The study used a convenience sample of patients undergoing surveillance following curative treatment for localized cancer who completed a paper survey to estimate the maximum copayment patients are willing to pay for better treatment outcomes. Results suggest that patients may be less willing to pay high copayments for treatments with modest benefit. In addition, sociodemographic factors such as education and employment status were associated with willingness to pay.
Abstract
Purpose.
Cost sharing, intended to control the “overuse” of health care resources, may also reduce use of necessary services. The influence of cost on the treatment choices of patients with life-threatening illness, such as cancer, is unknown.
Methods.
A convenience sample of patients undergoing surveillance following curative treatment for localized cancer completed a paper survey that included three scenarios to elicit the maximum copayment they would be willing to pay for better treatment outcomes. Scenario A described a treatment for a curable cancer in terms of recurrence risk. Scenarios B and C described treatments for noncurable cancer in terms of the 2-year survival probability and median life expectancy.
Results.
The sample (n = 60) was 78% female, 83% aged <65 years, and 58% college graduates. Thirteen percent reported making financial sacrifices to pay for treatment. Patients were willing to pay higher copayments for more effective treatments (p < .05 for all three scenarios). In scenario B, patients who were employed demonstrated a greater willingness to pay (WTP) (odds ratio [OR], 12.6; 95% confidence interval [CI], 2.0–80.4), when controlling for efficacy. In scenario C, college graduates showed greater WTP (OR, 5.0; 95% CI, 1.2–20.9) and patients who reported previous financial sacrifices showed lower WTP (OR, 0.2; 95% CI, 0.04–0.6).
Conclusion.
This pilot study suggests that patients may be less willing to pay high copayments for treatments with modest benefit. Even among this relatively young, affluent, and educated population, demographic variables were related to WTP. Larger studies in more diverse populations should be conducted to better understand how cost may influence treatment decisions and cancer treatment outcomes.
Background
Cost sharing, through copayments, coinsurance, or deductibles, is used by insurance companies to prevent the overuse of health care services and control costs [1]. Although effective in controlling costs, there is evidence that greater cost sharing is associated with worse outcomes in the sickest and poorest patients, perhaps by causing lower use of necessary services [2]. Given the highly emotional and life-threatening nature of a cancer diagnosis, cancer patients and their families may feel compelled to seek high-cost treatment and not be as responsive to higher out-of-pocket expenses.
Understanding the impact of cost on treatment decision making is especially important in the current era of high-cost treatments. With the introduction of many new anticancer treatments in recent years, patients are increasingly asked to choose among treatments that may have significantly different levels of cost sharing, efficacy, and toxicity. In addition, given the high cost of many new cancer treatments, cost sharing places a significant burden on cancer patients and their families [3].
As an initial effort to inform discussions regarding cost sharing and cancer treatment decision making, we conducted a pilot study to determine the feasibility of measuring cancer patients' willingness to pay (WTP) in hypothetical clinical scenarios. Cancer treatments may be used in the adjuvant setting (given following surgical resection to reduce the risk for recurrence) or in the palliative setting (noncurative treatments given to prolong life and relieve symptoms). Therefore, we constructed hypothetical “adjuvant” and “palliative” scenarios to determine whether patients expressed different preferences in the curative and noncurative settings. The objectives of this study were to obtain preliminary data to: (a) determine whether patients' out-of-pocket WTP for adjuvant or palliative chemotherapy is affected by cost and clinical outcome and (b) determine whether sociodemographic characteristics are associated with WTP for treatments. We hypothesized that patients would be willing to pay more for more effective cancer treatments in both the curative and palliative settings. In addition, we hypothesized that a higher socioeconomic status and education level would be associated with greater WTP, regardless of the clinical setting.
Methods
A convenience sample of patients at Fox Chase Cancer Center, a National Cancer Institute–designated comprehensive cancer center, were screened for eligibility based on the following criteria: (a) age >18, (b) documented malignancy, (c) ≤6 months from date of diagnosis, (d) without evidence of metastases or recurrence, and (e) completion of all adjuvant treatment (surgery, chemotherapy, biologic therapy, and radiation). In addition, patients were eligible if they were on adjuvant endocrine therapy for breast cancer or luteinizing-hormone-releasing hormone agonists for biochemical recurrence of prostate cancer. Potential participants were ascertained by review of patient schedules and medical information.
Consent to contact each patient was obtained from the attending physician or advanced practice clinician. A research assistant then contacted potential participants via telephone and invited them to participate. Patients provided written informed consent prior to enrollment. The study was reviewed and approved by the institutional review board at Fox Chase Cancer Center.
A development phase was conducted in 10 patients to gather feedback on the content and readability of the measures. No data collected from these 10 patients were included in the final analysis. Based on the initial feedback, editorial changes were made to improve the readability of the survey instrument. Patients were recruited to the main study in February to July of 2008. The study included a pen-and-paper questionnaire, which took approximately 40 minutes to complete. The patients were given a $20 gift card participant incentive. Patients completed the survey independently but could ask for assistance from family members or the research assistant.
Survey Instrument
The survey included the following domains.
Demographics
Data were collected on the age, gender, education, marital status, ethnicity, race, employment status, diagnosis, cancer treatment history, health insurance coverage, and current living situation of participants. Patients were also asked whether they had an important event in the next 2 years (i.e., family wedding, graduation, etc.) and if they had made sacrifices to pay for their cancer care.
Impact of Cost on Decision Making
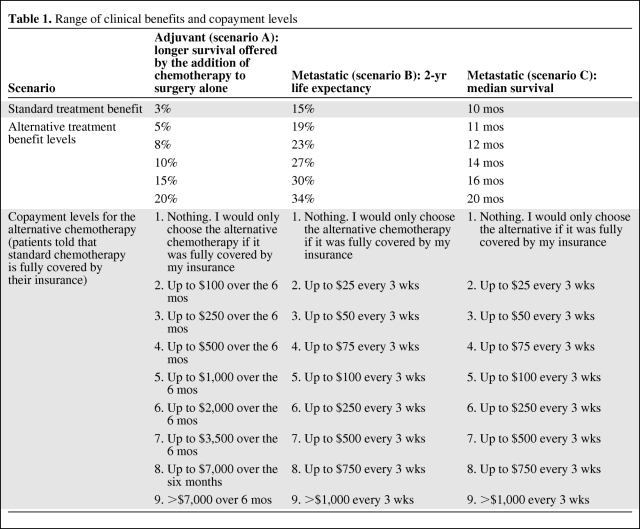
Patients were asked to compare a standard treatment option, covered by their insurance, with a superior alternative that would require a copayment. They were asked to select from a list of nine possible copayments the maximum they would be willing to pay for the better treatment. Three clinical scenarios (five questions each for a total of 15 questions) were presented. Within each scenario, the magnitude of benefit was varied for the alternative treatment option. Patients were given all three scenarios. Scenario A addressed adjuvant treatment (systemic chemotherapy given to reduce the risk for cancer recurrence) following surgical resection of a localized cancer. The patients were told that surgery alone offered a cure rate of 70%. Standard adjuvant treatment in addition to surgery increased the cure rate by 3% (73% cured, versus 70% with surgery alone), compared with the 5%–20% (75%–90% cured) cure rate offered by the alternative treatment. Scenario B addressed better treatment for metastatic disease. The standard treatment offered a 15% 2-year survival rate whereas the alternative treatment offered a 2-year survival rate in the range of 19%–34%. Scenario C addressed superior treatment for metastatic disease. The standard treatment offered a median survival time of 10 months whereas the alternative treatments offered median survival times in the range of 11–20 months. An example of the question format is presented in Figure 1. The range of clinical benefits and nine copayment levels are shown in Table 1. To avoid anchoring of responses, the alternative benefits were presented in random order within each scenario. The questions in scenario A were modeled on the addition of oxaliplatin to 5-fluorouracil and leucovorin for patients with localized colon cancer [4], which produces a higher 3-year disease-free survival rate, 78.2% versus 72.9%. The questions in scenarios B and C were modeled on the addition of bevacizumab to chemotherapy for patients with metastatic non-small cell lung cancer, which leads to a longer median survival time, 12.3 months versus 10.3 months, and higher 2-year survival rate, 23% versus 15% [5]. To avoid causing undue stress and respondent bias in patients with these diseases or who had received these medications, neither the specific disease sites nor treatments were referred to by name.
Figure 1.
Sample question from scenario B (metastatic).
Table 1.
Range of clinical benefits and copayment levels
Statistical Analyses
Sample Size Determination
We chose our sample size to have sufficient power to detect a relationship between WTP and the effectiveness variables of interest. With 60 study subjects, we had 90% power to detect a 13% population-level discordant rate and 80% power to detect an 11% discordant rate, comparing WTP for low versus high effectiveness drugs. The calculations assume the use of McNemar's test and assume that virtually no people who have low WTP for highly effective drugs would have a high WTP for lesser effective drugs. The sample size calculations were made assuming a one-sided 5% type 1 error rate.
Data Analysis
Data were entered into a Microsoft Access database and then exported to STATA (version 10 SE; STATA Corp., College Station, TX) for analysis. Results were analyzed dichotomizing WTP between low and high copayment (copayment preference was considered high if copayments level five through nine were chosen; otherwise it was considered low). Effectiveness was also analyzed as a dichotomous value (effectiveness was considered high for the fourth or fifth highest effectiveness levels; otherwise it was considered low). We performed sensitivity analyses to investigate if inferences about the relationship of effectiveness with WTP changed when including effectiveness in models as a continuous variable. In the primary analyses (reported in the tables and figures), we excluded patients who answered any of the questions inconsistently (i.e., were willing to pay more for a less effective treatment than a more effective treatment). However, we confirmed our inferences in models estimated using both consistent and inconsistent responders. To investigate associations, we used multiple logistic regression models of WTP estimated by generalized estimating equations (GEEs), assuming an exchangeable working correlation matrix and using robust standard errors. In these models, copayment preference was the outcome of interest. Patient characteristics and treatment efficacy (as a dichotomous variable) were the independent variables. The estimation of the models by GEE accounted for the correlation of responses among multiple scenarios within individuals. Because scenarios B and C both measured outcomes in the metastatic setting, we measured intrapatient correlation using Pearson correlations. We calculated p-values under a T-distribution assumption.
Results
Recruitment
We contacted 131 patients to enroll in the two phases of the study. Seventy-one patients agreed and completed the survey. Five patients agreed but did not arrive for their appointment. Fifty-six patients declined.
Development Phase
Ten patients were enrolled, with ages in the range of 42–81 years. Seven participants were female. The most prevalent cancer sites were lung (five patients) and breast (two patients). Three patients had a college education or greater. Eight were homeowners. One patient had dependent children. Four and three patients, respectively, in the metastatic scenarios (B and C) chose the two highest copayment levels (up to $350 every 3 weeks and >$350 every 3 weeks) for all five scenarios. Therefore, we increased the maximum copayment in the main study to ≥$1,000 every 3 weeks.
In debriefing sessions, patients reported that they understood the questions and were comfortable answering them. Several patients stated that they believed that patients of limited financial means might not be able to afford high copayments for cancer drugs, supporting the relevance of the subject matter to cancer patients. Several patients noted that treatment-related toxicity is an important component of their decision making. This was incorporated into a subsequent, larger, ongoing study of treatment decision making.
Main Study
Participant Characteristics
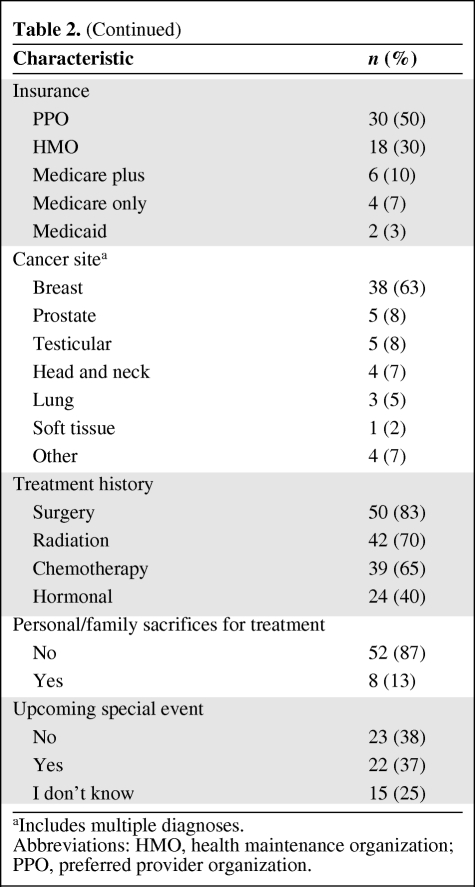
Sixty-one patients were enrolled; one patient was found to be ineligible and was not included in the analyses. Patient characteristics are shown in Table 2. The median age was 57 years, with 83% aged ≤65 years. The sample was predominately white (97%), female (78%), and college educated (58%). Sixty percent reported incomes >$75,000 annually. The most prevalent cancer of participants was breast cancer (68%).
Table 2.
Patient characteristics
Table 2.
(Continued)
aIncludes multiple diagnoses.
Abbreviations: HMO, health maintenance organization; PPO, preferred provider organization.
WTP
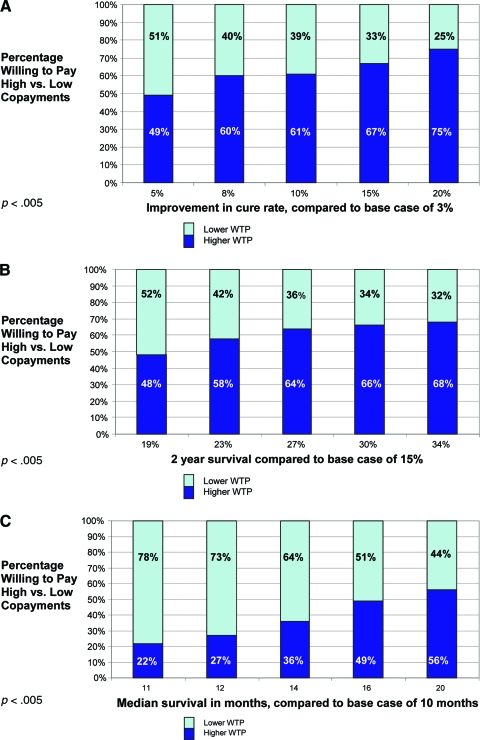
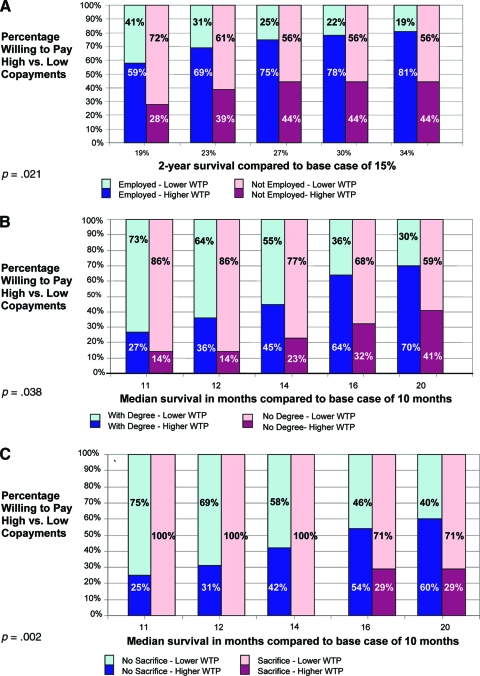
Participants were willing to pay more for cancer treatments as their potential benefit increased. Effects were consistent across all three scenarios and were observed whether the copayments were treated as dichotomized (high versus low) or continuous variables (p < .05 for both analyses). The percentage of patients willing to pay high (copayment level ≥5) versus low (copayment level ≤4) copayments for each level of clinical benefit is shown in Figure 2A–2C.
Figure 2.
Percentage of patients willing to pay high copayments (level ≥5) at each level of clinical benefit. (A): Scenario A. (B): Scenario B. (C): Scenario C.
Abbreviation: WTP, willingness to pay.
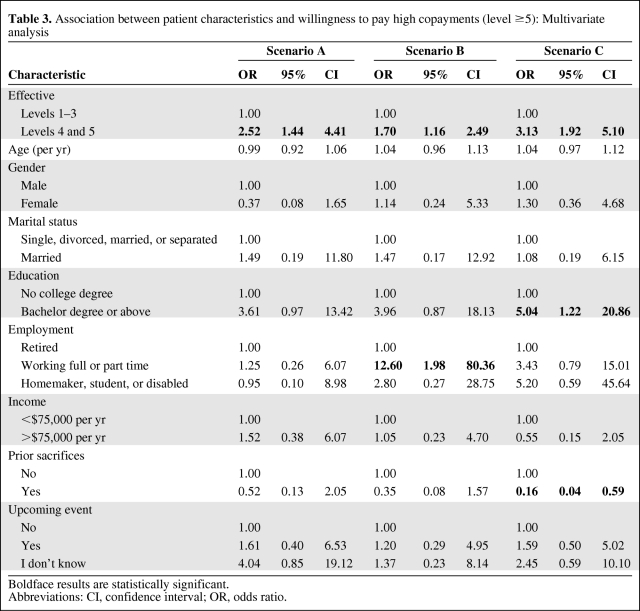
On multivariate analysis, several patient characteristics were associated with WTP when controlling for treatment efficacy as a dichotomized variable (Table 3). In these scenarios, high efficacy was defined as the fourth or fifth highest efficacy levels; all others were considered low. Participants who were working full or part time were more willing to pay higher copayments than those who were retired (odds ratio [OR], 12.6; 95% confidence interval [CI], 2.0–80.4) in scenario B. In scenario C, those who had a college degree were willing to pay higher copayments (OR, 5.0; 95% CI, 1.2–20.9) than those who did not. However, patients who described themselves as having made sacrifices to afford treatment in the past were less willing to accept higher copayments than those who did not describe themselves as having made such sacrifices (OR, 0.2; 95% CI, 0.04–0.6). As shown in Figure 3A, 3B, within each benefit level, patients who had a college degree or were working were more likely to be willing to pay high copayments than those who were not. Conversely, within each benefit level, participants who had made previous sacrifices were less willing to pay high copayments than those who had not (Fig. 3C). Although the results were not consistently statistically significant across all scenarios, the directions of the associations were similar. In addition, within the two metastatic scenarios (B and C), the intrapatient correlation was very high (p < .05).
Table 3.
Association between patient characteristics and willingness to pay high copayments (level ≥5): Multivariate analysis
Boldface results are statistically significant.
Abbreviations: CI, confidence interval; OR, odds ratio.
Figure 3.
Percentage of patients willing to pay high copayments (level ≥5) at each level of clinical benefit, by employment status, scenario B (A), by education status, scenario C (B), and by prior sacrifices, scenario C (C).
Abbreviation: WTP, willingness to pay.
There were no differences in inferences when we included inconsistent responders in the analysis (3, 11, and 5 participants for scenarios A, B, and C, respectively).
Discussion
These results suggest that cancer patients may be less willing to accept higher out-of-pocket expenses for cancer treatments with modest clinical benefits. This finding was present in both the adjuvant and metastatic scenarios. In addition, patients who did not have college degrees, were not currently working, or reported making previous financial sacrifices were less willing to pay higher copayments for cancer treatments in the metastatic scenarios, even when controlling for treatment efficacy. The findings that patients have a greater WTP for higher copayments for more effective treatments and that socioeconomic status affects WTP both support the face validity of our survey instrument.
Although not surprising, these results are important because they dispute the commonly held notion that physicians and patients have an “anything at all costs” approach to cancer treatment. It has been hypothesized that, given the life-threatening nature of a cancer diagnosis, patients may demand treatment regardless of cost; that is, higher prices do not result in lower use. This is thought to contribute to the rising cost of care, given the high cost and only modest activity of many new anticancer treatments [6]. However, these findings suggest that patients may not necessarily behave in this manner, but may instead assign a “value” to cancer treatments and be less willing to pay for less effective approaches.
This work begins to fill an important gap in the understanding of patient demand for cancer treatments, and hence has potential clinical and policy implications. To our knowledge, there has been only one study that examines the association between out-of-pocket payments and cancer drug use. Using insurance claims to measure the association between out-of-pocket payments and cancer drug use, Goldman et al. [7] found that cancer medication use did not decrease as the degree of cost sharing increased. This finding is in direct contrast to studies of other prescription medications, such as antihypertensive and antiasthmatic medications, for which higher copayments were shown to reduce use [1]. However, the study by Goldman et al. [7] examined the use of a heterogeneous group of cancer drugs, such as chemotherapy, antiemetics, and growth factors, that can be used across a variety of clinical settings. We specifically restricted our investigation to anticancer treatments and did not include supportive care medications. In addition, within each scenario, we asked patients to focus on a specific clinical setting (adjuvant versus metastatic settings), which allowed us to explore whether or not disease state may influence response. We found that patients assigned greater “value” to treatments that had a greater impact on overall survival in all three scenarios.
The impact of cost sharing for cancer patients is particularly important to understand given the severity of the diseases involved and the high cost of treatment [8, 9]. Although they are often covered by prescription plans, many oral cancer drugs are placed on high copayment tiers [10], potentially resulting in very high out-of-pocket costs for patients. In addition, because many oral cancer drugs were recently introduced, they do not have generic alternatives. Therefore, unlike choices about routine prescription drugs, cancer patients may not be able to select a “preferred drug” to save money. Some insurance plans require patients to pay a percentage of the costs for i.v. drugs as well; this can result in substantial costs for patients [11].
The impact of cost sharing may influence treatment outcomes by affecting patients' treatment choices. For example, if high out-of-pocket costs discourage patients from seeking adjuvant treatment, this might reduce overuse of medical services, but also compromise population-based outcomes. Higher cure rates for localized cancers have resulted, in part, from superior, but expensive, adjuvant therapies, such as trastuzumab for breast cancer [12, 13] and oxaliplatin for colorectal cancer [4]. Improvements in survival times for patients with metastatic cancer have also resulted from new but expensive drugs; for example, longer survival in advanced colorectal cancer has been shown to be associated with the availability of new chemotherapy drugs [14]. Because patients with metastatic cancer may be treated with sequential therapies until death, the cumulative out-of-pocket costs for patients and their families may be significant. Conversely, patients with “generous” coverage for cancer treatments may continue to pursue chemotherapy near the end of life rather than pursuing less costly supportive treatments through hospice.
Despite the relatively small sample size of this pilot study, we were able to identify potential associations between patient characteristics and WTP for cancer treatments in the metastatic setting. For example, education (which has been shown to be highly correlated with income in other studies [15]) and employment status were associated with greater WTP for treatments, even when controlling for efficacy. These findings may reflect the fact that employed and more educated patients have greater financial resources. On the other hand, patients who reported already making financial sacrifices to pay for their treatment were less willing to pay for longer survival. Although the sample size may have precluded seeing these findings in both metastatic scenarios, the directions of the effects were similar (Table 3). In addition, there was high intrapatient correlation for the responses in the two metastatic scenarios. Taken together, these findings suggest that, even among the relatively affluent and homogeneous population of well-insured patients in this small pilot study, sociodemographic factors may still influence patients' cost-related treatment choices. These choices may potentially exacerbate disparities in cancer care and outcomes. Therefore, discussions surrounding health care reform must be sensitive not only to the problem of uninsurance but to the problem of underinsurance as well.
We did not find statistically significant associations between patient characteristics and WTP in the adjuvant scenario. This may indicate that socioeconomic status is less likely to influence choices of adjuvant treatment than palliative treatments. However, the small sample size limits our ability to draw definitive conclusions.
These results should be interpreted within the limitations of the study design. Studies of WTP for cancer treatments have used proxies for patients. For example, one study of Australian oncology nurses as patient proxies used conjoint analysis to identify a greater WTP for a new chemotherapy, but this was driven more by the patients' preferences for toxicity rather than the ease of administration [16]. There have been few studies in cancer patients themselves. One study of Canadian patients with and without lung cancer found that only 5% were willing to pay the full cost for new treatments ($2,000 per month) for advanced lung cancer. However, these and many other WTP studies in health care are conducted in countries where differences in health care financing may affect patients' WTP out-of-pocket expenses [17]. Therefore, we conducted this pilot study to test the feasibility of assessing WTP in U.S. cancer patients. To avoid causing distress in acutely ill patients, we limited our patient population to those diagnosed at least 6 months prior who had completed all primary treatment for localized disease. By selecting a patient population of cancer survivors, we may have biased our results toward a lower WTP. As prospect theory suggests, individuals may be more willing to take on greater risk depending on their proximity to threat [18]. Therefore, patients who were recently diagnosed or are currently undergoing cancer treatments may be willing to take greater economic “risks” to treat their cancer than the patients in our study, who may consider themselves cancer “survivors” and further removed from the threatening nature of the cancer diagnosis. This study is also limited by the fact that we did not ask patients to consider toxicity associated with treatment, given the importance of quality of life for cancer patients [19]. We are currently undertaking a larger study in which we ask patients to consider both toxicity and benefits in assigning value to cancer treatments.
Because Fox Chase Cancer Center is a tertiary referral center, the study population may be more motivated and of higher than average socioeconomic status than the general population. In addition, most participants (87%) noted making no financially related sacrifices in order to afford treatment. This may bias our results toward a higher WTP, even for less effective treatments. However, as we noted earlier, even among this relatively homogeneous patient population, we still were able to identify several sociodemographic factors that influenced patients' WTP for treatments in the metastatic setting.
In conclusion, this study demonstrates that it is feasible to measure cancer patients' WTP for treatments in both the adjuvant and palliative settings. In addition, our results support the hypothesis that cancer patients' WTP for treatment may be influenced by both sociodemographic factors and their assessment of the treatment's value. If confirmed in a larger, more heterogeneous population, these findings suggest that insurance benefit designs based on treatment value may be feasible for cancer treatment. However, they also highlight the risk that higher out-of-pocket expenses may contribute to socioeconomic disparities in cancer care.
Acknowledgments
This project was supported by the American Cancer Society Institutional Research Grant 92-027-14 as well as P30 CA006927.
Author Contributions
Conception/Design: Yu-Ning Wong, Brian Egleston, Neal J. Meropol
Financial support: Yu-Ning Wong
Provision of study material or patients: Yu-Ning Wong
Collection and/or assembly of data: Yu-Ning Wong, Olivia Hamilton, Kevin Salador, Camara Murphy
Data analysis and interpretation: Yu-Ning Wong, Olivia Hamilton, Brian Egleston, Neal J. Meropol, Kevin Salador
Manuscript writing: Yu-Ning Wong, Olivia Hamilton, Brian Egleston, Neal J. Meropol
Final approval of manuscript: Yu-Ning Wong, Olivia Hamilton, Brian Egleston, Neal J. Meropol, Kevin Salador, Camara Murphy
References
- 1.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291:2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 2.Hsu J, Price M, Huang J, et al. Unintended consequences of caps on Medicare drug benefits. N Engl J Med. 2006;354:2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 3.National Survey of Households Affected by Cancer. Menlo Park, CA: The USA Today/Kaiser Family Foundation/Harvard School of Public Health; 2006. pp. 1–50. [Google Scholar]
- 4.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey SD. How should we pay the piper when he's calling the tune? On the long-term affordability of cancer care in the United States. J Clin Oncol. 2007;25:175–179. doi: 10.1200/JCO.2006.08.9805. [DOI] [PubMed] [Google Scholar]
- 7.Goldman DP, Joyce GF, Lawless G, et al. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25:1319–1331. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meropol NJ, Schulman KA. Cost of cancer care: Issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 9.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 10.Carroll J. When new drugs are costly, how high to raise copays? Manag Care. 2006;15:20–21. 25–26, 29–30. [PubMed] [Google Scholar]
- 11.Kaa K. Medicare challenges and solutions–reimbursement issues in treating the patient with colorectal cancer. J Manag Care Pharm. 2007;13(6 suppl C):S19–S26. doi: 10.18553/jmcp.2007.13.s6-c.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Day JC, Newburger EC. The big payoff: Educational attainment and synthetic estimates of work-life earnings. Curr Popul. 2002:23–210. Rep 23 Spec Stud. [Google Scholar]
- 16.Aristides M, Chen J, Schulz M, et al. Conjoint analysis of a new chemotherapy: Willingness to pay and preference for the features of raltitrexed versus standard therapy in advanced colorectal cancer. Pharmacoeconomics. 2002;20:775–784. doi: 10.2165/00019053-200220110-00006. [DOI] [PubMed] [Google Scholar]
- 17.Leighl NB, Tsao WS, Zawisza DL, et al. A willingness-to-pay study of oral epidermal growth factor tyrosine kinase inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2006;51:115–121. doi: 10.1016/j.lungcan.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Weinfurt KP. Value of high-cost cancer care: A behavioral science perspective. J Clin Oncol. 2007;25:223–227. doi: 10.1200/JCO.2006.08.9029. [DOI] [PubMed] [Google Scholar]
- 19.Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–3466. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]