Abstract
Rationale
The proepicardial organ (PE) contributes to the cellular diversity of the developing heart by giving rise to the epicardium as well as vascular smooth muscle cells and fibroblasts. Despite the importance of these cells in cardiac development, function and regeneration, the signals required for the specification of the PE remain largely unexplored.
Objective
We aim to identify the signaling molecules and transcription factors that regulate PE specification.
Methods and Results
Here, we present the first genetic evidence that Bmp signaling in conjunction with the T-box transcription factor Tbx5a is essential for PE specification in zebrafish. Specifically, Bmp4 from the cardiac region, but not the liver bud, acting through the type I BMP receptor Acvr1l, is required for PE specification. By overexpressing a dominant-negative form of a Bmp receptor at various embryonic stages, we determined when Bmp signaling was required for PE specification. We also found that overexpression of bmp2b right before PE specification led to the ectopic expression of PE specific markers including tbx18. Furthermore, using loss-of-function approaches, we discovered a previously unappreciated PE specification role for Tbx5a at early somite stages; this role occurs earlier than, and appears to be independent from, the requirement for Bmp signaling in this process.
Conclusion
Altogether, these data lead us to propose that Tbx5a confers anterior lateral plate mesodermal cells the competence to respond to Bmp signals and initiate PE development.
Keywords: proepicardial organ (PE), tbx18, tcf21, acvr1l, tbx5, zebrafish
Introduction
An increasing body of evidence has revealed that the PE contributes cells to the coronary vessels 1–6 and thus plays a critical role in cardiac development and function. In amniotes, the PE is an extracardiac grape-like structure associated with the septum transversum and located near the venous pole of the heart 7–9. Cells of the PE migrate to the postlooped heart to form its outermost layer, the epicardium. Subsequently, a fraction of the epicardial cells give rise to the subepicardial mesenchyme through an epithelial-mesenchymal transition (EMT) 1, 4, 10, 11. These epicardial-derived mesenchymal cells invade the heart and differentiate into coronary vascular smooth muscle cells, perivascular fibroblasts and intermyocardial fibroblasts 1–7.
Recent data indicate that the epicardium plays a critical role in cardiac regeneration in zebrafish. The epicardium re-activates expression of developmental genes upon partial amputation of the adult heart and participates in cardiac regeneration at least in part by apparently contributing to the new vasculature in the regenerating myocardium 12. Another study has shown that epicardial cells can be efficiently differentiated into cardiomyocytes in vitro 13, and two recent reports 14, 15 have claimed that the epicardium can give rise to cardiomyocytes during mouse embryonic development. Thus, the epicardium has generated substantial interest recently, especially as a potential source of new cardiomyocytes during homeostatic repair.
In addition to providing various cell types to the developing heart, the epicardium has important regulatory functions in the development of the myocardium 16–20. Targeted deletion of VCAM-1 or α4 Integrin results in the absence of the epicardial layer in the mouse heart 17, 18. This defect in epicardial formation in turn causes a significant reduction in the thickness of the ventricular myocardium. Migration of PE cells to form the epicardium appears to require the activity of the T-box transcription factor Tbx5 21. In chick embryos, PE cells transfected with morpholino antisense oligonucleotides for Tbx5 failed to migrate out of the PE 21. Interestingly, an epicardial specific knockout of the retinoid X receptor α (RXRα) gene also leads to the thinning of the ventricular myocardial wall 22. Thus, it has been proposed that the epicardium is a source of secreted mitogenic factors that promote cardiomyocyte proliferation. The epicardial derived signals include Fibroblast growth factors, such as Fgf9, whose expression appears to be regulated by retinoic acid signaling 23.
The zebrafish proepicardium, like that of higher vertebrates, is a transient epithelial structure that delivers mesothelial cells to form the epicardium 24. Despite the importance of the PE in coronary and cardiac development, little is known about the molecular mechanisms that govern its initial appearance or specification. In mouse embryos, Gata4 is highly expressed in the PE and genetic ablation of Gata4 results in a complete absence of PE formation 25. PE induction also seems to involve inductive signal(s) from neighboring tissues. In the chick embryo, the PE is closely apposed to the liver bud. Quail-to-chick transplantation data indicate that the liver bud has the capacity to induce ectopic PE marker gene expression when implanted into the posterior lateral plate mesoderm 26. Nevertheless, it is unclear whether the liver primordium is necessary for PE induction. And if it is, the exact nature of the liver bud-derived signal(s) required for PE specification also remains to be identified. In this study, we provide genetic evidence for a role for myocardium (but not liver bud) derived Bmp and the T-box transcription factor Tbx5a in PE specification in zebrafish. Loss of bmp4 activity causes a significant reduction in PE marker gene expression. Likewise, PE specification is severely compromised in acvr1l (aka alk8) mutants as well as in embryos overexpressing a dominant-negative form of a Bmp receptor right before PE specification. We further show that tbx5a mutants also have severe defects in PE specification. To delineate the temporal requirement for Tbx5a in PE development, we generated a dominant negative form of Tbx5a under the heat shock promoter, and used it to reveal that Tbx5a is required at early somite stages for this process. Furthermore, our data suggest that Tbx5a functions earlier than, and independently from, the requirement for Bmp signaling in PE specification. Thus, we propose that Tbx5a activity in the anterior lateral plate mesoderm confers competence to a subset of these cells to respond to Bmp signals and initiate PE development.
Materials and Methods
Zebrafish strains
Embryos and adult fish were raised and maintained under standard laboratory conditions 27. The mutant and transgenic lines used in this study are as follows: acvr1ltm110b 28, 29, bmp4 st72 30, hanc99 31, tbx5am21 32, wnt2bbs403 33, hnf1bahi2169 34, Tg(hsp70l:dnBmpr-GFP)w30 35, and Tg(hsp70:bmp2b) fr13 36.
Generation of a stable Dominant-negative tbx5a transgenic line
The truncated version of zebrafish tbx5a was amplified with the primers AGATCTATGGCGGACAGTGAAGACACC and GCGGCCGCTCAGAACGGGCTCTGACTGCT. This fragment was fused in frame with a myc tagged engrailed repressor domain 37 and then placed downstream of the hsp70 promoter 38.
The uncut DNA was co-injected with I-sceI enzyme into one cell stage AB embryos 39. F1 founders were screened for green fluorescent protein (GFP) expression in the eye driven by the crystallin promoter in the vector, and stable transgenic lines were generated.
Heat shock conditions
For the heat shock experiments, embryos obtained from outcrossing a hemizygous Tg(hsp70:dnBmpr-GFP)w30 fish were heat shocked around 36 hpf for 25 minutes at 40°C. After heat shock, the embryos were allowed to further develop in a 28°C incubator, and harvested for fixation at 57 hpf. Since GFP expression is maintained in these embryos for at least 24 hours after heat shock, hemizygous embryos expressing GFP were easily identifiable. Similarly, embryos from Tg(hsp70:dntbx5a)f23 outcross were heat shocked around 10 hpf or at later stages for 20 minute at 39.5°C. The embryos were allowed to further develop until harvested at 57 hpf for fixation. After in situ hybridization, the embryos were genotyped for the presence of the hsp70:dntbx5 transgene using the following primers: 5′-ATGAGCTCCAAGATGAGCGATGC-3′ and 5′-GACACCAGTTGCCTCATCCAG-3′. To hyperactivate Bmp signaling, embryos obtained from outcrossing a hemizygous Tg(hsp70:bmp2b) fr13 were heat shocked for 30 minute at 37°C. After in situ hybridization, genotyping for the presence of the hsp70:bmp2b transgene was carried out using the primers: 5′-CATGTGGACTGCCTATGTTCATC-3′ and 5′-GAGAGCGCGGACCACGGCGAC-3′.
In situ hybridization and RNA injections
Single or double whole-mount in situ hybridizations were performed as previously described40, using the following probes: tbx18 41, tcf21 24, cmlc2 42, tbx5 32, 43, 44 bmp4 45,46, and acvr1l29.
20 picograms of tcf21 mRNA was injected into one-cell stage wildtype or acvr1l mutant embryos. The acvr1l mutant embryos were easily identifiable by the lack of pectoral fin buds.
Results
Development of the proepicardium in zebrafish
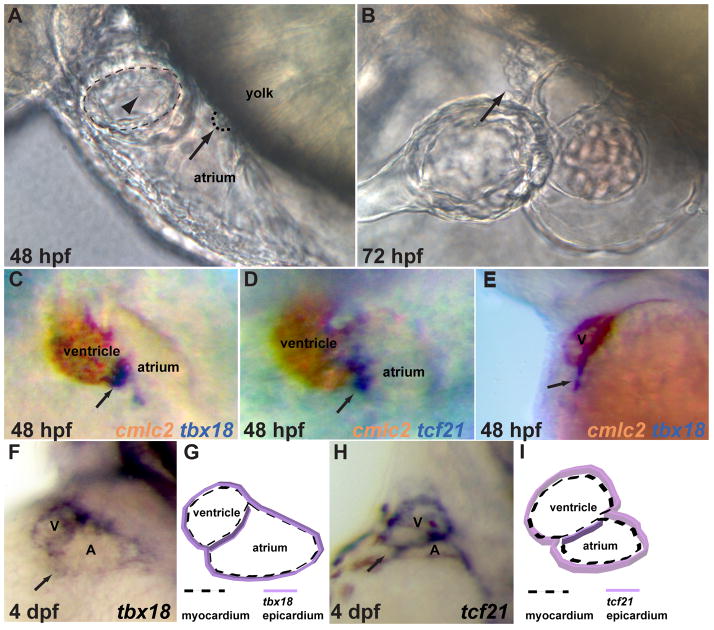
It is well documented that the PE in avian and mouse embryos becomes morphologically distinguishable as a cluster of cells located in close proximity to the sinus venosus of the looping-stage heart 7–9. To examine the progression of PE development in zebrafish embryos, we focused on the heart region and found that an extracardiac cluster of spherical cells which bears morphological resemblance to the PE was first detectable by light microscopy around 48 hpf. This cluster appears at the pericardial surface of the yolk and is located at the level of the atrioventricular junction 24 (Fig. 1A). By 72 hpf, this cluster has grown in size and has come in contact with the myocardium to initiate the formation of the epicardium (Fig. 1B).
Fig 1.
Proepicardial organ formation in zebrafish. A–B) Differential interference contrast (DIC) images of lateral views of the cardiac region. A) By 48 hpf, a grape like cluster of cells (arrow), characteristic of the proepicardium, is visible between the yolk and atrium and is located at a level just caudal to the atrioventricular junction (arrowhead). B) By 72 hpf, the proepicardial organ has grown in size and is in close contact with the ventricle. C–E) 48 hpf embryos were stained for expression of the proepicardial marker genes (purple) tbx18 (C, E), and tcf21 (D) together with the myocardial marker cmlc2 (brown). Ventral (C, D) and lateral (E) views show that tbx18 and tcf21 positive proepicardial cells (arrows) appear in between the developing atrium and ventricle. (F–I) Lateroventral views of 4 dpf larvae (F, H) with tbx18 (F) and tcf21 (H). At 4 dpf, the tbx18 (F, G) and tcf21 (H, I) positive cells are no longer organized in a cluster, but rather spread over the heart to form the epicardial layer (see also cartoons G, I). V (ventricle), A (atrium).
To determine the identity of this extracardiac cell population, we examined the expression of the common PE marker genes tbx18 26, 41, 47 and tcf21 24, 26 relative to that of the myocardial marker cmlc2 42 using double in situ hybridization. At 48 hpf, tbx18 and tcf21 expression is detected in the region between the developing atrium and ventricle (Fig. 1C, D). Lateral views at the same stage show that the tbx18 positive cells appear close to the atrioventricular junction and adjacent to the pericardial cavity (Fig. 1E). No expression of tbx18 or tcf21 is observed in the heart region at 36 hpf (data not shown). By 4 dpf, the tbx18 and tcf21 positive cells are no longer organized in clusters, but appear to be spread over the heart to cover the myocardium (Fig. 1F, G, H, I). Therefore, the expression pattern of the PE marker genes tbx18 and tcf21 corresponds precisely to the physical appearance of the extracardiac cell population, indicating their proepicardial identity.
Bmp signaling is essential for PE specification
In vitro culture studies have suggested that Bmp signaling regulates the differentiation of the proepicardial cells 13, 48. In chick embryos, reducing Bmp signaling levels by implantation of Noggin soaked beads into the sinus venosus region appears to reduce PE marker gene expression 48. However, the Bmp ligand and receptor potentially implicated in PE formation have not been identified. In order to determine whether Bmp signaling is indeed required for PE development, we first examined PE formation in acvr1l (activin A receptor, type I like) zebrafish mutants. acvr1l encodes a type I Bmp receptor and it is is expressed both maternally and zygotically 29. Zygotic acvr1l mutants display a weak dorsalized phenotype as shown by the absence of ventral tail fin formation 28, 29. We found that in acvr1l mutants the expression of tbx18 and tcf21 in the heart region was completely absent, whereas tbx18 expression in the pectoral fin and tcf21 expression in the pharyngeal arch appeared unaffected (Fig. 2B, D).
Fig 2.
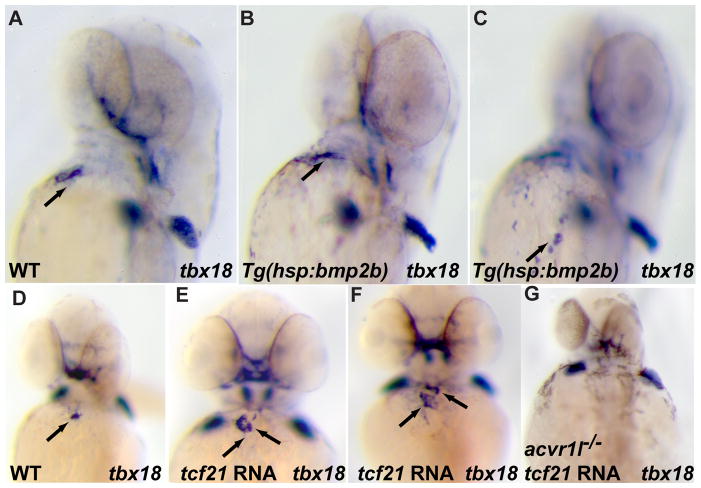
Bmp signaling is essential for proepicardial cell specification. acvr1l mutant embryos (B, D) and their wildtype siblings (A, C) were harvested at 57 hpf and the expression of tbx18 (A, B) and tcf21 (C, D) examined by whole mount in situ hybridization. The expression of tbx18 or tcf21 in the cardiac region (arrows) is completely absent in acvr1l mutants, whereas the expression in the pectoral fin (tbx18) and pharyngeal arch (tcf21) appears unaffected. (E–L) Embryos from a hemizygous Tg(hsp70l:dnBmpr-GFP)w30 outcross were heat-shocked at 36 hpf, and harvested and analyzed at 57 hpf. Heat shocked transgenic embryos had severe reduction (F, H, J, L; 17/23 for tbx18, 19/27 for tcf21) or loss (G, H, K, L; 6/23 for tbx18, 8/27 for tcf21) of expression of the proepicardial markers compared to their heat shocked non-transgenic siblings (E, H, I, L). The out of focus, more dorsal expression of tcf21 marks the pharyngeal arches.
In order to define the temporal requirement for Bmp signaling in the specification of the PE and circumvent the function of Bmp signaling in dorsoventral patterning, we heat shocked hemizygous Tg(hsp70l:dnBmpr-GFP)w30 embryos 35 and their wildtype siblings at 36 hpf, a stage before the formation of the PE and the onset of PE marker gene expression. We found that when examined at 57 hpf the expression of tbx18 and tcf21 was significantly reduced in the heart region of the heat-shocked transgenic embryos (Fig. 2F, G, H, J, K, L, arrows. Severe reduction: 17/23 for tbx18, 19/27 for tcf21; almost absent 6/23 for tbx18, 8/27 for tcf21) compared to their heat-shocked wildtype siblings. These data indicate that Bmp signaling occurring after 36 hpf is essential for PE specification.
In order to determine whether Bmp signaling is sufficient to induce PE marker gene expression, we overexpressed bmp2b by heat shocking hemizygous Tg(hsp70:bmp2b) fr13 embryos 36 at 36 hpf to hyperactivate Bmp signaling. In embryos with ubiquitous bmp2b expression, we observed ectopic tbx18 expression (19/25) around the heart region, whereas tcf21 expression appeared unaffected (Fig. 3B, C and data not shown). The weak PE-inducing activity of Bmp2b signaling suggests that it might cooperate with other signaling pathways to promote PE formation. This observation is consistent with a prior study showing that the liver bud, but not the lung bud, has the capacity to induce ectopic PE marker gene expression although both primordia express high levels of Bmp family member genes 26. The lack of response of tcf21 to bmp2b overexpression prompted us to examine whether tcf21 overexpression could induce tbx18 expression in the heart region. To this end, we injected 20 pg of tcf21 mRNA into one-cell stage wildtype embryos, and analyzed them at 57 hpf. Interestingly, overexpression of tcf21 expanded tbx18 expression in the heart region. In about 60% (16/25) of the tcf21 overexpressing embryos, two clusters of tbx18 positive cells were observed, each of which was about the same size as the cluster seen in wildtype embryos. Since both Bmp signaling and tcf21 can induce ectopic tbx18 expression, we wanted to investigate the relationship between Bmp signaling and tcf21 in regulating tbx18 expression. For this purpose, we injected tcf21 mRNA into embryos from acvr1l heterozygote incrosses and examined tbx18 expression at 57 hpf. We found that overexpression of tcf21 in embryos that lacked pectoral fin formation, a phenotype observed in acvr1l mutants, did not rescue the loss of tbx18 expression in the heart region, suggesting that the tbx18 inductive properties of Tcf21 rely on Bmp signaling.
Fig 3.
Expansion of tbx18 expression in the cardiac region by overexpression of bmp2b or tcf21. (A–C) Embryos from a hemizygous Tg(hsp70:bmp2b)fr13 outcross were heat-shocked at 36 hpf, and harvested and analyzed at 57 hpf. The expression of tbx18 in the cardiac region was expanded (19 expanded/25 scored, arrow in C points to ectopic tbx18 expression) in the heat shocked transgenic embryos (B, C) compared to their heat shocked non-transgenic siblings (A). (The same embryo is shown in (B) and (C), but at different focal planes.) (E–G) 20 pg of tcf21 mRNA was injected into one-cell stage wildtype (E, F) or acvr1l mutant (G) embryos, which were harvested at 57 hpf and processed for whole mount in situ hybridization for tbx18. Overexpression of tcf21 mRNA significantly expanded tbx18 expression (arrows) in the cardiac region of wildtype embryos (16 increased/25 scored) (E, F) but was not able to rescue the loss of tbx18 expression in acvr1l mutant embryos (G) compared to wildtype (D).
The liver is not required for PE induction in zebrafish
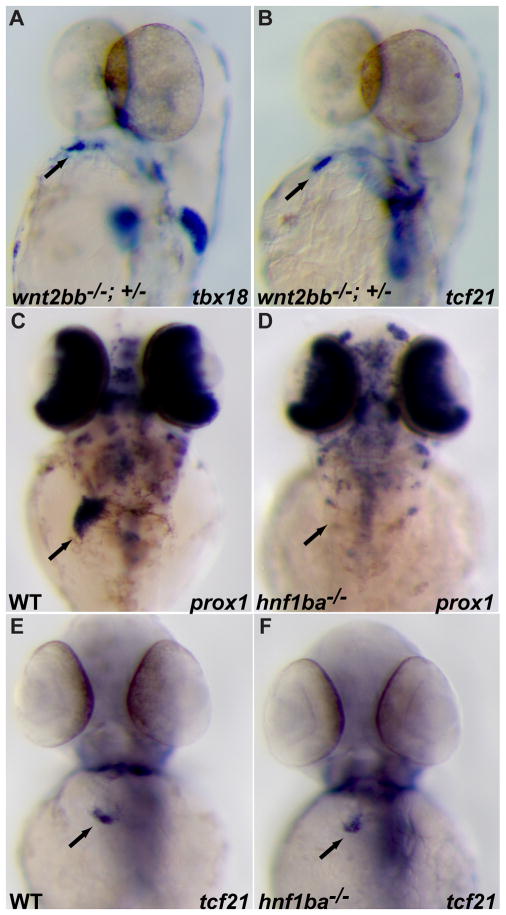
A prior study has shown that the liver bud can induce ectopic PE marker gene expression in chick embryos 26; however, it remains unclear whether the hepatic tissue is essential for PE induction. Attempts to address this issue genetically have been hampered by the fact that in addition to a complete absence of liver formation, loss of Foxa1;Foxa2 function in mouse embryos also causes a significant developmental delay of many internal organs at a stage when the PE starts to form 49. In zebrafish, wnt2bb has been recently identified as a positive regulator of hepatoblast specification 33. In wnt2bb mutants, development of the liver is significantly delayed. To explore a potential inductive role of the liver primordium on PE formation, we crossed a wnt2bb−/− female with a wnt2bb+/− male and analyzed PE marker gene expression at 57 hpf. Interestingly, tbx18 and tcf21 expression in the heart region appear unaffected (Fig. 4A, B). Since some hepatoblasts are present in wnt2bb mutants at 57 hpf 33, we examined hnf1ba (previously known as tcf2, hnf1b, vhnf1) mutants in order to further test the requirement of the liver bud in PE formation. The expression of the earliest hepatic marker genes prox1 and hhex is undetectable in the liver region of hnf1bahi2169 mutants at 57 hpf (Fig. 4D and data not shown), indicating an absence of liver bud formation 34. However, tbx18 and tcf21 are expressed at a wildtype level in the heart region of these embryos (Fig. 4G, H and data not shown), strongly arguing against an inductive role for the liver bud in PE formation in zebrafish.
Fig 4.
Proepicardial organ formation in zebrafish is not dependent on signals from the liver primordium. (A, B) Embryos obtained from crossing a wnt2bb+/−male with a wnt2bb−/− female were harvested at 57 hpf and examined for tbx18 (A) and tcf21 (B) expression. 28 out of 29 and 8 out of 8 embryos showed wildtype expression of tbx18 (A) or tcf21 (B) in the cardiac region, respectively. (C–F) 57 hpf wildtype and hnf1b mutant embryos were analyzed for prox1 (C, D) or tcf21 (E, F) expression. Although expression of the liver marker gene prox1 (D) is completely absent in hnf1b mutants, the proepicardial marker gene tcf21 (F) is expressed at wildtype levels.
Bmp4 is required for PE Specification
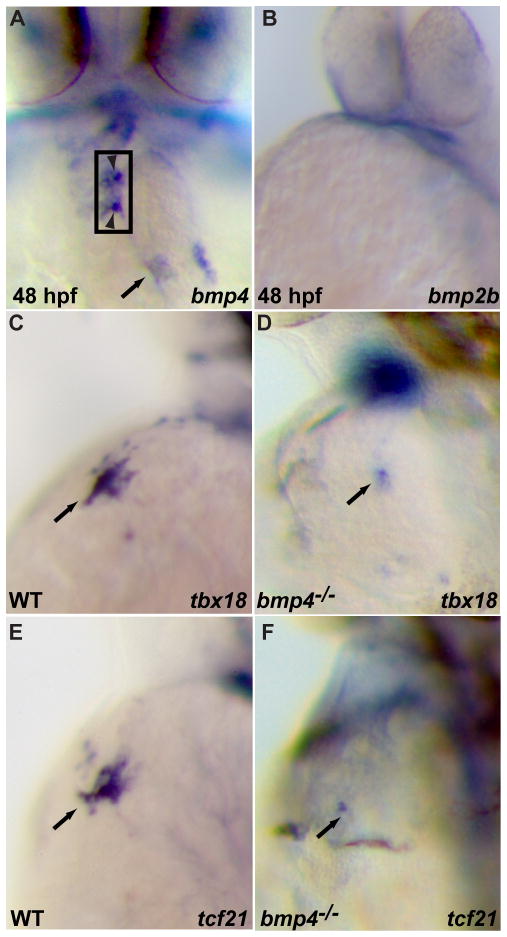
In chick and mouse embryos, the PE is closely apposed to the liver primordium and the sinoatrial myocardium; thus, both tissues have been postulated to play a role in PE induction. In zebrafish embryos, although the PE does not appear to be in close proximity to the liver when it arises, it does not rule out the possibility that the PE progenitors are in contact with the hepatoblasts at an earlier developmental time. Since our genetic data indicate that signal(s) from the liver bud are not essential for PE specification, we focused on the heart region to identify the Bmp ligand(s) involved in this process. We examined the expression pattern of several Bmp family members at the time of PE formation and earlier. bmp2a and bmp2b appear not to be expressed in the heart at 36 hpf and 48 hpf (Fig. 5A, B, and data not shown). In contrast, we found that bmp4 was expressed in the heart, mainly in the outflow tract, atrioventricular junction and sinoatrial myocardium by 48 hpf. The expression of bmp4 in the cardiac region (Fig. 5A arrow and arrowheads) is consistent with a potential role in PE specification. To determine whether bmp4 is involved in PE formation, we examined PE marker gene expression in bmp4 mutants and found a severe reduction of tbx18 and tcf21 expression in the heart region at 57 hpf (Fig. 5D, F) (19/21 for tbx18 and 16/17 for tcf21), whereas their expression in the neighboring tissues did not appear to be affected (data not shown). These data indicate that bmp4 regulates tbx18 and tcf21 expression in the heart region. To determine whether the effect of Bmp4 could be mediated through the Bmp receptor Acvr1l, since PE marker gene expression in the cardiac region is completely absent in acvr1l mutant embryos, we examined the expression pattern of acvr1l in wildtype embryos of 48 hpf, and found that acvr1l appears to be ubiquitously expressed in the cardiac region as well as neighboring tissues although its expression level seems to be relatively low in the cardiac region (data not shown).
Fig 5.
Bmp4 is required for proepicardial cell specification. (A–B) whole mount in situ hybridization of 48 hpf embryos for bmp4 (A) and bmp2b (B). bmp4 is expressed in the cardiac outflow tract, atrioventricular myocardium (between arrowheads) and inflow tract (arrow) regions (A), whereas bmp2b (B) does not appear to be expressed in the cardiac region around 48 hpf. (C–F) bmp4 mutant embryos and their wildtype siblings were harvested at 57 hpf and processed for in situ hybridization for tbx18 (C, D) or tcf21(E, F). tbx18 (D) and tcf21 (F) expression in the cardiac region is severely reduced in bmp4 mutants (19 reduced/21 scored for tbx18, and 16 reduced/17 scored for tcf21) compared to their wildtype siblings (C, E).
Hand2 and Tbx5a are essential for PE formation
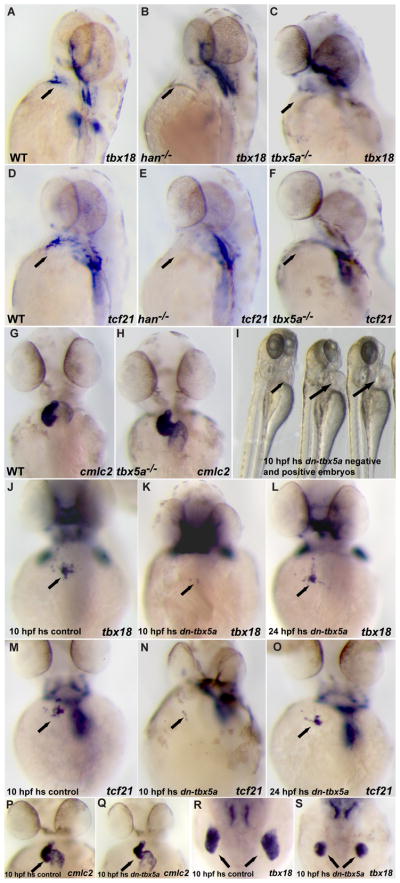
Lineage tracing studies suggest that the myocardial and PE cells likely share a common embryonic origin. In mouse embryos, it has been shown that virtually all cells of the developing heart, including the myocardial, endocardial and epicardial cells, are derived from Mesp1 expressing mesodermal cells 50–52. However, the genetic program that drives the diversification of the various heart cell lineages remains poorly understood. In zebrafish, hand2 is expressed in the lateral plate mesoderm (LPM) and appears to be critical for the differentiation of the LPM derivatives, such as the myocardium and pectoral fin 31. In hand2 mutants, the formation of the pre-cardiac mesoderm delineated by nkx2.5 expression appears unaffected; however myocardial differentiation is severely disrupted. Interestingly, we found that PE formation, as assessed by tbx18 and tcf21 expression is completely absent in hand2 mutants (Fig. 6B, E). These data suggest that hand2 might also be required for the differentiation of mesodermal cells into PE cells. However, given the severe cardiac malformations in hand2 mutants, it is possible that the observed absence of PE formation is secondary to the myocardial defects.
Fig 6.
Hand2 and Tbx5a are required for the induction of proepicardial marker gene expression. (A–F) 52 hpf wildtype, hand2 or tbx5a mutant embryos were analyzed for tbx18 (A–C) or tcf21 (D–F) expression. tbx18 (B, C) and tcf21 (E, F) expression (arrows) is completely absent in hand2 (B, E) and tbx5a (C, F) mutants. (G, H) cmlc2 expression in 52 hpf tbx5a mutant embryos and their wildtype siblings. Cardiac components including the ventricle, atrium, and inflow tract are formed properly although cardiac looping is delayed. (H–O) Tg(hsp70l:dntbx5a)f32 hemizygous embryos heat shocked at 10 hpf showed mild cardiac edema (H, arrows) with relatively normal cardiac morphogenesis (P, Q) and reduction of the pectoral fins (R, S) by 52 hpf. These embryos also exhibited a significant reduction of PE marker gene expression (K, N; 18/22 for tbx18 and 21/25 for tcf21). By contrast, Tg(hsp70l:dntbx5a)f32 hemizygous embryos exhibited wildtype levels of tbx18 and tcf21 expression when heat shocked at 24 hpf (L, O).
The T-box transcription factor Tbx5a has also been reported to play a critical role in cardiac and pectoral fin development in zebrafish 32. Similar to hand2, tbx5a is expressed in the lateral plate mesoderm, but its expression is restricted to a narrower domain extending from the pre-cardiac mesoderm to the pectoral fin bud 43. The major cardiac morphogenetic defect of tbx5a mutants at the stage when the PE starts to form in wildtype is a failure to complete cardiac looping (Fig. 6G, H), but otherwise the mutant heart appears morphologically intact before it eventually deteriorates to exhibit a string-like morphology 32. These milder cardiac phenotypes observed in zebrafish tbx5a mutants are likely due to functional redundancy between tbx5a and its newly identified paralogue tbx5b53. In order to determine whether tbx5a function is required for PE formation, we examined the expression of PE marker genes at 52 hpf and found that the expression of tbx18 and tcf21 in the heart region was barely detectable in tbx5a mutant embryos (Fig. 6C, F). Altogether, these data suggest that tbx5a might have a direct function for PE specification, rather than affecting PE formation indirectly through its role in myocardial development. To further explore tbx5a function in PE formation, we generated a putative dominant negative form of Tbx5a by fusing the Drosophila Engrailed repressor domain to a truncated Tbx5a containing its N terminus and T-box domains 37, 54. This construct was placed under the control of a heat-shock promoter to generate transgenic lines 35, 55. We heat shocked hemizygous Tg(hsp70l:dntbx5a)f23 embryos at various developmental stages and found that overexpression of the dominant negative Tbx5a around 10 hpf resulted in mild cardiac edema with relatively normal heart morphogenesis and reduction in the size of the pectoral fin at 52 hpf (Fig. 6I, compare the right two embryos with their wildtype sibling on the left, and Fig. 6P, Q, R, S). These data are consistent with a role for tbx5a in cardiac and pectoral fin development. Importantly, these embryos also showed a significant reduction in the PE marker gene expression (Fig. 6K, N; 18/22 for tbx18 and 21/25 for tcf21). By contrast, Tg(hsp70l:dntbx5a)f23 embryos exhibited wildtype levels of tbx18 and tcf21 expression when heat shocked after 24 hpf (Fig. 6L, O). This requirement for Tbx5a at early somite stages in PE specification is in sharp contrast to its role in PE cells migration towards the myocardium 21. Thus, we have revealed a previously unappreciated role for tbx5a during PE formation, and propose that tbx5a functions at early somite stages to provide competence to a subset of lateral plate mesodermal cells to differentiate into the PE lineage.
The above data show that manipulating Bmp signaling and Tbx5a activity both affect PE marker gene expression. These observations raise the possibility that Bmp signaling and Tbx5a might function in the same pathway to regulate PE specification. To test this possibility, we monitored the expression of bmp4 in the cardiac region including the atrioventricular and sinoatrial myocardium of tbx5a mutants. However, we did not find any significant changes in the level or pattern of bmp4 expression in the mutants (Fig. 7A, B). Conversely, we examined tbx5a expression in acvr1l mutants at early somite stages since tbx5 is required at these stages for PE specification. The expression of tbx5a in the lateral plate mesoderm in acvr1l mutants at the 10-somite stage appears unaffected compared to wildtype (Fig. 7C, D). These data suggest that Bmp signaling and Tbx5a function independently and further corroborate the idea that they are required at different times to regulate PE formation.
Fig 7.
Tbx5a and Bmp signaling may act independently during PE specification. (A, B) 57 hpf wildtype and tbx5a mutant embryos analyzed for bmp4 expression. bmp4 expression in tbx5a mutants (B) does not appear to be reduced in the outflow tract (white arrowhead), atrioventricular myocardium (between arrowheads ) or inflow tract (arrow) regions compared to wildtype (A). (C) Schematic representation of the heart and bmp4 expression. (D, E) 12 somite stage (15 hpf) wildtype and acvr1l mutant embryos analyzed for tbx5a expression. tbx5a expression in the lateral plate mesoderm of acvr1l mutant embryos (E) is not significantly altered compared to wildtype (D).
Discussion
The PE is known to be the primary extracardiac source for several cardiac cell types including cardiac fibroblasts and coronary smooth muscle cells 1–6. In addition, the PE derived epicardium secretes mitogenic factors that promote ventricular cardiomyocyte proliferation during development. In this study, we focused on the earliest step of PE development and presented genetic evidence that Bmp signaling is essential for PE specification in zebrafish. We also revealed a critical role for Tbx5 in PE specification. Loss of Tbx5 function leads to the absence of PE formation. Based on its expression pattern and the heat shock experiments that blocked Tbx5 function at various stages, we propose that Tbx5 is required at early somite stages to provide competence to a subset of tbx5 expressing mesodermal cells to become PE cells at a later developmental stage.
Evolutionary conservation and differences during PE development
PE formation has been investigated in mouse and chick embryos, but there are only limited studies on PE development in other model systems 7–9. Our data suggest that in zebrafish embryos, the appearance of the PE coincides with the transition of the heart from a primitive linear heart tube to a post-looped two-chambered pumping organ. The zebrafish PE cells are clustered into a cauliflower like structure which is positioned close to the sinus venosus and at the level of the atrioventricular junction. These observations suggest that the PE in zebrafish arises in a similar fashion to that in mouse and chick. Strikingly, PE development is also conserved at a molecular level. The PE marker genes tbx18, tcf21 and wt1 identified in mouse and chick also appear to be expressed in the zebrafish PE 24, 26, 41, 42, 47, 48. However, differences in PE formation between zebrafish, mouse and chick do exist. In chick and mouse embryos, the PE is located adjacent to the developing liver bud. The close apposition of the liver to the PE suggests an inductive role for the liver primordium in PE formation. Indeed, a recent study by Ishii et al (2007) showed that liver bud implantation could induce ectopic PE marker gene expression in chick embryos26. In zebrafish, however, the liver primordium seems to be dispensable for PE development. PE specification still occurs in zebrafish wnt2bb−/− and hnf1ba−/−mutants, in which hepatoblast specification is significantly delayed or completely absent, respectively, a result consistent with the fact that the zebrafish PE is not located close to the liver when it arises. Similarly, the heart is not required for liver induction in zebrafish 56 although it appears to be in mouse 57.
Tbx5 function in PE development
It has been reported that Tbx5 plays a role in regulating PE cell migration. In chick embryos, the PE comes in contact with the myocardium around HH stage 20 to initiate epicardium formation. At this stage, Tbx5 expression is turned on in a subset of the PE cells that have made contact with the myocardium 21. Mosaic overexpression of wildtype Tbx5 or inhibition of Tbx5 translation by transfecting PE cells with Tbx5 morpholino antisense oligonucleotides inhibits PE cell migration out of the PE, suggesting that Tbx5 levels or activity in the PE cells are tightly regulated to ensure proper PE cell migration and epicardialization of the developing heart 21.
Our study extends the role of Tbx5 in PE development to include regulation of PE specification. A role for Tbx5 in PE specification has not been examined before largely because mouse Tbx5 null mutants display severe cardiac defects with hypoplastic sinoatrial structures and left ventricle. The development of mouse Tbx5 null mutants is arrested at E9.5, precluding the examination of PE formation in these animals 58. The data presented in our study suggest that zebrafish tbx5a mutants display much less severe cardiac phenotypes possibly due to functional compensation by a second tbx5 gene (tbx5b) until the time of PE specification53, suggesting that the absence of PE formation in tbx5a mutants is not secondary to the myocardial abnormalities.
Bmp signaling is required for PE specification
In this study, we have revealed an essential role for Bmp signaling in PE specification. A complete loss or significant reduction of PE marker gene expression was observed in acvr1l mutants as well as in embryos in which Bmp signaling was blocked around 36 hpf. The reduction of PE marker gene expression by blocking Bmp signaling at 36 hpf in zebrafish is somewhat reminiscent of the loss of PE marker gene expression observed after implanting Noggin beads into the sinus venosus region of HH stage 11 chick embryos, suggesting that the role of Bmp signaling in PE specification, or maintenance of PE cell identity, is conserved. In chick embryos, bmp2 which is expressed in the sinoatrial myocardium and bmp4 which is expressed in the PE cells are likely to be the source of Bmp signaling, although specific knock down studies need to be carried out to test this hypothesis 48. In zebrafish, we found that bmp4 is expressed in the atrioventricular junction and sinoatrial myocardium, and that loss of Bmp4 activity results in a reduction PE marker gene expression, indicating that bmp4 in zebrafish might be the functional orthologue of bmp2 or bmp4 in chick during PE specification.
A recent study by van Wijk et al59, suggests that in chick the role of Bmp signaling in PE specification may be more complicated. This study shows that proepicardial cells and inflow myocardial cells originate from a common progenitor pool. The segregation of these progenitors into the proepicardium or inflow myocardium lineages appears to involve an opposing effect of BMP and FGF signaling; Bmp signaling promotes inflow myocardium formation while FGF signaling favors PE formation. This reported difference in the role of BMP signaling in PE specification may in part reflect the multiple roles it plays during PE development, as is observed during pancreas development60.
In summary, we provided the first genetic evidence that Bmp4 and its receptor Acvr1l are essential for PE formation in zebrafish. In this paper, we also showed that Tbx5a is required at early somite stages for PE specification. Therefore, we propose that Tbx5a is necessary to confer competence to anterior lateral plate mesodermal cells, thereby allowing some of them to respond to Bmp signals to initiate PE specification. Giving that the re-activation of the adult epicardium is critical for cardiac regeneration in zebrafish 12, our study may provide a new paradigm to stimulate the epicardium to respond to cardiac injury for functional repair.
Novelty and significance.
What Is Known?
The proepicardium (PE)/epicardium provides precursor cells that differentiate into coronary vascular smooth muscle cells, fibroblasts, and possibly, endothelial cells and cardiomyocytes during cardiac development.
The PE derived epicardium is a source of secreted factors that stimulate cardiomyocyte proliferation.
The proepicardium/epicardium-derived cells appear to play an important role during cardiac tissue homeostasis and regeneration.
What New Information Does This Article Contribute?
Our study shows that Bmp4, which is expressed in the developing heart, functions through the type I Bmp receptor Acvr1l to regulate PE development.
Our findings reveal a previously unappreciated role for Tbx5 at early somite stages for PE specification.
Despite the importance of PE in cardiac development, function and regeneration, the signaling pathways that regulate the development of PE remain unidentified. In this study, we focused on the earliest step of PE development and our work represents the first in vivo identification of signaling molecules and transcription factor that regulate PE formation. We identified myocardial Bmp4 as a positive regulator for PE specification. In addition, we found that Tbx5 plays a critical role in PE specification. Based on the expression pattern of these factors and the heat shock experiments that blocked the activity of Tbx5 or Bmp signaling at various stages, we propose that Tbx5 is necessary to confer competence to anterior lateral plate mesodermal cells, thereby allowing some of them to respond to Bmp signals to initiate PE specification. Given that the re-activation of the adult epicardium is critical for cardiac regeneration in zebrafish, our study provides a new paradigm for stimulating the epicardium to respond to cardiac injury for functional repair
Acknowledgments
We thank Drs. Deborah Garrity, Ian Scott, Gerrit Begemann, David Kimelman and Jeroen Bakkers for fish stocks and reagents; Dr. Takashi Mikawa, Dr. Sven Reischauer and David Staudt for critical reading of the manuscript; Ana Ayala and Milly Alva for excellent fish care.
Sources of Funding
J.L. was supported by a postdoctoral fellowship from the American Heart Association and is currently supported by a NIH (National Institutes of Health) training grant (Shaun R. Coughlin, T32 HL007731). This work was funded by grants from the National Institutes of Health (HL54737) and Packard foundation to D.Y.R.S.
Non-standard Abbreviations and Acronyms
- PE
proepicardial organ
- Bmp
bone morphogenetic protein
- Tbx
T-box
- acvr1l
activin A receptor, type I like
- hpf
hours post fertilization
- wnt2bb
wingless-type MMTV integration site family, member 2Bb
- hnf1ba
HNF1 homeobox Ba
- prox1
prospero-related homeobox gene 1
- hhex
hematopoietically expressed homeobox
- LPM
lateral plate mesoderm
- Hand2
heart and neural crest derivatives expressed transcript 2
- Nkx2.5
NK2 transcription factor related 5
Footnotes
Disclosures
None.
Bibliography
- 1.Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 2.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 5.Reese DE, Mikawa T, Bader DM. Development of the coronary vessel system. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- 6.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 7.Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 8.Manner J. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 1992;186:379–385. doi: 10.1007/BF00185988. [DOI] [PubMed] [Google Scholar]
- 9.Komiyama M, Ito K, Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat Embryol (Berl) 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Pomares JM, Munoz-Chapuli R. Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec. 2002;268:343–351. doi: 10.1002/ar.10165. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Pomares JM, Phelps A, Sedmerova M, Wessels A. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev Dyn. 2003;227:56–68. doi: 10.1002/dvdy.10284. [DOI] [PubMed] [Google Scholar]
- 12.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 13.Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 18.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 20.Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JB, Eid H, Sucov HM. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 21.Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM, Mikawa T, Basson CT. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiol Genomics. 2004;18:129–140. doi: 10.1152/physiolgenomics.00060.2004. [DOI] [PubMed] [Google Scholar]
- 22.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Serluca FC. Development of the proepicardial organ in the zebrafish. Dev Biol. 2008;315:18–27. doi: 10.1016/j.ydbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii Y, Langberg JD, Hurtado R, Lee S, Mikawa T. Induction of proepicardial marker gene expression by the liver bud. Development. 2007;134:3627–3637. doi: 10.1242/dev.005280. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene: University of Oregon Press; 2000. [Google Scholar]
- 28.Bauer H, Lele Z, Rauch GJ, Geisler R, Hammerschmidt M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development. 2001;128:849–858. doi: 10.1242/dev.128.6.849. [DOI] [PubMed] [Google Scholar]
- 29.Mintzer KA, Lee MA, Runke G, Trout J, Whitman M, Mullins MC. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 30.Stickney HL, Imai Y, Draper B, Moens C, Talbot WS. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev Biol. 2007;310:71–84. doi: 10.1016/j.ydbio.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, Stainier DY. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- 32.Garrity DM, Childs S, Fishman MC. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development. 2002;129:4635–4645. doi: 10.1242/dev.129.19.4635. [DOI] [PubMed] [Google Scholar]
- 33.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001;15:3217–3229. doi: 10.1101/gad946701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. 2006;133:2275–2284. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- 36.Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Jaynes JB, O’Farrell PH. Active repression of transcription by the engrailed homeodomain protein. Embo J. 1991;10:1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halloran MC, Sato-Maeda M, Warren JT, Su F, Lele Z, Krone PH, Kuwada JY, Shoji W. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- 39.Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 40.Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Begemann G, Gibert Y, Meyer A, Ingham PW. Cloning of zebrafish T-box genes tbx15 and tbx18 and their expression during embryonic development. Mech Dev. 2002;114:137–141. doi: 10.1016/s0925-4773(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 42.Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 43.Begemann G, Ingham PW. Developmental regulation of Tbx5 in zebrafish embryogenesis. Mech Dev. 2000;90:299–304. doi: 10.1016/s0925-4773(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 44.Tamura K, Yonei-Tamura S, Belmonte JC. Differential expression of Tbx4 and Tbx5 in Zebrafish fin buds. Mech Dev. 1999;87:181–184. doi: 10.1016/s0925-4773(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- 46.Hwang SP, Tsou MF, Lin YC, Liu CH. The zebrafish BMP4 gene: sequence analysis and expression pattern during embryonic development. DNA Cell Biol. 1997;16:1003–1011. doi: 10.1089/dna.1997.16.1003. [DOI] [PubMed] [Google Scholar]
- 47.Haenig B, Kispert A. Analysis of TBX18 expression in chick embryos. Dev Genes Evol. 2004;214:407–411. doi: 10.1007/s00427-004-0415-3. [DOI] [PubMed] [Google Scholar]
- 48.Schlueter J, Manner J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 50.Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development. 2000;127:3215–3226. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 51.Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- 52.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 53.Albalat R, Baquero M, Minguillon C. Identification and characterisation of the developmental expression pattern of tbx5b, a novel tbx5 gene in zebrafish. Gene Expr Patterns. 2009 doi: 10.1016/j.gep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Rallis C, Bruneau BG, Del Buono J, Seidman CE, Seidman JG, Nissim S, Tabin CJ, Logan MP. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- 55.Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating MT. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- 56.Scott IC, Masri B, D’Amico LA, Jin SW, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DY. The G protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 58.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 59.van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AF, van den Hoff MJ. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res. 2009;105:431–441. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung WS, Andersson O, Row R, Kimelman D, Stainier DY. Suppression of Alk8-mediated Bmp signaling cell-autonomously induces pancreatic beta-cells in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 107:1142–1147. doi: 10.1073/pnas.0910205107. [DOI] [PMC free article] [PubMed] [Google Scholar]