Abstract
The design, synthesis and structural analysis of two macrocyclic D,L-alternating hexapyrrolinones has been achieved. These cyclic peptide mimics adopt a flat, hexagonal conformation, stabilized by intramolecular hydrogen bonding between adjacent pyrrolinone rings. Extensive NMR studies and X-ray analysis reveal respectively that the macrocyclic hexapyrrolinones aggregate in solution, and in the solid state form staggered stacked nanotube-like assemblies.
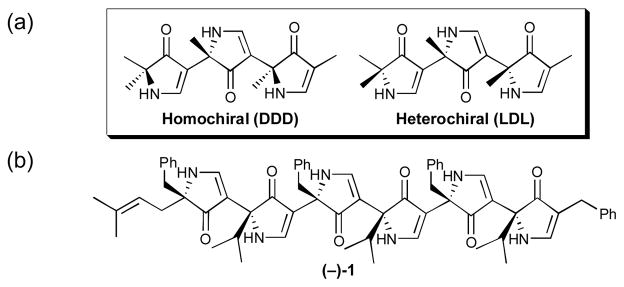
The evolution of the pyrrolinone scaffold towards a universal peptidomimetic possessing both conformational control and diversity was a significant theme of the Hirschmann-Smith collaboration for over 15 years. Through these efforts, we learned that the combined effects of α-stereogenicity of the pyrrolinone ring, intramolecular hydrogen bonding, and choice of side-chains determined the global minimum energy conformation of the polypyrrolinone chain. Homochiral polypyrrolinones (eg., all D, Figure 1),1 that preferentially adopt an extended conformation, proved to be excellent β-strand/β-sheet mimics,2 and as such, led to potent, orally bioavailable pyrrolinone-based enzyme inhibitors of aspartic acid proteases,3 as well as modest metalloprotease inhibitors,4 and peptide-pyrrolinone hybrid ligands for the class II MHC protein HLA–DR1.5 Alternatively, heterochiral polypyrrolinones (e.g., alternating D,L pyrrolinones; Figure 1), much like heterochiral polypeptides, adopt a turn structure,6 and as such have been employed to generate functional β-turn mimetics.7 Subsequent investigations of the extended heterochiral pyrrolinone motif led to the discovery that hexapyrrolinone (−)-1 adopts a flat G-shaped conformation that aggregates in solution and in the solid state self-assembles into a nanotube-like stucture.8
Figure 1.
(a) Homochiral (DDD) and Heterochiral Pyrrolinones (LDL); (b) Structure of D,L-Hexapyrrolinone (−)-1.
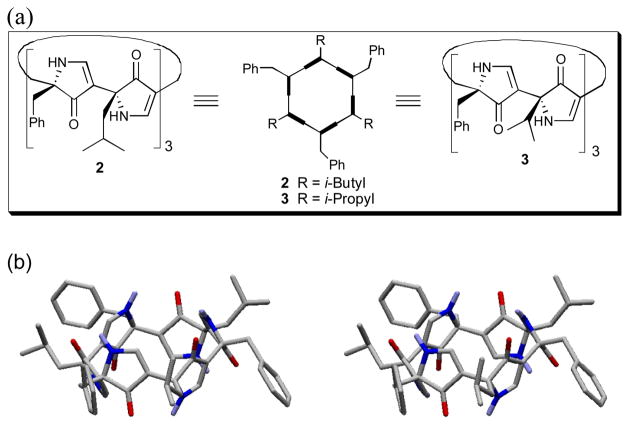
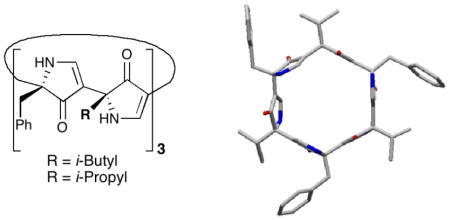
The nanotube-like architecture of (−)-1 in the solid-state, possessing termini in close proximity, readily suggested the design of macrocyclic hexapyrrolinones 2 and 3 (Figure 2a). Unencumbered with terminal substituents, we reasoned that such cyclic polypyrolinones might self-assemble into nanotubes.9 Pleasingly, Monte Carlo conformational searches10 for 2 predicted that the low energy conformations would possess a flat, hexagonal conformation (Figure 2b), in agreement with previous structural analysis of the acyclic heterochiral pyrrolinones such as (−)-1.
Figure 2.
(a) Prospective macrocyclic hexapyrrolinones 2 and 3; b) Stereoview of the lowest energy conformation of 2 derived via Monte Carlo conformational analysis.
Importantly, the predicted conformation presents hydrogen bonding acceptors and donors (cf. C=O and N-H, respectively) in an alternating pattern directed above and below the plane of the molecule, thus providing the potential for intermolecular hydrogen bonding in a nanotube-like array.
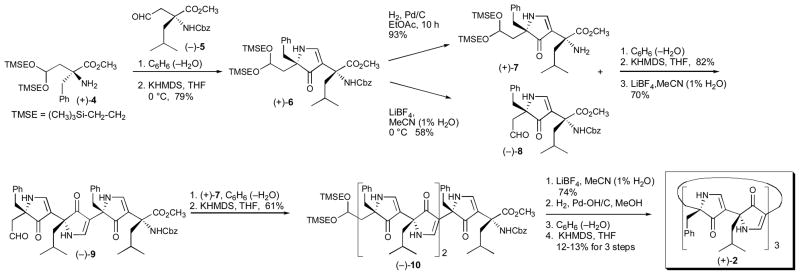
To access 2, we initially employed our iterative polypyrrolinone synthetic tactic in a linear fashion,2,6 beginning with the C terminus to generate the open-chain pentamer (−)-10. Although this approach to (+)-2 eventually proved successful (Supporting Information), we subsequently designed a more effective, convergent synthesis, beginning with (+)-411 and (−)-5 (Scheme 1).12 Condensation to afford an intermediate imine, followed by treatment with KHMDS generated monopyrrolinone (+)-6, a common precursor for both (+)-7 and (−)-8. Hydrogenolysis furnished amine (+)-7, while treatment with LiBF4 led to aldehyde (−)-8. Union of these two pyrrolinone building blocks was achieved in 82% yield by imine formation, followed by treatment with KHMDS. Acetal hydrolysis furnished trispyrrolinone (−)-9; a two-step sequence with pyrrolinone amine (+)-7 then delivered the pentapyrrolinone (−)-10. The critical final pyrrolinone ring construction, leading to macrocycle (+)-2 was achieved in a similar fashion, albeit in this case the yield was at best modest (ca. 12–13%). Notwithstanding the efficiency of the final cyclization, a sample (ca. 100 mg) of (+)-2 was prepared for structural analysis.
Scheme 1.
Convergent Synthesis of Macrocyclic Hexapyrrolinone (+)-2.
Assignment of structure (+)-2 was based principally on simplification of both the 1H and 13C NMR spectra, in conjunction with HRMS identification of the parent ion. Pentapyrrolinone (−)-10 (an unsymmetrical molecule, Scheme 1) displays a distinct set of signals for the five chemically (and magnetically) different pyrrolinone units (e.g., vinyl and benzyl hydrogens, etc). Conversion to the cyclic C3-symmetrical hexamer (+)-2 (Figure 3, Scheme 1) renders each benzyl and isobutyl pyrrolinone chemically and magnetically identical, resulting in isochronous NMR signals for the three monomeric units. Indeed, only two sets of signals are observed in both 1H and 13C NMR spectra of (+)-2, corresponding to the two types of pyrrolinone rings.
Figure 3.
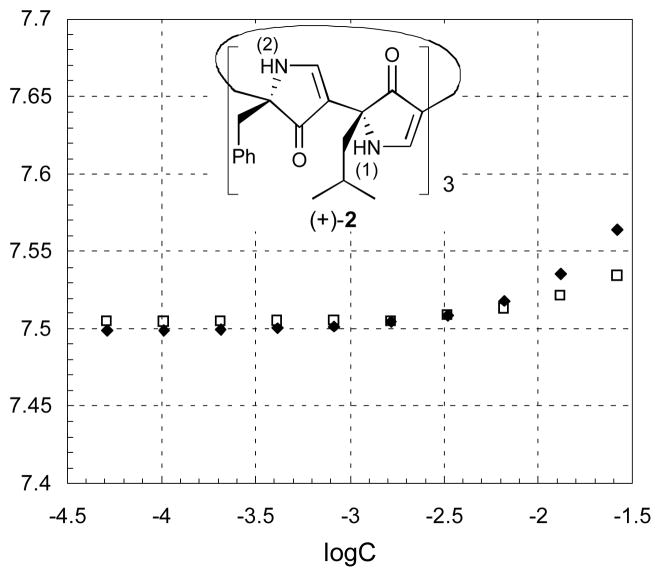
Concentration dependence of 1H NMR chemical shifts in CDCl3 of N-H(1) (□) and NH(2) (◆) protons of (+)-2.
The propensity of macrocycle (+)-2 to self-assemble in solution was demonstrated via a series of 1HNMR studies in CDCl3 similarly employed for in the study of (−)-1.8,13 The cyclic structure of (+)-2 permits each N-H of the individual macrocycles to be involved in intramolecular hydrogen bonding, thereby lessening their solvent exposure, and thus a relatively small effect of concentration was observed on the chemical shifts.6 Nonetheless, a concentration dependence of the N-H protons was observed (Figure 3) despite the intramolecular interactions. The measurable down-field shift for the N-H signals with increasing concentration is consistent with aggregation mediated by intermolecular hydrogen bonding.
Single crystal X-ray analysis of (+)-2 would provide the strongest evidence possible for nanotube assembly; unfortunately, crystals of (+)-2 suitable for X-ray analysis have not, as yet, been forthcoming. The failure to obtain suitable crystals of (+)-2 prompted the synthesis of hexapyrrolinone 3, wherein the isobutyl groups were substituted for isopropyl groups, with the expectation that the reduced flexibility of the isopropyl side-chains might facilitate crystal growth.
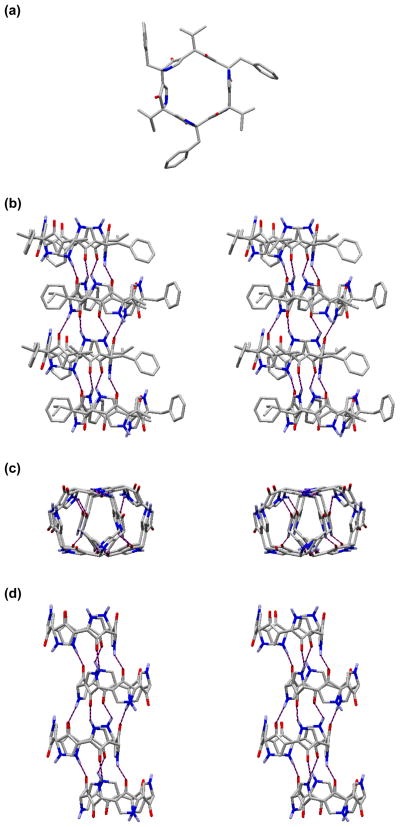
The synthesis of (+)-3 was completed in a convergent fashion, similar to that employed for (+)-2 (see Supporting Information). Not surprisingly, the macrocyclization step proceeded in even lower yield given the steric encumberance of the isopropyl side-chains (ca. 2% yield). Concentration dependent NMR analysis of (+)-3 revealed nearly identical solution-state aggregation as observed for (+)-2 (see Supporting Information). Gratifyingly, compared to (+)-2, superior crystals of (+)-3 could be obtained by slow evaporation in 1:1 CHCl3:trifluoro- ethanol, and the solid-state structure analysis using synchrotron X-ray diffraction was achieved.14 Interestingly, unlike the open chain hexapyrrolinone (−)-1, (+)-3 assembles into a staggered nanotube-like array (Figure 4). Comparison of this structure, with that of (−)-1,8 provides both interesting similarities and differences. The monomers of (+)-3 assemble in an antiparallel fashion, as observed for (−)-1. Alternatively, the staggered array adopted by (+)-3, possesses only four intermolecular pyrrolinone-pyrrolinone hydrogen bonds between each pair of monomers, compared to six for (−)-1. Additionally, the staggered nanotube structure of (+)-3 forms an infinite array in the crystal lattice (four molecules/unit cell as illustrated in Figure 4b–d). Given the pyrrolinone scaffold comprises a designed peptidomimetic, our work has obvious parallels to the cyclic peptides pioneered by Karle,15 Lorenzi,16 and Ghadiri.17,9 Of particular interest, the 1987 report by G. P. Lorenzi et al. discloses a series of D,L-alternating cyclic hexapeptides.16 In contrast to the hexapeptides, which were reported to be completely insoluble in all common organic solvents, (+)-2 and (+)-3 are moderately soluble in most polar organic solvents (i.e., EtOAc, EtOH), and display excellent solubility in CHCl3. Importantly the structure of cyclic hexapyrrolinone (+)-3 overlays remarkably well with the Lorenzi cyclic hexapeptides (Figure 5), illustrating the close correspondence between the pyrrolinone and peptide units, and suggesting the possibility that the two macrocycles would form heterogenous aggregates.
Figure 4.
The X-ray structure of (+)-3: (a) a single molecule; (b) representative stereoview of the infinite staggered nanotube array, viewed from the side. The staggered nanotube structure is more clearly visible with the side-chains removed, from the top (c), and from the side (d).
Figure 5.
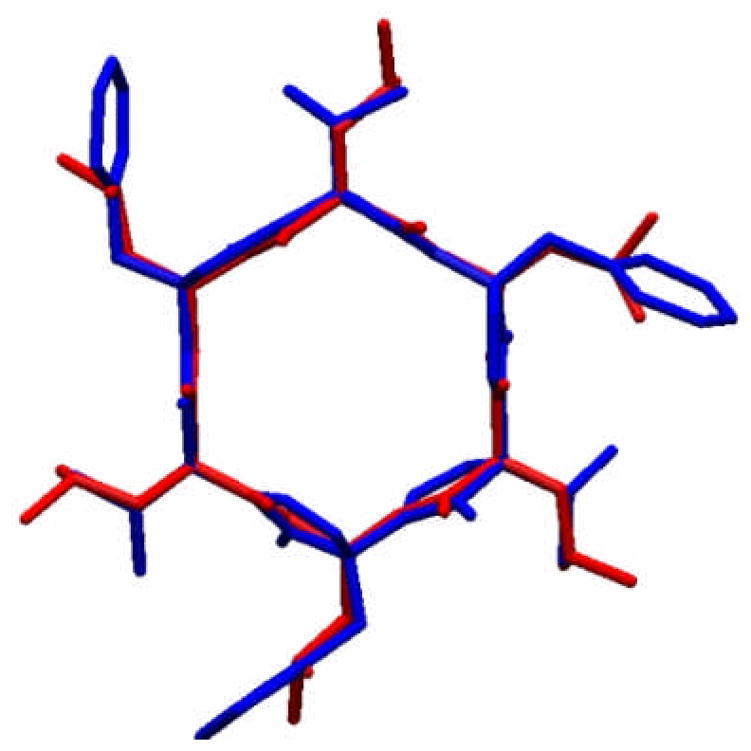
Overlay of the X-ray structure of cyclic hexapyrrolinone (+)-3 (blue) with the X-ray structure of cyclo(-D-Leu-L-MeLeu-D-Leu-L-MeLeu-D-Leu-L-MeLeu-) (red).16
In summary, the design, syntheses and structural analysis of macrocyclic D,L-hexapyrrolinones (+)-2 and (+)-3 have been achieved. Studies by NMR suggest that the macrocyclic hexapyrrolinones aggregate in solution, while the solid-state structure of (+)-3, determined via X-ray crystallography, revealed an extended, staggered nanotube-like array, stabilized by four intermolecular hydrogen bonds between pairs of pyrrolinone rings. Importantly, the solid state structure of (+)-3 demonstrates the ability of macrocyclic pyrrolinones to assemble into nanotube-like structures. Studies to both improve the final macrocyclization as well as to exploit the structural properties of such cyclic heterochiral polypyrrolinones continue in our laboratory.
Supplementary Material
Acknowledgments
Support was provided by the NIH through Grants AI-42010 and GM-49758. Additional support was provided by Postdoctoral Fellowships from the Department of Defense (PC-030136) to E.F.M., from the Swiss National Science Foundation to M.B., and Graduate Fellowships from Bristol-Myers Squibb and the University of Pennsylvania to A.K.C. We thank the Advanced Light Source (Berkeley, CA) for access to synchrotron beamline 5.0.2. We also thank Samuel H. Gellman, the Ralph Hirschmann Professor of Chemistry at the University of Wisconsin, for insightful discussion.
Footnotes
Supporting Information Available Experimental procedures and spectroscopic data for all new compounds are available.
References
- 1.While D,L descriptors are not directly applicable to the pyrrolinone units, their use simplifies comparison with peptidal structures.
- 2.(a) Smith AB, III, Keenan TP, Holcomb RC, Sprengler PA, Guzman MC, Wood JL, Carrol PJ, Hirschmann R. J Am Chem Soc. 1992;114:10672. [Google Scholar]; (b) Smith AB, III, Holcomb RC, Guzman MC, Keenan TP, Sprengeler PA, Hirschmann R. Tetrahedron Lett. 1993;34:63. [Google Scholar]; (c) Smith AB, III, Guzman MC, Sprengler PA, Keenan TP, Holcomb RC, Wood JL, Carrol PJ, Hirschmann R. J Am Chem Soc. 1994;116:9947. [Google Scholar]
- 3.(a) Smith AB, III, Akaishi R, Jones DR, Keenan TP, Guzman MC, Holcomb RC, Sprengeler PA, Wood J, Hirschmann R, Holloway MK. Biopolymers (Peptide Science) 1995;37:29. doi: 10.1002/bip.360370106. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, III, Cantin L-D, Pasternak A, Guise-Zawacki L, Yao WQ, Charnley AK, Barbosa J, Sprengeler PA, Hirschmann R, Munshi S, Olsen DB, Schleif WA, Kuo LC. J Med Chem. 2003;46:1831. doi: 10.1021/jm0204587. and references cited therein. [DOI] [PubMed] [Google Scholar]; (c) Smith AB, III, Charnley AK, Harada H, Beiger JJ, Cantin LD, Kenesky CS, Hirschmann R, Munshi S, Olsen DB, Stahlhut MW, Schleif WA, Kuo LC. Bioorg Med Chem Lett. 2006;16:859. doi: 10.1016/j.bmcl.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Smith AB, III, Nittoli T, Sprengeler PA, Duan JW, Liu RQ, Hirschmann R. Org Lett. 2000;2:3809. doi: 10.1021/ol000254p. [DOI] [PubMed] [Google Scholar]
- 5.Lee KH, Olson GL, Bolin DR, Benowitz AB, Sprengeler PA, Smith AB, III, Hirschmann RF, Wiley DC. J Am Chem Soc. 2000;122:8370. and references cited therin. [Google Scholar]
- 6.Smith AB, III, Wang W, Sprengler PA, Hirschmann R. J Am Chem Soc. 2000;122:11037. [Google Scholar]
- 7.Smith AB, III, Charnley AK, Mesaros EF, Kikuchi O, Wang W, Benowitz A, Chu CL, Feng JJ, Chen KH, Lin A, Cheng FC, Taylor L, Hirschmann R. Org Lett. 2005;7:399. doi: 10.1021/ol0476974. [DOI] [PubMed] [Google Scholar]
- 8.See preceeding article in this journal.
- 9.(a) Scanlon S, Aggeli A. Nano Today. 2008;3:22. [Google Scholar]; (b) Fischer L, Decossas M, Briand JP, Didierjean C, Guichard G. Angew Chem Int Ed. 2009;48:1625. doi: 10.1002/anie.200804019. [DOI] [PubMed] [Google Scholar]; (c) Bong DT, Clark TD, Granja JR, Ghadiri MR. Angew Chem Int Ed. 2001;40:988. [PubMed] [Google Scholar]
- 10.Chang G, Guida WC, Still WC. J Am Chem Soc. 1989;111:4379. [Google Scholar]
- 11.We employed the bis(2-TMS-ethyl) acetal (+)-4 in the synthesis of (+)-2, as opposed to a dimethyl acetal, due to incompatibility of acid cleavage of the latter with the Cbz-protected amines. See: Paquette LA, Backhaus D, Braun R. J Am Chem Soc. 1996;118:11990.Walkup RD, Obeyesekere NU. Synthesis. 1987:607.. For hydrolysis of acetals with LiBF4 see: Lipshutz BH, Harvey DF. Synthetic Commun. 1982;12:267.
- 12.The amino ester building blocks are readily available via the methods of: Karady S, Amato JS, Weinstock LM. Tetrahedron Lett. 1984;25:4337.Seebach D, Fadel A. Helv Chim Acta. 1985;68:1243.
- 13.Haque TS, Little JC, Gellman SH. J Am Chem Soc. 1996;118:6975. [Google Scholar]
- 14.For crystallographic data for hexapyrrolinone (+)-3 see Supporting Information.
- 15.Karle IL, Handa BK, Hassall CH. Acta Crystallogr. 1975;B31:555. [Google Scholar]
- 16.(a) Tomasic L, Lorenzi GP. Helv Chim Acta. 1987;70:1012. [Google Scholar]; (b) Sun X, Lorenzi GP. Helv Chim Acta. 1994;77:1520. and references cited therein. [Google Scholar]
- 17.(a) Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Nature. 1993;366:324. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]; (b) Ghadiri MR, Kobayashi K, Granja JR, Chadha RK, McRee DE. Angew Chem Int Ed Engl. 1995;34:93. [Google Scholar]; (c) Clark TD, Buriak JM, Kobayashi K, Isler MP, McRee DE, Ghadiri MR. J Am Chem Soc. 1998;120:8949. and references cited therein. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.