Abstract
Aims/hypothesis
To examine whether retinal vessel diameter in persons with type 1 diabetes mellitus (T1DM) is associated with changes in subclinical anatomic and functional diabetic nephropathy indicators.
Methods
Persons with T1DM had both gradable fundus photographs and renal biopsy data at baseline and 5-year follow-up (n=234). Retinal arteriolar and venular diameters were measured at baseline and follow-up. Central retinal arteriolar (CRAE) and central retinal venule equivalent (CRVE) were computed. Baseline and 5-year follow-up renal structural parameters (e.g., mesangial fractional volume and glomerular basement membrane width (GBMW) and the mesangial matrix fractional volume and GBMW composite glomerulopathy index) were assessed by masked electron microscopic morphometric analyses from percutaneous renal biopsy specimens.
Results
While controlling for other covariates, baseline CRAE was positively associated with change in the glomerulopathy index over the 5 year period. Change in CRAE was inversely related to a change in mesangial matrix fractional volume while change in CRVE was directly related to change in the volume fractions of cortex which was interstitium [Vv(Int/cortex)] over the 5-year period. Baseline CRAE or CRVE or changes in these diameters were not related to changes in other renal anatomic or functional endpoints.
Conclusions/interpretation
In our study, independent of other factors, baseline CRAE correlated with changes in glomerulopathy index, a composite measure of extracellular matrix accumulation in the mesangium and GBM, while a narrowing of the CRAE was related to mesangial matrix accumulation. Changes in CRVE were related to changes in Vv(Int/cortex), a measure of interstitial expansion in persons with T1DM.
Keywords: Areas 1.02, Research field epidemiology 3.01.01, 3.06.05 nephropathy, 3.06.10 microvascular disease
Despite intensive glycemic and blood pressure (BP) control, diabetic nephropathy (DN) remains an important cause of morbidity and mortality in persons with type 1 diabetes mellitus (T1DM) [1–6]. Detection of persons at risk of DN prior to the development of clinical signs of glomerulopathy is essential in developing preventive approaches at an early reversible stage of the disease [6]. It is, therefore, important to anticipate progression of DN at its preclinical stages. In particular, renal lesions, through much of the natural history of DN, develop in clinical silence (Figure 1). Because carefully measured renal biopsies are not likely to become the standard in the care of persons with T1DM and because renal lesions may already be present before standard tests of renal dysfunction (e.g., microalbuminuria [MA]) reveal it, it is important to have earlier DN risk predictors [7]. Moreover, additional indicators are needed because MA, although useful, is an imprecise predictor of progression to proteinuria (P). We have previously reported significant associations between diabetic retinopathy (DR) and preclinical morphologic changes of DN in baseline assessments of normoalbuminuric (NA), normotensive T1DM persons in the Renin-angiotensin System Study (RASS) [8]. There is suggestive evidence that an additional measurement, assessment of retinal vessel diameter, might also be a useful predictor of chronic kidney disease in persons with T1DM [9,10]. In this report we examine whether retinal vessel diameter (Figure 2) in RASS patients is associated with changes in subclinical anatomic and functional signs of DN.
Figure 1.
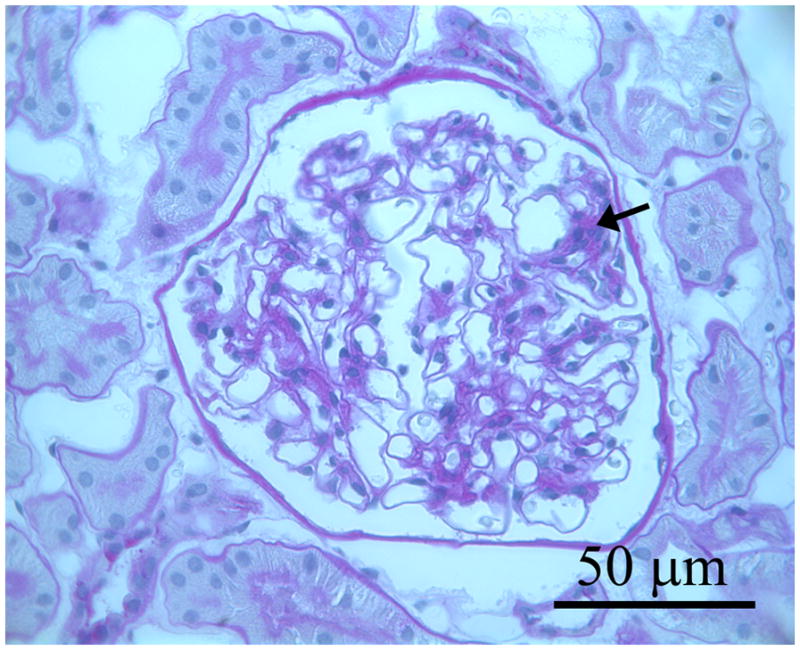
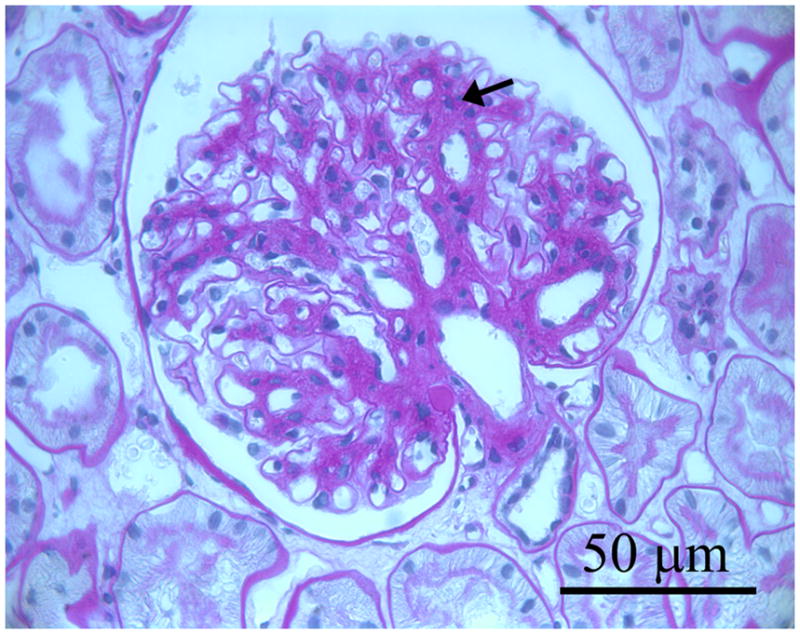
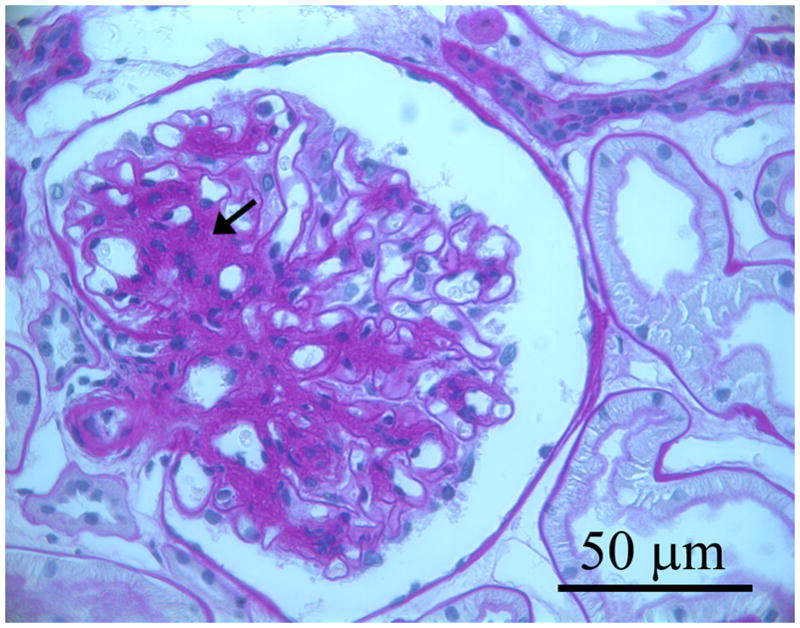
Three photomicrographs from three separate baseline biopsies of glomerular tissues for light microscopy that were fixed in Zenker’s solution and embedded in paraffin and stained with periodic acid Schiff (PAS): a. glomerulus in which the mesangium appears normal; b. shows a glomerulus with mild mesangial expansion with increased PAS positive matrix material between the capillary loops and branching from the base of the glomerulus (hilus) to the periphery (black arrows); c. shows a glomerulus with moderate mesangial expansion (black arrows).
Figure 2.
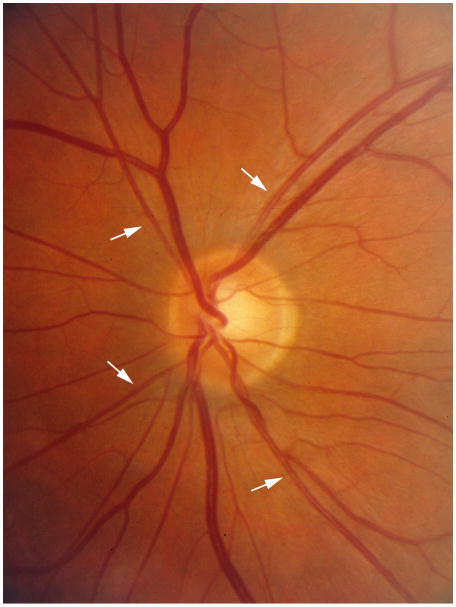
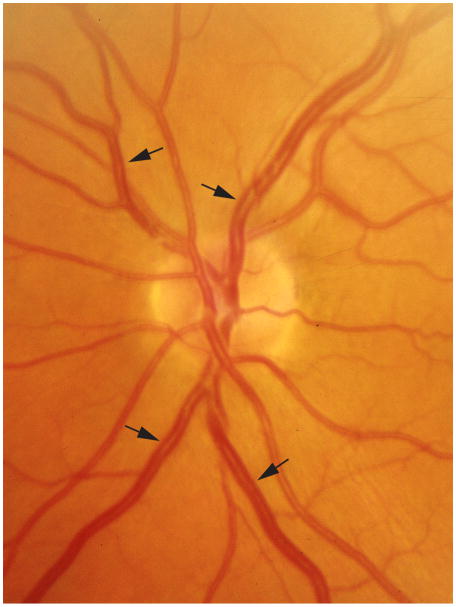
a. Retinal image of left eye showing narrow retinal arterioles (white arrows, CRAE 99:m, mean and standard deviation in the RASS 159±13:m) and normal retinal venules (CRVE 222:m, mean and standard deviation in the RASS 228±22:m) and b. Retinal image of a left eye showing wide retinal venules (black arrows, CRAE 177 :m, CRVE 304 :m) and normal retinal arterioles.
Research Design and Methods
Description of Cohort
The RASS was a parallel, double-blind, placebo controlled, multicenter, clinical trial of DN primary prevention and retinopathy development and progression conducted at three clinical centers in Minneapolis, Minnesota, United States and Montreal, Quebec and Toronto, Ontario, Canada. The study design and cohort description have been detailed elsewhere [11]. All data were collected with Institutional Review Board approval in conformity with all federal, state, and provincial laws, and the study was in adherence with the tenets of the Declaration of Helsinki as revised in 1983. Informed consent was obtained. Subjects were 15 years of age or older with 2 to 20 years of T1DM and onset before their 45th birthday. All were normotensive (<135/85 mmHg), NA (AER < 20 μg/min on at least 2 of 3 timed overnight urine collections), and had normal or increased GFR (GFR ≥ 90 ml/min/1.73 m2). Two hundred and eighty-five subjects were randomized into one of the following three treatment groups: losartan (an angiotensin-receptor blocker or ARB), enalapril (an angiotensin converting enzyme inhibitor or ACEI), or placebo [11].
Blood Pressure, Weight, Height, and Retinal Measurements
The baseline examination included measurement of BP with the participant seated after resting for 5 minutes, using an automated BP device (DinaMap Vital Signs Monitor #18465X, Critikon Inc., Palatine, IL, USA). Height and weight were measured according to standard anthropometric procedures. Pupils were dilated, and 30° color stereoscopic fundus photographs were taken of the seven standard fields as defined in the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol [12]. The photographs were graded in a masked fashion by the University of Wisconsin Ocular Epidemiology Reading Center using the modified Airlie House Classification scheme and the ETDRS retinopathy severity scale. Grading protocols and retinopathy severity scales have been described in detail elsewhere [12,13]. DR severity, based on the more severe eye, was grouped as follows: None (Level 10), Mild DR (Levels 20–43) and Moderate to Severe DR (Levels ≥ 47).
Retinal vessel diameters were measured at baseline and at the 5-year follow-up RASS examinations using a computer-assisted technique based on a standard protocol which entailed the following: retinal photographs of field 1 (centered at the optic nerve head), were converted to digital images by a high-resolution scanner using identical settings for all photographs [14]. Retinal vessel measurements were done independently for each examination and each eye. Trained graders masked to participant characteristics measured the diameters of all arterioles and venules coursing through a specified area one-half to one disc diameter surrounding the optic disc using a computer software program. On average, between 7 and 14 arterioles and an equal number of venules were measured per eye. Individual arteriolar and venular measurements were combined into summary indices that reflect the average retinal arteriolar (central retinal arteriole equivalent [CRAE]) and venular (central retinal venule equivalent [CRVE]) diameter of an eye based on the Parr-Hubbard-Knudtson formula [15]. Graders regularly participated in quality control exercises; the inter-and intra-grader variability was small (interclass and intraclass correlations > 0.90 for CRAE and CRVE.
Measures of Renal Function and Glycemia
GFR was estimated at baseline and follow-up by plasma disappearance of nonradioactive iohexol (Omnipaque 300, Shearing Co. as detailed elsewhere and expressed in ml/min adjusted up to 1.73m2) [16,17]. AER was measured in timed overnight urine collections using a sensitive fluorescence immunoassay [18] and expressed in μg/minute. NA was defined as having at least two of three AER readings < 20 μg/min. Glycosylated hemoglobin was measured by HPLC (DIAMAT glycosylated hemoglobin analyzer, BioRad, Hercules, CA, USA) until April 2002 when the TOSOH method was introduced (Tosoh Medics Inc., San Francisco, CA, USA) [19].
Electron Microscopy (EM)
Baseline percutaneous renal biopsies were obtained within six months prior to and follow-up biopsies five years after randomization [20]. Tissue processing was as described elsewhere [8]. The center-most intact glomeruli in the EM blocks were selected for study and an image calibration grid was obtained at the beginning of each microscopy session [21]. EM digital images (X3900) stored in tagged image file format (TIFF) format (Adobe Photoshop 6.0 software, San Jose, CA, USA) were used to build montages of entire glomerular cross-sections on which counting grids were superimposed. These montages were used for the measurement of the fraction of the glomerulus occupied by the mesangium or mesangial fractional volume [Vv(Mes/glom)], mesangial matrix fractional volume [Vv(MM/glom)], mesangial cell fractional volume (Vv(MC/glom)], and surface density (surface/volume) of peripheral glomerular capillary GBM [Sv(PGBM)/glom] [8,21–26]. After the center-most glomerulus in a block was randomly entered, 10 to 20 evenly-spaced digital images representing an unbiased systematic sample of approximately 30% of the glomerular profile was obtained at x11,000 for measurement of GBM width [21,22,24,25] and mesangial composition [8,21,26].
A glomerulopathy index (GI = GBM width/10 + [Vv(MM/glom) × 100]), a composite expression of glomerular extracellular matrix accumulation in diabetes, was computed using the formula as described by Rudberg et al. [27]. All EM determinations were by a single masked observer.
Light microscopy
Zenker’s fixed periodic acid-Schiff stained light microscopy slides were randomly selected for determining the index of arteriolar hyalinosis (IAH) in all cortical arterioles ≤ 1 average tubular diameter in size based on the fraction of each arteriolar wall replaced by hyaline as simultaneously estimated by two masked observers. IAH was calculated as published [22]. Volume fractions of cortex which was interstitium [Vv(Int/cortex)] and of cortical tubules per total tubules which were atrophic [Vv(AT/TT)] were estimated by a masked observer at X300 by point counting [28].
Statistics
Statistical analyses were conducted in SAS version 9 (SAS Institute Inc., Cary, NC, USA). Means were compared for statistically significant differences by the t-test and analysis of variance when two or more than two groups, respectively, were involved. Pearson correlation coefficients were computed for pair-wise associations of variables. Multivariable associations between renal anatomic and functional endpoints (and changes in these measures) with CRAE and CRVE and other independent variables such as age, sex, duration of diabetes, glycosylated hemoglobin, BP, and body mass were explored by multiple linear regression. Similarly, multivariable relationships between renal anatomic and functional characteristics defined as normal or abnormal with CRAE or CRVE and other independent variables were evaluated by logistic regression and selected covariates in the model were chosen because they were statistically significantly associated with the specific outcome.
Results
Characteristics of the cohort baseline examination
Of the 285 participants, one had insufficient glomeruli for renal morphometric measurements, 28 had renal biopsy data without gradable fundus photographs for retinal vessel measurement, and 257 had both gradable fundus photographs and biopsy data at baseline. Persons without gradable fundus photographs and biopsy data were older than those without gradable fundus photographs and biopsy data (p=0.002); there were no statistically significant differences between groups for other characteristics [8].
Baseline renal and retinal characteristics of the cohort are presented in Table 1 for 257 of the 285 persons with data available for both vessel measurement and at least one of the anatomic or functional DN measures. The cohort had a mean age of 30.2 ± 9.6 years, a mean duration of T1DM of 11.3 ± 4.7 years, and a mean glycosylated hemoglobin level of 8.5 ± 1.6%. Ninety-two per cent of the cohort had no (36.6%) or mild (55.3%) DR (Table 1).
Table 1.
Baseline Characteristics of the Renin-angiotensin System Study Cohort.
|
N=257 |
||
|---|---|---|
| Variable | Mean or % | SD |
| Age (years) | 30.2 | 9.6 |
| Sex (% male) | 48 | |
| Diabetes duration (years) | 11.3 | 4.7 |
| Glycosylated hemoglobin(%) | 8.5 | 1.6 |
| Body mass (kg/m2) | 25.8 | 3.7 |
| Systolic blood pressure (mmHg) | 120 | 12 |
| Diastolic blood pressure (mmHg) | 70 | 8 |
| Mean arterial blood pressure (mmHg) | 87 | 9 |
| Serum creatinine (mg/dl) | 0.81 | 0.14 |
| Glomerular filtration rate (ml • min • 1.73 m2) | 129 | 20 |
| Albumin excretion rate (μg/min) | 6.4 | 5.9 |
| Mesangial fractional volume/glomerulusa | 0.21 | 0.04 |
| Mesangial matrix fractional volume/glomerulusa | 0.11 | 0.03 |
| Mesangial cell fractional volume/glomerulusa | 0.072 | 0.020 |
| Glomerular basement membrane widtha (nm) | 476 | 92 |
| Peripheral GBMb surface density/glomerulusa | 0.13 | 0.02 |
| Volume fractions of cortex which was interstitiumb | 0.110 | 0.036 |
| Volume fraction cortical tubules per total tubules which were atrophicc | 0.004 | 0.019 |
| Index of arteriolar hyalinosisd | 1.1 | 0.2 |
| Glomerulopathy indexa | 58.1 | 10.6 |
| Diabetic Retinopathy Severity | ||
| None, % | 34.6 | |
| Mild, % | 55.3 | |
| Moderate to Severe, % | 10.2 | |
| Central retinal arteriole equivalent (μm) | 159 | 13 |
| Central retinal venule equivalent (μm) | 228 | 22 |
GBM: glomerular basement membrane.
N = 256
N = 223
N = 210
N = 231
Relationships of CRAE and CRVE to Change in Renal Endpoints
Of the 257 participants with both gradable retinal vessel measurements and biopsy data at baseline, 234 had both gradable fundus photographs and biopsy data at follow-up. Persons with biopsy data at follow-up were younger than those without (p=0.03); additionally, persons without both follow-up biopsy data and gradable fundus photographs had larger baseline Vv(Mes/glom) (p=0.04) and Vv(MM/glom) (p=0.002) than those with biopsy data, otherwise, there were no statistically significant differences between groups for other characteristics [8].
The univariate relationships of baseline retinal characteristics to changes in renal endpoints are presented in Table 2. CRAE was associated with change in glomerulopathy index (GI), while CRVE was associated with change in Vv(MM/glom), Sv(PGBM/glom), and GI. Relationships of CRAE to changes in renal endpoints, controlling for other factors are presented in Tables 3 and 4. While controlling only for duration of diabetes, CRAE was associated with change in GBM width, Vv(MM/glom), GI, and Sv(PGBM/glom). The relationship of CRAE with change in GI remained statistically significant after adding other covariates to the model. These relationships did not change when DR severity was added to the models (data not shown). Other relationships of CRAE and changes in renal structural endpoints were not statistically significant.
Table 2.
Correlations of Central Retinal Arteriole Equivalent (CRAE) and Central Retinal Venule Equivalent (CRVE) at Baseline with Change in Diabetic Nephropathy Measures and Incident Abnormal Values over 5-year of Follow-up.
| CRAE |
CRVE |
||||
|---|---|---|---|---|---|
| N at riska | rb | P | rb | P | |
| Change in: | |||||
| Glomerular filtration rate | 236 | −0.03 | 0.61 | 0.00 | 0.98 |
| Albumin excretion rate | 234 | 0.00 | 0.96 | 0.01 | 0.93 |
| Mesangial fractional volume/glomerulus | 234 | 0.08 | 0.20 | 0.07 | 0.28 |
| Mesangial matrix fractional volume/glomerulus | 234 | 0.12 | 0.07 | 0.13 | 0.05 |
| Mesangial cell fractional volume/glomerulus | 234 | −0.02 | 0.71 | −0.00 | 0.99 |
| Glomerular basement membrane (GBM) width (nm) | 234 | 0.11 | 0.08 | 0.10 | 0.12 |
| Peripheral GBM surface density/glomerulus | 234 | −0.11 | 0.09 | −0.16 | 0.02 |
| Volume fraction of cortex which was interstitium | 183 | −0.03 | 0.64 | 0.08 | 0.26 |
| Volume fraction cortical tubules per total tubules which were atrophic | 165 | −0.09 | 0.23 | −0.011 | 0.16 |
| Glomerulopathy index | 234 | 0.14 | 0.03 | 0.13 | 0.04 |
| Incident abnormal: | |||||
| Mesangial fractional volume/glomerulus (n = 30) | 206 | −0.05 | 0.50 | −0.04 | 0.61 |
| Mesangial matrix fractional volume/glomerulus (n = 43) | 165 | 0.11 | 0.14 | 0.12 | 0.13 |
| Mesangial cell fractional volume/glomerulus (n = 20) | 216 | 0.02 | 0.74 | −0.00 | 0.95 |
| GBM width (n = 27) | 93 | 0.09 | 0.41 | 0.10 | 0.35 |
| Peripheral GBM surface density/glomerulus (n = 61) | 206 | 0.08 | 0.25 | 0.14 | 0.05 |
| Glomerulopathy index (n = 27) | 86 | 0.23 | 0.04 | 0.22 | 0.04 |
Numbers at risk vary due to gradable measurement of nephropathy measures and those with prevalent abnormal values at baseline.
r = Pearson correlation for continuous measures (top 9 rows) and Spearman correlation for categorical measures (bottom 6 rows).
Table 3.
Relation of Central Retinal Arteriole Equivalent and Central Retinal Venule Equivalent at Baseline to Change in Renal Endpoint Models.
| Dependent Variable | Independent Variable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Central retinal arteriole equivalent per SD |
Central retinal venule equivalent per SD |
|||||||
| Duration adjusted |
Multivariatea adjusted |
Duration adjusted |
Multivariatea adjusted |
|||||
| Est (SE) | P | Est (SE) | P | Est (SE) | P | Est (SE) | P | |
| Change in glomerular filtration rate | −0.52 (1.15) | 0.65 | 0.08 (1.13) | 0.94 | ||||
| Change in albumin excretion rate | 0.04 (1.39) | 0.97 | 0.09 (1.37) | 0.95 | ||||
| Change in mesangial fractional volume/glomerulus | 0.0051 (0.0033) | 0.13 | 0.0042 (0.0033) | 0.20 | ||||
| Change in mesangial matrix fractional volume/glomerulus | 0.0045 (0.0022) | 0.04 | 0.0032 (0.0022) | 0.14 | 0.0046 (0.0021) | 0.03 | 0.0033 (0.0021) | 0.13 |
| Change in mesangial cell fractional volume/glomerulus | −0.0003 (0.0015) | 0.86 | 0.0002 (0.0015) | 0.89 | ||||
| Change in volume fraction of cortex which was interstitium | −0.0032 (0.0044) | 0.46 | 0.0040 (0.0043) | 0.36 | ||||
| Change in volume fraction cortical tubules per total tubules which were atrophic | −0.0012 (0.0036) | 0.73 | −0.0019 (0.0035) | 0.58 | ||||
| Change in glomerular basement membrane width (nm) | 9.30 (4.47) | 0.04 | 7.29 (4.43) | 0.10 | 8.06 (4.38) | 0.07 | 5.03 (4.36) | 0.25 |
| Change in glomerulopathy index | 1.38 (0.55) | 0.01 | 1.06 (0.53) | 0.05 | 1.27 (0.54) | 0.02 | 0.81 (0.52) | 0.12 |
From linear regression model.
Multivariate adjustment:
Change in glomerular basement membrane (GBM) width (nm): diabetes duration, glycosylated hemoglobin, sex.
Change in glomerulopathy index: diabetes duration, glycosylated hemoglobin, sex, drug trial treatment.
Change in peripheral GBM surface density/glomerulus: diabetes duration, glycosylated hemoglobin, study site.
Change in mesangial matrix fractional volume/glomerulus: diabetes duration, glycosylated hemoglobin.
Table 4.
Relation of Central Retinal Arteriole Equivalent and Central Retinal Venule Equivalent at Baseline to 5-year Incidence of Renal Abnormality Models.
| Incident Variable | Independent Variable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Central retinal arteriole equivalent per SD |
Central retinal venule equivalent per SD |
|||||||
| Duration adjusted |
Multivariate adjusteda |
Duration adjusted |
Multivariate adjusteda |
|||||
| OR (95% CI)b | P | OR (95% CI) b | P | OR (95% CI)b | P | OR (95% CI)b | P | |
| Abnormal mesangial fractional volume/glomerulus | 0.86 (0.58, 1.27) | 0.45 | 0.88 (0.59, 1.31) | 0.53 | ||||
| Abnormal mesangial matrix fractional volume/glomerulus | 1.38 (0.96, 1.98) | 0.08 | 1.30 (0.92, 1.85) | 0.14 | ||||
| Abnormal mesangial cell fractional volume | 1.03 (0.64, 1.64) | 0.91 | 1.00 (0.63, 1.58) | 0.99 | ||||
| Abnormal glomerular basement membrane (GBM) width | 1.36 (0.85, 2.19) | 0.20 | 1.38 (0.85, 2.23) | 0.19 | ||||
| Abnormal peripheral GBM surface density/glomerulus | 1.23 (0.90, 1.67) | 0.19 | 1.37 (1.01, 1.85) | 0.04 | 1.07 (0.76, 1.50) | 0.70 | ||
| Abnormal glomerulopathy index | 1.93 (1.16, 3.20) | 0.01 | 1.48 (0.76, 2.86) | 0.25 | 1.81 (1.07, 3.05) | 0.03 | 1.25 (0.64, 2.45) | 0.51 |
Multivariate adjustment:
Abnormal peripheral GBM surface density/glomerulus: diabetes duration, glycosylated hemoglobin, study site.
Abnormal glomerulopathy index: diabetes duration, glycosylated hemoglobin, age.
OR (95% CI) from logistic regression model.
While controlling for duration of diabetes, CRVE was positively associated with change in Vv(MM/Glom), GI, Sv(PGBM), and with the incidence of abnormal GBM, and abnormal GI (Tables 3 and 4). None of these relationships remained statistically significant while controlling for other covariates in the model.
Relationships of Change in CRAE and CRVE to Changes in Renal Endpoints
Changes in CRAE over the five year period were related to changes in Vv(MM/glom) (r=−0.14, p=0.04) and incident abnormal Vv(MM/glom) (r=−0.17, p=0.03) while changes in CRVE were related to changes in Vv(Int/cortex) (r=0.20, p<0.01). The relation of changes in CRAE remained associated with changes in Vv(MM/glom) and incident abnormal Vv(MM/glom) and that between changes in CRVE and changes in Vv(Int/cortex) while controlling for other risk factors (data not shown). Changes in CRAE or CRVE were not statistically significantly associated with changes in other renal parameters (data not shown).
Discussion
Changes in retinal vessel diameters occur early in the course of T1DM, possibly reflecting structural and/or functional alterations resulting from changes in retinal blood flow and the effects of chronic hyperglycemia, inflammation, and endothelial dysfunction [29,30]. Retinal arteriolar narrowing (a small CRAE), for example, is thought to be related to cumulative small vessel damage from the effects of hypertension while retinal venular widening (a large CRVE) has been thought to reflect retinal hyperperfusion and/or lactic acidosis associated with hyperglycemia and hypoxia (Figure 2) [14,29,30]. In persons with T1DM, independent of DR severity, narrower retinal arteriolar and wider retinal venular diameters are thought to reflect microvascular changes from processes described above [31–38]. These may be markers of similar changes in the microvascular diameters in the cerebral, coronary, peripheral, and renal circulations in persons with or without T1DM or indicators of pathogenetic processes damaging to other targets of diabetic microvascular injury [32,38,39].
The RASS is the first study to examine the relationship of CRAE and CRVE with changes in diabetic renal structural parameters in sequential research biopsies in T1DM persons. CRAE and CRVE were associated with abnormalities and increases in renal structural parameters which reflect extracellular matrix accumulation, the central pathologic processes leading to renal dysfunction in DN [7,40]. These included increases in GBM width and mesangial matrix fractional volume that, as the composite GI, are closely associated with albuminuria in T1DM and decrease in glomerular filtration surface density [Sv(PGBM] which results from mesangial expansion [40] and are ultimately associated with GFR loss in T1DM (Figure 1) [23].
In the main, these relationships were independent of diabetes duration or DR severity. Speculation on these associations could take several directions. Increased CRVE and CRAE could be related to increased retinal blood flow which was perhaps mirrored by increases in renal blood flow, long posited as a candidate mechanism for DN pathogenesis through hemodynamic injury [41]. Alternately, both increased retinal and renal blood flow could reflect compensatory responses to metabolic disturbances which could be the drivers of tissue injury in diabetes, such as relative tissue hypoxia [42] or oxidative stress [43].
Regardless of the mechanism, the RASS, which directly compares structural changes at relatively early stages of DN and DR, adds information indicating important relationships between these two microvascular complications which could not be derived from renal functional studies, since these were all normal at RASS baseline. It is not clear from these data whether the associations between the baseline retinal diameters parameters and the change in important DN structural parameters in T1DM patients without renal functional abnormalities will provide added precision to early DN risk predictions [44]. In contrast to the relationship of baseline CRAE and renal structural change as measured by GI, an increase in the CRAE over the 5 years of RASS was inversely related to Vv(MM/glom). Clearly, longer-term studies involving larger numbers of type 1 patients with hypertension and albuminuria would be necessary to further describe and unravel these complex dynamic relationships.
In the RASS, an increase in the CRVE was related to an increase in Vv(Int/cortex). The latter is measure of interstitial expansion which at these earlier stages of DN would mainly result from an increase in the cellular component of the interstitium [28]. Wider retinal venules are associated with systemic inflammation, consistent with the finding that the latter may be involved in the early increase in Vv(Int/cortex) [45].
In the RASS, there were no relationships found between retinal vessel diameters and renal functional endpoints. This was in contrast to the findings in persons with T1DM in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) [9]. In that study, there was an increased risk of incident proteinuria (RR comparing 4th to 1–3rd quartiles, 1.53, 95% CI 1.19, 1.97], and renal insufficiency (RR 1.41, 95% CI 1.05, 2.17) in persons with wider retinal venular diameters independent of age, sex, duration of diabetes, glycosylated hemoglobin levels, baseline DR severity, and other factors. No relation was found with retinal arteriolar diameter at baseline with these renal endpoints in that study. Comparisons of RASS findings with those from the WESDR are limited due to differences in the two cohorts. The findings from the WESDR are with functional outcomes in a diabetic cohort that included persons with hypertension and microalbuminuria at baseline, while those in the RASS cohort were all normotensive and NA at baseline. Moreover, WESDR participants were studied in a period of poorer glycemic control. Perhaps if the RASS had included hypertensive albuminuric persons with longer duration of type 1 diabetes, as in the WESDR, stronger associations of retinal vessel diameters with renal anatomic abnormalities might have been found.
There are a number of strengths to our study, including the objective determination of retinal vessel caliber using standardized protocols and the use of careful morphometric techniques in 2 research renal biopsies spaced 5 years apart. However, caution should be observed in interpreting the findings herein. It is possible that the ARB or ACEI treatment used in the RASS masked a possible relationship of retinal vessel measurements to renal endpoints. Although, neither treatment arm in our study was shown to have a protective affect on renal structural or functional endpoints [46], both ARB and ACEI treatment significantly reduced retinopathy progression in RASS. This may have limited detection of associations of changes in CRAE or CRVE and renal structural or functional endpoints that might have been present in a natural history study. However, when the analyses were limited to the 85 persons in the placebo group the associations of CRAE or CRVE to the renal endpoints were essentially unchanged (Klein R. Unpublished data, 2010). Also, our study included estimates of CRAE and CRVE taken at a single and random point in the pulse cycle. However, we have previously shown that the effect of variability from the pulse cycle was not large and was unlikely to affect any of the associations under study [47]. It is possible that the measure chosen as a marker of narrowing of the retinal arterioles, CRAE, may not have been as sensitive as other measures of structural and physiological changes in the microvasculature, e.g., retinal arteriole wall-to-lumen ratio, resulting in diminishing our ability to demonstrate stronger relationships with renal structural abnormalities [48].
In summary, retinal vessel diameters were related to the early stages of DN lesion development in matrix accumulation and interstitial expansion but were unrelated to changes in renal function in normotensive NA persons with T1DM. At this point in time we can only speculate that widened retinal venular diameter reflects the cumulative effects of various diabetic processes on the systemic microvasculature, including the kidney. However, it is too early to use this measure clinically to determine risk for renal anatomic damage prior to the appearance of functional changes, e.g., albuminuria and decreasing glomerular filtration in people with type 1 diabetes
Acknowledgments
This study was funded by research grants from Juvenile Diabetes Research Foundation (R. Klein), National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease (NIH #DK51975); Merck (in the United States); Merck Frosst (in Canada); and Canadian Institutes of Health Research (CIHR) (#DCT 14281), Canada. The University of Minnesota General Clinical Research Center (GCRC) is supported by NIH (#M01 RR 00400). Additional support was given by the National Institutes of Health, National Eye Institute (# EY12198) and Research to Prevent Blindness (R. Klein, Senior Scientific Investigator Award), New York, NY. Dr. Suissa was the recipient of a Distinguished Investigator Award from the CIHR. The sponsor or funding organizations had no role in the design or conduct of this research.
Abbreviations used
- ACEI
Angiotensin-receptor-converting enzyme inhibitor
- ARB
Angiotensin-receptor blocker
- BP
Blood pressure
- CRAE
Central retinal arteriole equivalent
- CRVE
Central retinal venule equivalent
- DN
Diabetic nephropathy
- DR
Diabetic retinopathy
- ETDRS
Early Treatment Diabetic Retinopathy Study
- GBMW
Glomerular basement membrane width
- GI
Glomerulopathy index
- H
Retinal hemorrhages
- IAH
Index of arteriolar hyalinosis
- IRMA
Intraretinal microvascular abnormalities
- MA
Microalbuminuria
- NA
Normoalbuminuric
- P
Proteinuria
- RASS
Renin-angiotensin System Study
- SCr
Serum creatinine
- SP
Standard photo
- Sv(PGBM)/glom
Surface density (surface/volume) of peripheral glomerular capillary glomerular basement membrane
- T1DM
Type 1 diabetes mellitus
- TIFF
Tagged image file format
- Vv(AT/TT)
Cortical tubules per total tubules which were atrophic
- Vv(Int/cortex)
Volume fraction of cortex which was interstitium
- Vv(Mes/glom)
Mesangial fractional volume per glomerulus
- Vv(MM/glom)
Mesangial matrix fractional volume per glomerulus
- Vv(MC/glom)
Mesangial cell fractional volume per glomerulus
- WESDR
Wisconsin Epidemiologic Study of Diabetic Retinopathy
Footnotes
Duality of Interest:
Dr. Zinman, lecture fees, consulting fees, and research grants from Merck; and Dr. Klein reports being an advisory board member for AstraZeneca (through the DIRECT study), Pfizer, Lilly, and Novartis.
No other dualities of interest relevant to this article were reported.
No reprints
References
- 1.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Renal Data System, USRDS 2006 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 2.Reddi AS, Camerini-Davalos RA. Diabetic nephropathy. An update. Arch Intern Med. 1990;150:31–43. [PubMed] [Google Scholar]
- 3.Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- 4.Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am. 2004;88:1001–36. xi. doi: 10.1016/j.mcna.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 6.Rossing P. Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia. 2006;49:11–19. doi: 10.1007/s00125-005-0077-3. [DOI] [PubMed] [Google Scholar]
- 7.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17:339–352. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Zinman B, Gardiner R, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes. 2005;54:527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53:179–184. doi: 10.2337/diabetes.53.1.179. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114:1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Mauer M, Zinman B, Gardiner R, et al. ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2002;3:262–269. doi: 10.3317/jraas.2002.048. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(suppl):786–806. [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 15.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 16.Gaspari F, Perico N, Matalone M, et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol. 1998;9:310–313. doi: 10.1681/ASN.V92310. [DOI] [PubMed] [Google Scholar]
- 17.Gaspari F, Perico N, Remuzzi G. Application of newer clearance techniques for the determination of glomerular filtration rate. Curr Opin Nephrol Hypertens. 1998;7:675–680. doi: 10.1097/00041552-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Chavers BM, Simonson J, Michael AF. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984;25:576–578. doi: 10.1038/ki.1984.57. [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly S, Goodyer P, Mauer M. Comparing the automated versus manual method of needle biopsy for renal histology artefacts. Nephrol DialTransplant. 2008;23:2098–2100. doi: 10.1093/ndt/gfn061. [DOI] [PubMed] [Google Scholar]
- 21.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 22.Brito PL, Fioretto P, Drummond K, et al. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998;53:754–761. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 23.Ellis EN, Steffes MW, Goetz FC, Sutherland DE, Mauer SM. Glomerular filtration surface in type I diabetes mellitus. Kidney Int. 1986;29:889–894. doi: 10.1038/ki.1986.82. [DOI] [PubMed] [Google Scholar]
- 24.Jensen EB, Gundersen HJ, Østerby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979;115:19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 25.Steffes MW, Barbosa J, Basgen JM, Sutherland DE, Najarian JS, Mauer SM. Quantitative glomerular morphology of the normal human kidney. Lab Invest. 1983;49:82–86. [PubMed] [Google Scholar]
- 26.Steffes MW, Bilous RW, Sutherland DE, Mauer SM. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992;41:679–684. doi: 10.2337/diab.41.6.679. [DOI] [PubMed] [Google Scholar]
- 27.Rudberg S, Osterby R, Østerby R, Dahlquist G, Nyberg G, Persson B. Predictors of renal morphological changes in the early stage of microalbuminuria in adolescents with IDDM. Diabetes Care. 1997;20:265–271. doi: 10.2337/diacare.20.3.265. [DOI] [PubMed] [Google Scholar]
- 28.Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61:2058–2066. doi: 10.1046/j.1523-1755.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Moss SE, et al. Retinal vascular abnormalities in persons with type 1 diabetes. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110:2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122:76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 31.Wise GN, Dollery CT, Henkind P. The retinal circulation. Harper and Row; New York, NY: 1971. p. 325. [Google Scholar]
- 32.Wells RE, Herman M, Gorlin R. Microvascular changes in coronary heart disease. Circulation. 1966;237:33–34. [Google Scholar]
- 33.Wendland JP. Retinal arteriolosclerosis in age, essential hypertension, and diabetes mellitus. Trans Am Ophthalmol Soc. 1966;64:735–761. [PMC free article] [PubMed] [Google Scholar]
- 34.Dollery CT, Ramalho PS, Patterson JW. Retinal vasculary alterations in hypertension. In: Gross F, editor. Antihypertensive therapy; principles and practice, and international symposium. Springer; New York: 1966. p. 152. [Google Scholar]
- 35.Tso MO, Abrams GW, Jampol LM. Hypertensive retinopathy, choroidopathy, and optic neruopathy: clinical and pathophysiological approach to classification. In: Singerman LJ, Jampol LM, editors. Retinal and choroidal manifestations of systemic disease. Williams and Wilkins; Baltimore, MD: 1991. pp. 79–127. [Google Scholar]
- 36.Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 37.Apple DJ, Naumann GO. Retina. In: Naumann GO, Apple DJ, editors. Pathology of the eye. Springer-Verlag; New York, NY: 1986. pp. 580–583. [Google Scholar]
- 38.Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6:263–269. doi: 10.1161/01.str.6.3.263. [DOI] [PubMed] [Google Scholar]
- 39.Carlson EC. Scanning and transmission electron microscopic studies of normal and diabetic acellular glomerular and retinal microvessel basement membranes. Microsc Res Tech. 1994;28:165–177. doi: 10.1002/jemt.1070280302. [DOI] [PubMed] [Google Scholar]
- 40.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens. 2009 doi: 10.1097/MNH.0b013e32833240fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson JR, Chang K, Frangos M, et al. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 43.Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69–84. doi: 10.1055/s-2007-949721. [DOI] [PubMed] [Google Scholar]
- 44.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–709. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 45.Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 2008;74:860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 46.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knudtson MD, Klein BE, Klein R, et al. Variation associated with measurement of retinal vessel diameters at different points in the pulse cycle. Br J Ophthalmol. 2004;88:57–61. doi: 10.1136/bjo.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritt M, Harazny JM, Ott C, et al. Wall-to-lumen ratio of retinal arterioles is related with urinary albumin excretion and altered vascular reactivity to infusion of the nitric oxide synthase inhibitor N-monomethyl-L-arginine. J Hypertens. 2009;27:2201–2208. doi: 10.1097/HJH.0b013e32833013fd. [DOI] [PubMed] [Google Scholar]