 nmol/L) only among participants living at northern US latitudes (i.e., Michigan, Minnesota, and Wisconsin) (28). Persons who live at low latitudes with warm, sunlit environments might maintain efficient vitamin D status throughout the year (28, 43). This could contribute to more within-person variability in ambulatory populations and attenuate risk estimates (28). Further, participants in the Shanghai and MEC studies, who lived at low latitudes, tended to have lower 25(OH)D concentrations overall (i.e., Shanghai) or because of race/ethnicity (e.g., African Americans). As a result, few participants residing at low latitudes had 25(OH)D concentrations greater than 100 nmol/L (Table 4; 5 cases and 8 controls), which limited our statistical power to observe associations.
The present analysis differs from the previous 2 studies (27, 28) in that clinically relevant vitamin D status cutpoints were used to examine associations, with 50–<75 nmol/L being used as the referent category, rather than quintiles based on the distribution among study controls (27, 28). In the current pooled analysis, a threshold pattern of association was observed, with an increased risk being seen only at the highest vitamin D concentrations (≥100 nmol/L), which corresponds to the top 2.3% of the distribution in the controls. In contrast, the previous ATBC Study showed a significant positive trend with increasing quintiles of vitamin D concentration (27). On average, men in the ATBC Study had lower 25(OH)D concentrations than those of the US-based cohorts (Table 1), and in a range where positive associations were not observed in the VDPP. Few ATBC participants were in the high 25(OH)D category (≥100 nmol/L). Excluding the ATBC participants from the present analysis increased the strength of the association (>100 nmol/L vs. 50–<75 nmol/L: OR = 2.95, 95% CI: 1.47, 5.93), because the ATBC men were removed from the referent category. Therefore, the increased pancreatic cancer risk with high vitamin D status in the present pooled study is not explained by the ATBC Study participants. The PLCO Study contributed 19% of the cases (n = 183) and 27% of the controls (n = 364), with both cases and controls having higher 25(OH)D concentrations than the other cohorts (Table 1). With the PLCO participants excluded, the association remained positive but nonsignificant because of reduced study power (OR = 1.60, 95% CI: 0.83, 3.08). Excluding both the ATBC and PLCO participants (52% of the cases and 67% of the controls) resulted in no change in the strength of the association, but the significance was diminished (OR = 2.23, 95% CI: 0.82, 6.08) also because of reduced statistical power. The VDPP's advantage for examining pancreatic cancer is the “pooling” of data from 8 cohorts covering multiple geographic regions, which in total includes a larger number of cases and a wider range of vitamin D concentrations than the individual studies alone and provides greater power to observe associations.
A major strength of the VDPP is the prospective design, thereby reducing the likelihood of reverse causality. In addition, the cohort study investigators followed a protocol for case-control selection, and 25(OH)D levels were measured centrally at the same laboratory (except in the ATBC Study). Conclusions concerning associations among Asians or African Americans separately are limited because of their small numbers in the present analysis. The measurement of serum 25(OH)D concentration reflects internal dose and actual vitamin D status and is considered more valid than evaluation of vitamin D intake alone or predictors of vitamin D status. Although a single measure of 25(OH)D in adulthood may not correspond to long-term vitamin D status, it can reflect exposure over the past several weeks or months (44). In addition, high intraclass correlations ranging from 0.70 to 0.78 have been observed in both men and women for measures taken over several years (45, 46), suggesting that 1 sample may provide a good measure of long-term status. Controls were matched to the cases by month of blood collection to reduce misclassification of vitamin D status due to seasonal variation. Residual confounding by cigarette smoking is not likely, because there was no significant interaction of the vitamin D association with smoking status, and positive associations were observed among never smokers. Both men and women were included in this study, as well as never, former, and current smokers; therefore, our results should be generalizable to Caucasian populations.
The biologic basis for the observed associations is speculative, because there is an incomplete understanding of the molecular mechanisms by which the vitamin D receptor–1,25-dihydroxyvitamin D3 (1,25(OH)2D3) complex regulates the expression of genes involved in pancreatic carcinogenesis. The active form of vitamin D is a steroid hormone, and it is likely that the vitamin D receptor–1,25(OH)2D3 complex affects and interacts with other regulators and endogenous hormones (47), particularly in an autocrine or paracrine manner within the pancreas and/or pancreatic tumor tissue. The active form of vitamin D, 1,25(OH)2D3, might influence growth factors (19, 20, 48) which promote tumor growth (27). Toxic effects of hypervitaminosis D are thought to be mediated through hypercalcemia, including calcification of soft tissues; however, the range of 25(OH)D levels associated with risk in this study was below that considered to reflect hypervitaminosis D (400–1,250 nmol/L) (49). Alternatively, a mechanism directly related to solar ultraviolet radiation or an unknown surrogate risk factor that is correlated with sun exposure is possible.
In conclusion, the previously reported positive association between 25(OH)D and pancreatic cancer observed in the earlier studies (27, 28) was confirmed in this large pooled study; however, the increased risk was observed only among subjects with the highest circulating 25(OH)D concentrations. Before any conclusions regarding vitamin D's potential role(s) in the etiology of pancreatic cancer can be reached, more research is needed, including prospective studies and laboratory investigations of biologically plausible mechanisms that may explain the observations. Given the present study's pooled results and research gaps in the understanding of vitamin D's role in carcinogenesis, recommendations to increase vitamin D concentrations in healthy persons for cancer prevention seem premature.
Acknowledgments
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Rachael Z. Stolzenberg-Solomon, Stephanie J. Weinstein, Mark P. Purdue, Kai Yu, Demetrius Albanes, Richard Hayes); Epidemiology Research Program, American Cancer Society, Atlanta, Georgia (Eric J. Jacobs, Alpa V. Patel, Marjorie L. McCullough); Department of Environmental Medicine, School of Medicine, New York University, New York, New York (Alan A. Arslan, Anne Zeleniuch-Jacquotte, Karen Koenig); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, School of Medicine, Vanderbilt University, Nashville, Tennessee (Dai Qi, Xiao-Ou Shu, Wei Zheng, Qiuyin Cai); Prevention and Research Center, Weinberg Center for Women's Health and Medicine, Mercy Medical Center, Baltimore, Maryland (Kathy J. Helzlsouer, Sandra Clipp); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Kathy J. Helzlsouer, Sandra Clipp); Information Management Services, Inc., Silver Spring, Maryland (Kirk Snyder, Lonn Irish); Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland (Jarmo Virtamo); Epidemiology Program, Cancer Research Center, University of Hawaii, Honolulu, Hawaii (Lynn R. Wilkins, Laurence N. Kolonel, Loic Le Marchand); Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland (Chinonye Harvey); and Heartland Assays, Inc., Ames, Iowa (Ronald L. Horst).
This work was supported by the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute (NCI) (Bethesda, Maryland) and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, NCI. The New York University Women's Health Study was supported by the NCI (grant R01 CA098661). The Multiethnic Cohort Study was supported by the NCI (grants R37 CA54281, P01 CA33619, R01 CA063464, and N01-PC35137). The Shanghai Men's Health Study was supported by the NCI (grant R01 CA82729). The Shanghai Women's Health Study was supported by the NCI (grants R37 CA70867 and N02-CP-11010-66). The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial was supported by contracts from the NCI to the University of Colorado, Denver, Colorado (grant N01-CN-25514); Georgetown University, Washington, DC (grant N01-CN-25522); the Pacific Health Research Institute, Honolulu, Hawaii (grant N01-CN-25515); the Henry Ford Health System, Detroit, Michigan (grant N01-CN-25512); the University of Minnesota, Minneapolis, Minnesota (grant N01-CN-25513); Washington University, St. Louis, Missouri (grant NO1-CN-25516); the University of Pittsburgh, Pittsburgh, Pennsylvania (grant N01-CN-25511); the University of Utah, Salt Lake City, Utah (grant N01-CN-25524); the Marshfield Clinic Research Foundation, Marshfield, Wisconsin (grant N01-CN-25518); the University of Alabama, Birmingham, Alabama (grant NO1-CN-75022); Westat, Inc., Rockville, Maryland (grant N01-CN-25476); and the University of California, Los Angeles, Los Angeles, California (grant NO1-CN-25404). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the NCI and US Public Health Service contracts (grants N01-CN-45165, N01-RC-45035, and N01-RC-37004). CLUE was supported by the National Institute on Aging (grant U01 AG018033) and the NCI (grant R01 CA105069 and K07 CA73790). The participation of CLUE investigators was also supported by an NCI contract awarded to Mercy Medical Center through the University of Hawaii (Honolulu, Hawaii). The Cancer Prevention Study II Nutrition Cohort was supported by the American Cancer Society (Atlanta, Georgia).
The authors thank Dr. Karen Phinney of the National Institute of Standards and Technology for providing the Vitamin D in Human Serum (SRM 972) used in this work.
Members of the VDPP Pancreatic Cancer Writing Committee: Rachael Z. Stolzenberg-Solomon, Eric J. Jacobs, Alan A. Arslan, Dai Qi, Alpa V. Patel, Kathy J. Helzlsouer, Stephanie J. Weinstein, and Laurence N. Kolonel.
This report is based at least in part on information provided by the Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene.
Dr. Ronald L. Horst is the president and chief executive officer of Heartland Assays, Inc. (Ames, Iowa).
Glossary
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
- CI
confidence interval
- CPS-II
Cancer Prevention Study II Nutrition Cohort
- MEC
Multiethnic Cohort Study
- NIST
National Institute of Standards and Technology
- NYU-WHS
New York University Women's Health Study
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- OR
odds ratio
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- SMHS
Shanghai Men's Health Study
- SWHS
Shanghai Women's Health Study
- VDPP
Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
References
- 1.Grant WB. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UVB irradiance and smoking. Int J Cancer. 2007;120(5):1123–1128. doi: 10.1002/ijc.22386. [DOI] [PubMed] [Google Scholar]
- 2.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. (doi: 10.1186/1471-2407-6-264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuohimaa P, Pukkala E, Scélo G, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer. 2007;43(11):1701–1712. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004;87(5):532–538. doi: 10.1097/01.hp.0000137179.03423.0b. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita S, Wagatsuma Y, Okada M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int J Health Geogr. 2007;6:34. doi: 10.1186/1476-072X-6-34. (doi: 10.1186/1476-072X-6-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 7.Neale RE, Youlden DR, Krnjacki L, et al. Latitude variation in pancreatic cancer mortality in Australia. Pancreas. 2009;38(4):387–390. doi: 10.1097/MPA.0b013e31819975f4. [DOI] [PubMed] [Google Scholar]
- 8.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89(5):552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 10.Office of Dietary Supplements, National Institutes of Health. Dietary Supplement Fact Sheet: Vitamin D. Bethesda, MD: National Institutes of Health; 2008. [Google Scholar]
- 11.Bland R, Markovic D, Hills CE, et al. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90(1–5):121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 12.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GG, Eads D, Rao A, et al. Pancreatic cancer cells express 25-hydroxyvitamin D-1α-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 2004;25(6):1015–1026. doi: 10.1093/carcin/bgh086. [DOI] [PubMed] [Google Scholar]
- 14.Zugmaier G, Jäger R, Grage B, et al. Growth-inhibitory effects of vitamin D analogues and retinoids on human pancreatic cancer cells. Br J Cancer. 1996;73(11):1341–1346. doi: 10.1038/bjc.1996.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersson F, Colston KW, Dalgleish AG. Differential and antagonistic effects of 9-cis-retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br J Cancer. 2000;83(2):239–245. doi: 10.1054/bjoc.2000.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GG, Eads D, Naczki C, et al. 19-nor-1α,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther. 2008;7(3):430–436. doi: 10.4161/cbt.7.3.5418. [DOI] [PubMed] [Google Scholar]
- 17.Kawa S, Yoshizawa K, Tokoo M, et al. Inhibitory effect of 22-oxa-1,25-dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology. 1996;110(5):1605–1613. doi: 10.1053/gast.1996.v110.pm8613068. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu C, Gysemans C, Giulietti A, et al. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 19.Maestro B, Campión J, Dávila N, et al. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47(4):383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 20.Maestro B, Dávila N, Carranza MC, et al. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2-3):223–230. doi: 10.1016/s0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 21.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 22.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud DS, Wolpin B, Giovannucci E, et al. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2101–2109. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 24.Hennig R, Ding XZ, Adrian TE. On the role of the islets of Langerhans in pancreatic cancer. Histol Histopathol. 2004;19(3):999–1011. doi: 10.14670/HH-19.999. [DOI] [PubMed] [Google Scholar]
- 25.Skinner HG, Michaud DS, Giovannucci E, et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 27.Stolzenberg-Solomon RZ, Vieth R, Azad A, et al. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res. 2006;66(20):10213–10219. doi: 10.1158/0008-5472.CAN-06-1876. [DOI] [PubMed] [Google Scholar]
- 28.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the Prostate, Lung, Colorectal, and Ovarian Screening Trial. Cancer Res. 2009;69(4):1439–1447. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 30.Helzlsouer KJ, Alberg AJ, Huang HY, et al. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(6):525–532. [PubMed] [Google Scholar]
- 31.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 32.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87(3):190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 33.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes RB, Sigurdson A, Moore L, et al. Methods for etiologic and early marker investigations in the PLCO Trial. Mutat Res. 2005;592(1-2):147–154. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Chow WH, Yang G, et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 36.Lee SA, Xu WH, Zheng W, et al. Physical activity patterns and their correlates among Chinese men in Shanghai. Med Sci Sports Exerc. 2007;39(10):1700–1707. doi: 10.1249/mss.0b013e3181238a52. [DOI] [PubMed] [Google Scholar]
- 37.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Fears TR, Ziegler RG, Donaldson JL, et al. Reproducibility studies and interlaboratory concordance for androgen assays in female plasma. Cancer Epidemiol Biomarkers Prev. 2000;9(4):403–412. [PubMed] [Google Scholar]
- 39.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 40.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 41.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallicchio L, Helzlsouer KJ, Chow W-H, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010 doi: 10.1093/aje/kwq116. 172(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimlin MG. The climatology of vitamin D producing ultraviolet radiation over the United States. J Steroid Biochem Mol Biol. 2004;89-90(1–5):479–483. doi: 10.1016/j.jsbmb.2004.03.111. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr. 1990;120(suppl 11):1464–1469. doi: 10.1093/jn/120.suppl_11.1464. [DOI] [PubMed] [Google Scholar]
- 45.Zeleniuch-Jacquotte A, Gallicchio L, Hartmuller V, et al. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010 doi: 10.1093/aje/kwq114. 172(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platz EA, Leitzmann MF, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 47.Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7 doi: 10.1621/nrs.07001. e001. (doi: 10.1621/nrs.07001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gómez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7(2):125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- 49.Dietary Reference Intakes: For Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press; 1997. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Vitamin D; pp. 250–287. [Google Scholar]