Abstract
Rationale
Prostaglandin E (PGE)2, which increases intracellular cyclic AMP via activation of adenylyl cyclases (ACs), induces vasodilation and hyaluronan-mediated intimal thickening (IT) in the ductus arteriosus (DA) during late gestation. After birth, however, differential regulation of vasodilation and IT is preferable for treatment of patients with patent DA and DA-dependent congenital cardiac malformations.
Objective
Our objectives were to examine whether AC isoforms play differential roles in DA vasodilation and IT.
Methods and Results
AC2 and AC6 were more highly expressed in rat DA than in the aorta during the perinatal period. AC6-targeted siRNA counteracted PGE1-induced hyaluronan production in rat DA smooth muscle cells. Overexpression of AC6 enhanced PGE1-induced hyaluronan production and induced IT in DA explants. Furthermore, IT of the DA was less marked in mice lacking AC6 than in wild-type and AC5-deficient mice. Stimulation of AC2 attenuated AC6-induced hyaluronan production via inhibition of the p38 mitogen-activated protein kinase pathway and AC6-induced IT of the DA. An AC2/6 activator, 6-[N-(2-isothiocyanatoethyl) aminocarbonyl] forskolin (FD1), did not induce hyaluronan-mediated IT in DA explants, although an AC5/6 activator, 6-[3-(dimethylamino)propionyl]-14 15-dihydroforskolin (FD6) did. Moreover, FD1 induced longer vasodilation of the DA than did PGE1 without significant adverse effects in vivo.
Conclusions
AC6 is responsible for hyaluronan-mediated IT of the DA and AC2 inhibited AC6-induced hyaluronan production. Stimulation of both AC2 and AC6 by FD1 induced longer vasodilation without hyaluronan-mediated IT in the DA in vivo. FD1 may be a novel alternative therapy to current PGE therapy for patients with DA-dependent congenital heart disease.
Keywords: ductus arteriosus, patent, prostaglandins, muscle, smooth, vasodilation, remodeling
Introduction
Prostaglandin E (PGE)2 and PGE1 play principal roles in maintaining the patency of the ductus arteriosus (DA) during gestation. PGE1 is widely used to keep the DA open in patients with DA-dependent congenital heart diseases, since both PGE1 and PGE2 increase the intracellular concentration of cyclic AMP (cAMP), resulting in vasodilation in the DA1, 2. On the other hand, we have demonstrated that PGE-EP4-cAMP signals during late gestation increased hyaluronan production in the DA and consequently induced intimal thickening (IT), which is critical for permanent closure of the DA after birth3. Therefore, the effects of PGE1/2 on vasodilation and remodeling oppose each other in terms of regulation of the DA after birth. Differential regulation of vasodilation and IT in the DA would be preferable for patients who need PGE/anti-PGE therapy.
Since intracellular cAMP is synthesized by adenylyl cyclases (ACs), which are transmembrane enzymes activated by G protein-coupled receptors, including PGE receptors, ACs must play an important role in regulating vasodilation and remodeling in the DA. To date, nine different isoforms of membrane-bound forms of ACs (AC1 through AC9) have been identified in vertebrate tissues4. Most tissues express several AC isoforms, which exhibit remarkable diversities in their biochemical properties5, 6. Since smooth muscle cells (SMCs) in the DA exert biological properties distinct from SMCs in other vessels such as the aorta, we hypothesized that such properties are due, at least in part, to the distinct roles of specific AC isoforms in the DA.
In addition to the role of PGE1/2 in vasodilation, the PGE-AC-cAMP signal cascade has been shown to regulate vascular remodeling7, 8. For example, cAMP markedly inhibits proliferation of SMCs9 and reduces IT after arterial injury in vivo10, a process that shares many aspects with IT in the DA1, 11. Interestingly, several studies have demonstrated that PGE1/2 inhibits the proliferation of vascular SMCs7, 8, whereas others have reported that PGE2 stimulates the growth of vascular SMCs12, 13. Such diversities in the effects of PGE signaling might be related to differential expression of AC isoforms among vascular tissues. It has been difficult, however, to evaluate the contribution of ACs to relevant phenomena in an AC isoform-dependent manner, since multiple isoforms of ACs are co-expressed. This is partially due to the lack of available AC isoform-selective pharmacological regulators. In previous studies, we synthesized more than 200 new derivatives of forskolin (a non-isoform selective AC activator) and identified derivatives that are selective to specific AC isoforms6, 14, 15. Such AC isoform-selective activators enable us to explore the role of each AC isoform in vascular tone and remodeling especially in vivo. In the present study, using such AC isoform-specific activators, overexpression or selective silencing of AC isoforms and AC-isoform deficient-mice, we have investigated the role of AC isoforms and the availability of AC isoform-selective activators in regulating DA vascular tone and remodeling.
Methods
See the online-only Data Supplement for additional details.
Reagents
Forskolin derivatives: 6-[N-(2-isothiocyanatoethyl) aminocarbonyl]forskolin (FD1)14, 16, and 6-[3-(dimethylamino)propionyl]-14 15-dihydroforskolin (FD6)14 were kindly provided by Nippon Kayaku Co., Ltd (Tokyo, Japan).
Animals and Tissues
All animals were cared for in compliance with the guiding principles of the American Physiologic Society. The experiments were approved by the ethical committee of animal experiments at Yokohama City University School of Medicine. Wistar rat embryos were obtained from timed-pregnant mothers (Japan SLC Inc., Shizuoka, Japan). Pooled tissues of DA and aorta were obtained from rat embryos on embryonic day 19 (e19, n>60) and day 21 (e21, n>60) and neonates on the day of birth (day0, n>60). Generation and phenotypes of AC5 knockout mice (AC5KO) and AC6 knockout mice (AC6KO) have been described previously17, 18. All mice were littermates from heterozygote crosses.
Isolation and Culture of Rat Ductus Arteriosus Smooth Muscle Cells (DASMCs)
Vascular SMCs were obtained from DA and aorta of Wistar rat embryos at e21 as previously described19.
Quantitative and Semi Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Isolation of total RNA and generation of cDNA were performed and RT-PCR analysis was done as previously described19. The primers were designed based on the rat nucleotide sequences of AC isoforms. Each primer set was designed between multiple exons (Online Table I), and PCR products were confirmed by sequencing. The abundance of each gene was determined relative to the GAPDH transcript using TaqMan Rodent GAPDH control reagents kits (Applied Biosystems, Foster City, CA).
Immunoblot Analysis
Proteins from whole cells were analyzed by immunoblotting as previously described19.
RNA Interference (siRNA)
Double-stranded siRNAs to the selected regions of AC2-7 and the negative siRNA used as a control were purchased from QIAGEN (Hilden, Germany) or Invitrogen (San Diego, CA) (Online Table II). According to the manufacturer’s instruction, cells were transfected with siRNA (300 pmol), using Lipofectamin RNAiMAX (Invitrogen).
Adenovirus Construction
Full-length cDNA-encoding rat AC2 was cloned into the shuttle vector for construction of an adenoviral vector harboring AC2 through the use of an AdenoX adenovirus construction kit (Clontech, Tokyo, Japan). Adenovirus encoding murine AC6 driven by a cytomegalovirus promoter was generated by homologous recombination as previously described20. Adenovirus encoding MKK3 was kindly provided by Dr. Yibin Wang at University of California, Los Angeles21.
cAMP Production by Radioimmunoassay
DASMCs were serum-starved for 48 h and assayed for cAMP production by RIA after incubation with drugs of interest (Supplemental methods).
Quantitation of Hyaluronan
The amount of hyaluronan in the cell culture supernatant was measured by the latex agglutination method as previously described3.
Organ Culture
DA organ culture was performed as previously described3, 22.
Measurement of Isometric Tension of the Vascular Rings of DA
Isometric tension of the vascular rings of DA was measured as previously described23.
Rapid Whole-Body Freezing Method
To study the in situ morphology and inner diameter of neonatal DA, a rapid whole-body freezing method was used as previously described24. The fetuses at e21 were delivered by cesarean section and intraperitoneally injected 1 h after birth with PGE1, FD1 or FD6 in 200 μL of saline. The minimal dose of FD1 (10.8 mg/kg of body weight) and FD6 (1.29 mg/kg of body weight) that caused maximal dilation in the DA were used.
Protein Kinase A (PKA) Activity
PKA activity was measured using an assay kit (StressGen Biotechnologies, Ann Arbor, MI) according to the manufacturer’s instructions as previously described25.
Statistical Analysis
Data are shown as the mean ± SEM of independent experiments. Statistical analysis was performed between two groups by unpaired Student t-test or between multi-groups by one-way ANOVA followed by Student-Newmans-Keuls multiple comparison test. A value of P<0.05 was considered significant.
Results
Multiple Transcripts of AC Isoforms in Rat DA
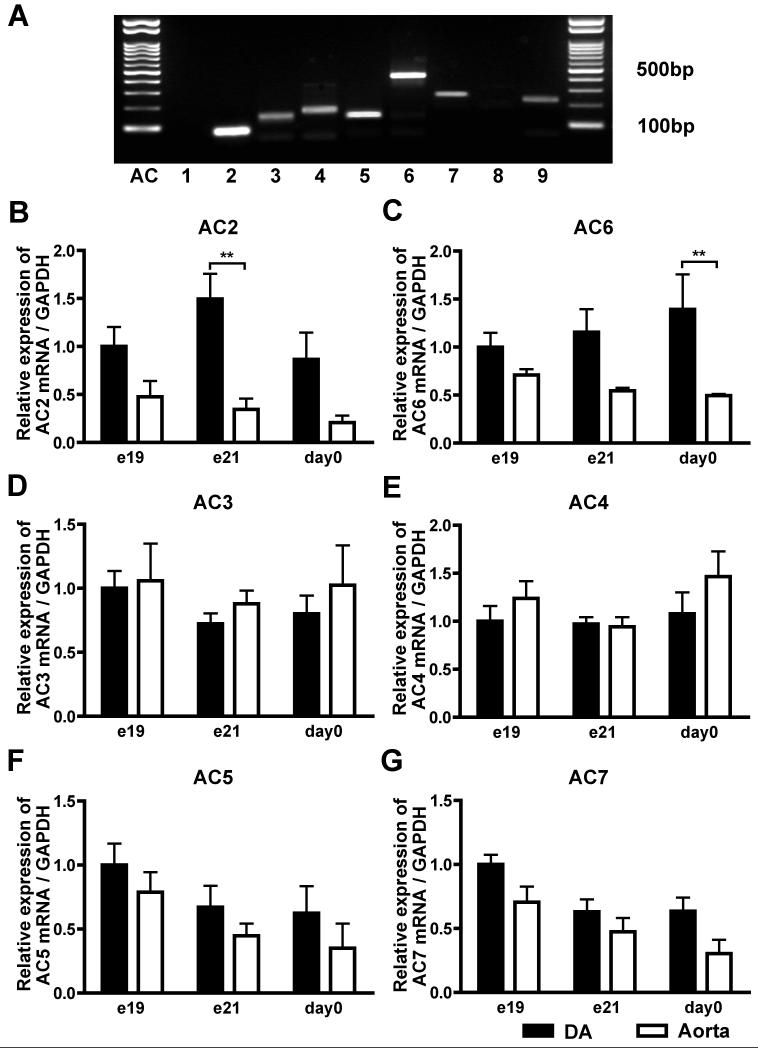
First, we detected all isoforms except for AC1 and AC8 in rat e21 DA by semi-quantitative analyses (Figure 1A). Next, quantitative RT-PCR analyses of AC2-7 showed that AC2, AC5, and AC6 were abundantly expressed in rat DA and that the expression levels of AC2 and AC6 were significantly higher in the DA than in the aorta during the perinatal period, whereas those of AC5 were comparable between the DA and the aorta. The expression of AC2 reached maximal level in e21 DA (Figures 1B), whereas that of AC6 was increased during development in rat DA (Figures 1C).
Figure 1.
Multiple transcripts of AC isoforms in rat DA. (A) mRNA expression of AC isoforms using semi-quantitative RT-PCR in rat e21 DA. (B) Quantitative RT-PCR analyses of AC2-7. **P<0.01. Data are from six independent experiments.
AC6 is Responsible for Hyaluronan Production in DASMCs
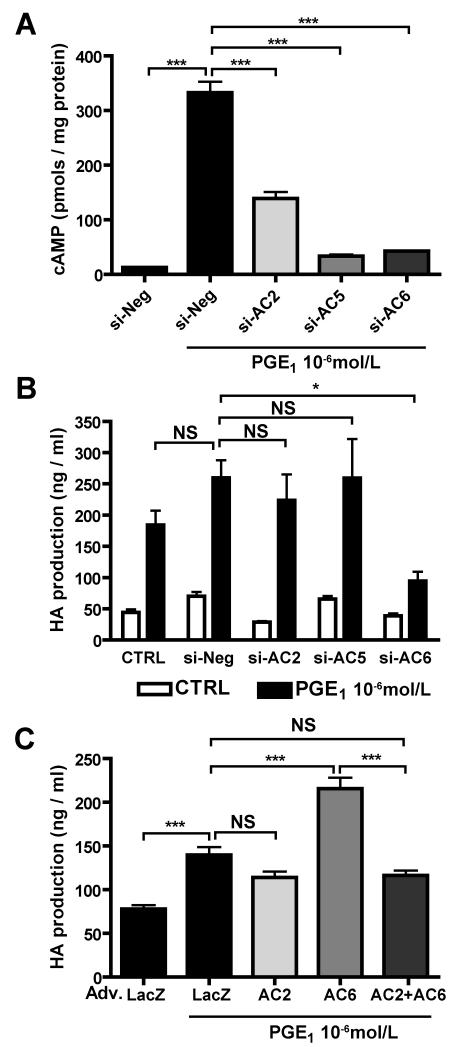
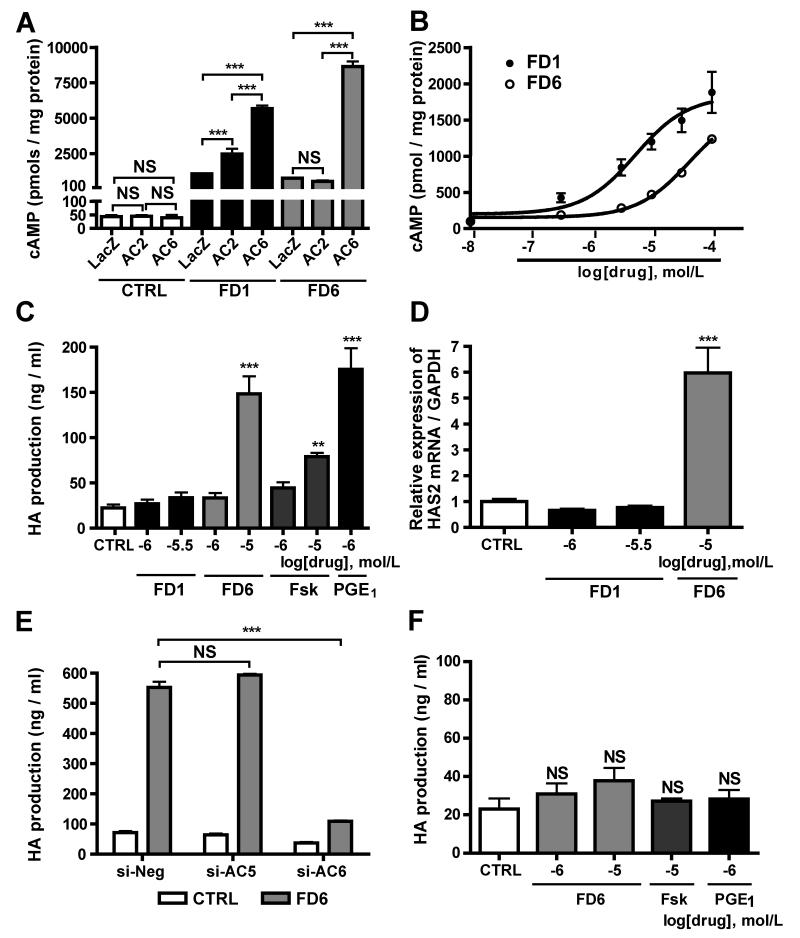
We examined the contribution of AC2, AC5 and AC6 to PGE1-induced cAMP production in DASMCs by using AC2-, AC5-and AC6-targeted siRNAs. The expression levels of ACs mRNAs using the siRNAs are shown in Online Figure I. Silencing of AC5 or AC6 dramatically decreased PGE1-induced cAMP production and that of AC2 also decreased PGE1-induced cAMP production by 58% (Figure 2A), indicating that AC2, AC5 and AC6 are major isoforms responsible for cAMP production by PGE1 in DASMCs. We then examined the effect of ACs on hyaluronan production in DASMCs. AC6-targeted siRNA weakened PGE1-induced hyaluronan production, whereas AC2-and AC5-targeted siRNA did not (Figure 2B). Neither AC3-, AC4-nor AC7-targeted siRNA weakened PGE1-induced hyaluronan production (Online Figure II). Using adenovirus-mediated gene transfer of AC2 and AC6 (Adv.AC2 and Adv.AC6), efficacy of which is shown in Online Figure III, we found that the overexpression of AC6, but not of AC2, further enhanced PGE1-induced hyaluronan production when compared with the overexpression of LacZ as a control (Figure 2C). Interestingly, co-overexpression of both AC2 and AC6 negated AC6-mediated enhancement of hyaluronan production.
Figure 2.
AC6 is responsible for hyaluronan (HA) production in DASMCs. (A) PGE1 induced cAMP accumulation in DASMCs in the cells treated with negative siRNA. AC2-, AC5-and AC6-targeted siRNA decreased PGE1-induced cAMP production (n=4). (B) AC6-targeted siRNA attenuated PGE1-induced hyaluronan production, whereas AC2-and AC5-targeted siRNA did not (n=7-11). (C) Adv.AC6 enhanced PGE1-induced hyaluronan production. Adv.AC2 abolished the Adv.AC6-induced enhancement of hyaluronan production (n=4). *P<0.05 and ***P<0.001. NS: not significant.
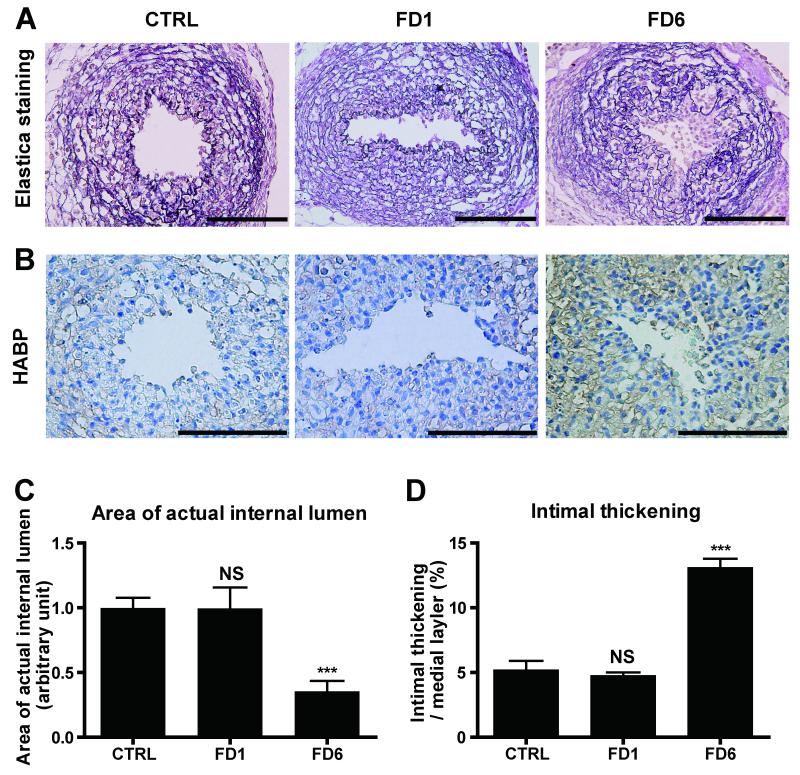
AC6 Gene Transfer, but not AC2, Promoted IT in Rat DA Explants
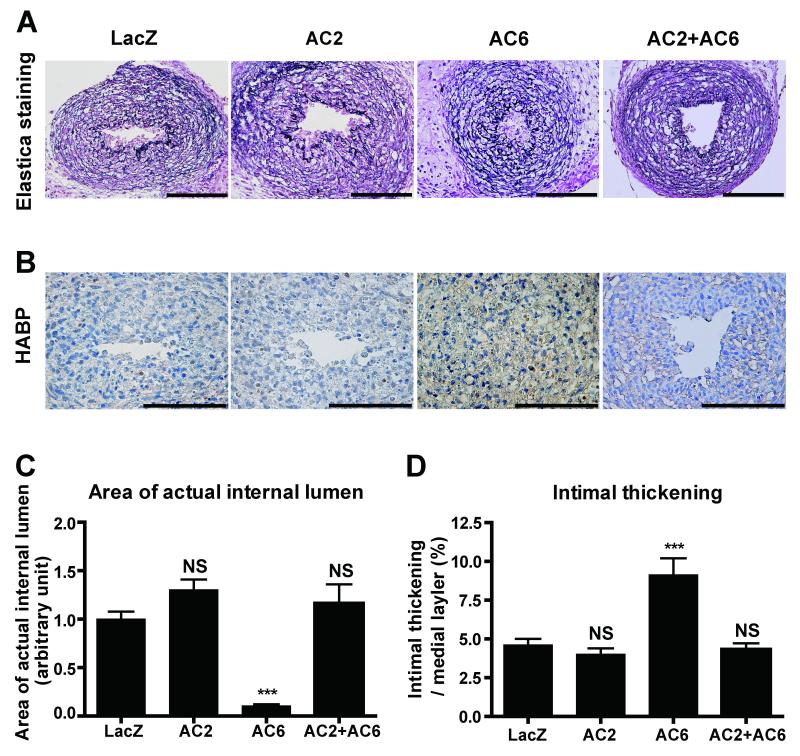
When AC6 was overexpressed in immature rat DA explants in which IT had not yet formed, prominent IT with strong hyaluronan deposition was observed in AC6-overexpressed DA explants, as compared to LacZ controls (Figures 3A, B and D). The internal lumen of the DA treated with Adv.AC6 was almost completely closed (Figure 3C). However, overexpression of AC2 did not promote hyaluronan deposition and IT formation. Further, Adv.AC2 abrogated AC6 overexpression-induced hyaluronan production and IT ex vivo, which is consistent with the data in Figure 2C. Taken together, these results indicate that AC2 has an inhibitory effect on AC6-induced hyaluronan-mediated IT in DA explants.
Figure 3.
Adenovirus-mediated AC6 gene transfer promoted IT in rat DA explants. (A) Elastica van Gieson staining for cultured DA explants overexpressed with Adv.LacZ, Adv.AC2, Adv.AC6 or Adv.AC2+Adv.AC6. (B) Strong immunoreaction to hyaluronan in DA explants cultured with Adv.AC6. Bars=100 μm. (C) The area of the internal lumen of the DA treated with Adv.AC6 was significantly decreased (n=8-9). (D) The ratio of IT to the thickness of the medial layer was increased in the DA treated with Adv.AC6, but not with Adv.AC2 (n=8-9). ***P<0.001. HABP: hyaluronan binding protein.
AC6 Deficiency Decreased IT in Mouse DA
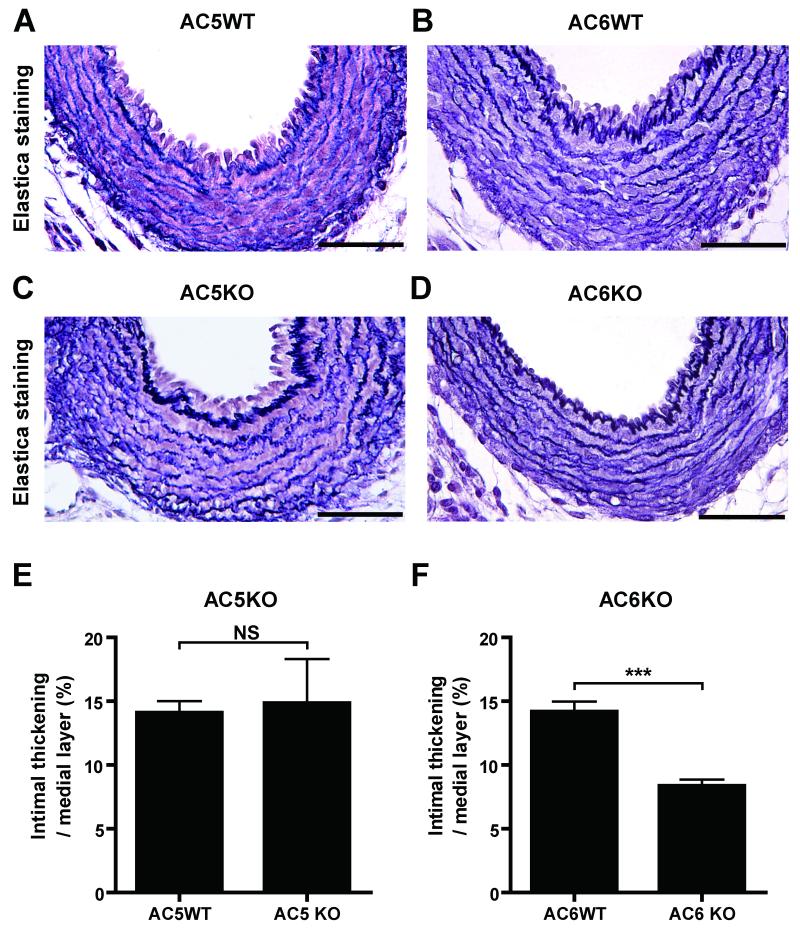
Although AC5 and AC6 share an extremely high amino acid homology, the above experiments suggested that AC6 is a major isoform for DA remodeling. We therefore examined whether AC6 indeed plays a major role in in vivo IT of the DA and found that genetic disruption of the AC6 isoform resulted in less IT during late gestation (e18.5) (Figures 4B, D and F). It should be noted that DAs closed after birth in AC6KO mice (data not shown). The IT of AC5KO mice was normally developed at e18.5 (Figures 4A, C and E) and the DA of AC5KO mice closed after birth (data not shown). These findings support the conclusion that AC6 plays a primary role in IT and, thus, the vascular remodeling in the mouse DA.
Figure 4.
Impaired IT in the mouse DA due to AC6, but not AC5, deficiency. (A and C) DAs from AC5KO mice at e18.5 were stained with Elastica van Gieson stain. Both AC5KO and WT mice showed IT in the DA (n=4-5). (B and D) DAs from AC6KO mice at e18.5 had less IT compared to WT mice (n=8). Bars=50 mm. ***P<0.001.
Effect of Isoform-Selective AC Activators on cAMP Accumulation in Rat DASMCs
Based on the findings of previous crystallographic studies and computer-assisted drug design, we identified forskolin derivatives (FD1 or FD6) that have enhanced selectivity for AC2 or AC5 in regulating tissue AC catalytic activity14.However, the ability of cAMP production via AC6 of FD1 and FD6 has not been demonstrated. FD1 enhanced LacZ control-induced cAMP accumulation in DASMCs infected with Adv.AC2 or Adv.AC6 (Figure 5A). FD6 enhanced cAMP accumulation in DASMCs with Adv.AC6, but not with Adv.AC2. These data suggest that FD1 stimulates both AC2 and AC6 and that FD6 stimulates AC5 and AC6. We confirmed that FD1 (AC2/6 stimulator) and FD6 (AC5/6 stimulator) increased cAMP accumulation in DASMCs in a dose-dependent manner (Figure 5B).
Figure 5.
The effects of FD1 and FD6 on cAMP and hyaluronan production in DASMCs. (A) the effect of overexpression of AC2 or AC6 on FD1- or FD6-induced cAMP accumulation (n=6). (B) FD1 and FD6 increased cAMP accumulation in DASMCs in a dose-dependent manner (n=4). (C) FD6, but not FD1, increased hyaluronan production (n=8-11). (D) FD6 significantly increased HAS2 mRNA (n=6). (E) AC6-targeted siRNA negated FD6-induced hyaluronan production (n=4). (F) FD6, PGE1 and forskolin did not induce hyaluronan production in aortic SMCs. (n=6) **P<0.01 and ***P<0.001 vs. CTRL. Fsk: forskolin.
The Effects of Isoform-Selective AC Activators on DASMC Hyaluronan Production
We then found that FD6 significantly increased hyaluronan production (Figures 5C) and transcripts of hyaluronan synthase type 2 (HAS2) in DASMCs at 10−5 mol/L (Figures 5D). In contrast, FD1, in doses up to 10−5.5 mol/L, did not increase hyaluronan production or HAS2 transcripts up. It should be noted that production of cAMP by FD1 at a concentration of 10−5.5 mol/L was equivalent to that by FD6 at 10−5 mol/L (Figures 5B and C) and that FD1 significantly decreased DASMC viability at a concentration higher than 10−5 mol/L. Silencing of AC6, but not of AC5, abolished FD6-induced hyaluronan production (Figure 5E), indicating that AC6 is responsible for FD6-induced hyaluronan production. Furthermore, to examine whether the effect of FD6 on hyaluronan production is specific to DASMCs, we found that FD6 did not induce hyaluronan production in SMCs from the rat aorta (Figure 5F), because expression of AC6 mRNA in aortic SMCs was approximately 60% lower than in DASMCs. However, when AC6 was overexpressed in the aortic SMCs, hyaluronan production was significantly increased by 1.4 ± 0.1-fold (n=6) in the presence of FD6 (10−5M), suggesting that this data can provide insight into a more general vascular remodeling by AC6.
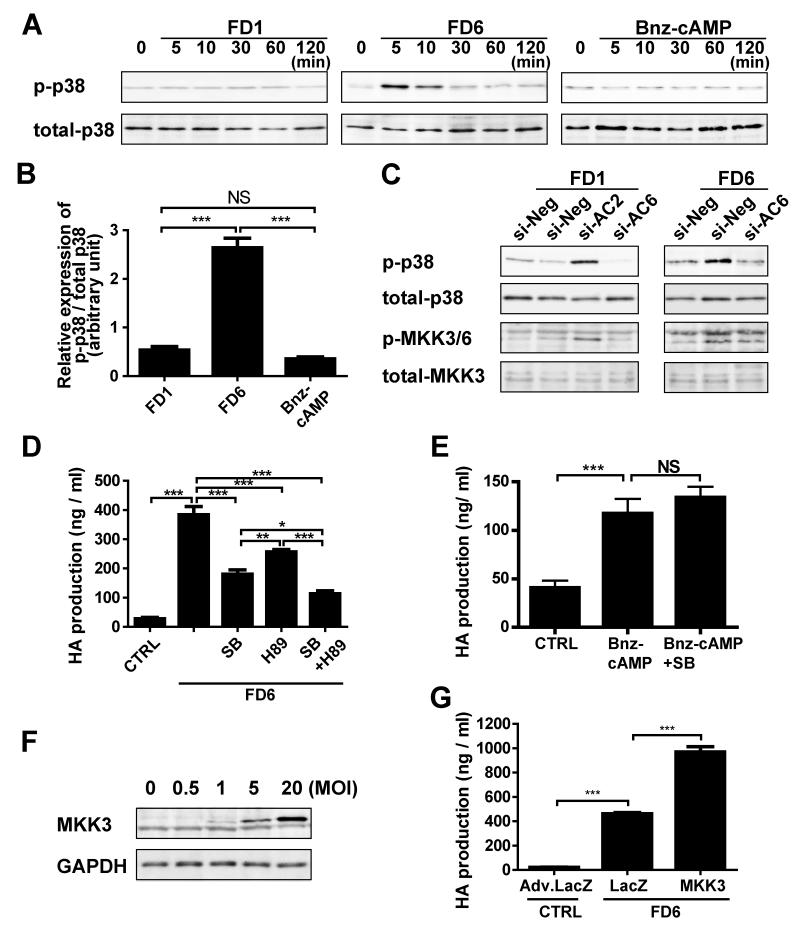
Involvement of MKK3-p38 MAPK in AC6-Induced Hyaluronan Production
To examine the mechanism by which AC2 inhibits AC6-induced hyaluronan production, we focused on several signal pathways such as p38 mitogen-activated protein kinase (MAPK). We found that FD6 increased phosphorylation of p38 protein in DASMCs, whereas FD1 and N6-Benzoyladenosine-cAMP (Bnz-cAMP), a PKA selective cAMP analog, did not (Figures 6A and B). FD6-induced phosphorylation of p38 and MKK3/6 was negated in DASMCs treated with AC6-targeted siRNA (Figure 6C). FD1 increased phosphorylation of p38 and MKK3/6 when AC2 expression was downregulated by AC2-targeted siRNA (Figure 6C). FD6-induced hyaluronan production was attenuated by SB203580, a p38 inhibitor, or H89, a PKA inhibitor. Combined treatment of SB203580 and H89 further inhibited hyaluronan production (Figure 6D). In contrast, SB203580 did not affect PKA-induced hyaluronan production (Figure 6E). These data suggest that p38 MAPK and PKA independently regulate hyaluronan production. Furthermore, overexpression of MKK3, the efficacy of which is demonstrated by Adv.MKK3 (Figure 6F), enhanced FD6-induced hyaluronan production in DASMCs (Figure 6G). Extracellular signal-related kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK) were not phosphorylated by FD6 (data not shown). Phospholipase C (PLC), protein kinase C (PKC), IP3 receptor, PI3 kinase, and Epac signaling were not involved in AC6-induced hyaluronan production, as shown using specific agonists or inhibitors for each pathway (Online Figure IV). These data indicate that stimulation of AC6 promotes hyaluronan production via both p38 and PKA pathways and that AC2 inhibits the AC6-activated MKK3-p38 pathway.
Figure 6.
The signaling pathway of AC6-induced hyaluronan production in DASMC. (A) Phosphorylation of p38 protein (p-p38) by FD1 (10-5.5mol/L), FD6 (10-5mol/L) or Bnz-cAMP (10-5mol/L) (n=4). (B) Quantification of the ratio of p-p38 to total p38 after 5 min stimulation by FD1, FD6 or Bnz-cAMP. (n=4) (C) Phosphorylation of p38 and MKK3/6 induced by 5 min treatment of FD1 (10-5.5mol/L) or FD6 (10-5mol/L) in DASMCs treated with si-negative, si-AC2 or si-AC6 RNA (n=4). (D) FD6-induced hyaluronan production was attenuated by SB203580 (SB, 2X10-5mol/L) and H89 (10-6mol/L). (n=6) (E) SB203580 (2x10-5mol/L) did not affect Bnz-cAMP-induced hyaluronan production. (n=6) (F) MKK3 protein expression by Adv.MKK3. (G) Adv.MKK3 enhanced FD6-induced hyaluronan production. (n=6) *P<0.05, **P<0.01 and ***P<0.001.
The Effects of Isoform-Selective AC Activators on IT ex vivo
We then examined the effects on FD1 and FD6 on IT using DA explants. Consistent with other data (Figures 5 and 6), 48 h incubation with FD6 significantly induced IT, increased hyaluronan production, and narrowed the internal lumen in DA explants (Figures 7). It should be noted that FD1 and FD6, similarly to forskolin and PGE1, inhibited proliferation of DASMCs. Overexpression of AC2 or AC6 also inhibited DNA synthesis in DASMCs (Online Figure V), indicating that FD6 does not directly promote IT by proliferation of DASMCs.
Figure 7.
IT in rat DA explants is promoted by FD6. (A) Elastica van Gieson staining for DA explants treated with FD1 or FD6. (B) Strong immunoreaction to hyaluronan in DA explants cultured with FD6. (C) The area of the internal lumen of the DA treated with FD6 was significantly decreased (n=6-7). (D) The increased IT in the DA treated with FD6 (n=6-7). ***P<0.001 vs. control (CTRL). Bars=100 μm. HABP: hyaluronan binding protein.
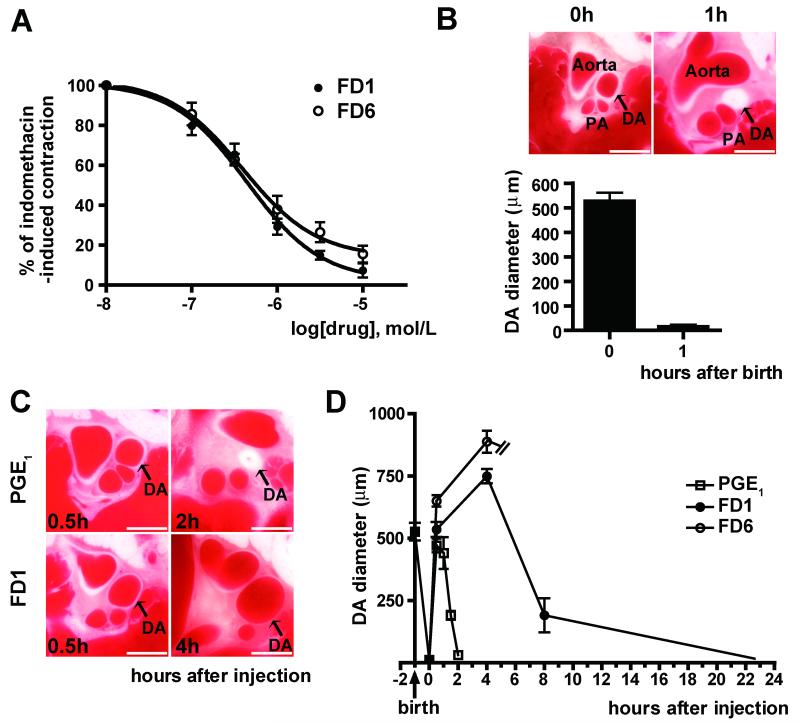
The Effects of Isoform-selective AC Activators on Vasodilation
Since PGE1/2 strongly dilates the DA via activation of ACs, AC activators should be potent vasodilators for the DA as well. We found that FD1 and FD6 similarly attenuated indomethacin-induced contraction in DA explants (Figure 8A). We then examined the vasodilatory effect of FD1 and FD6 in vivo using a whole body freezing method. Here, the DA closed completely 1 h after birth (Figure 8B) when PGE1, FD1 or FD6 were administrated by intraperitoneal injection. Consistent with previous data24, PGE1 caused maximal dilatation 30 min after injection and then the DA completely closed within 2 h (Figures 8C and D). FD1 induced maximal dilatation of the DA up to 4 h and then the DA gradually contracted, suggesting that FD1 has a longer vasodilatory effect on the DA than dose PGE1. Although FD6 also dilated the DA 30 min after injection, all neonates died around 4h after injection due to suppression of respiration, which is the same side effect caused by PGE1 and was not caused by FD1. We also found that FD1 and FD6 did not affect the diameter of the ascending aorta, whereas FD6, but not FD1, significantly dilated the main pulmonary artery up to 4 h after administration (Online Figures VIA-C). Using cultured DASMCs, AC6-targeted siRNA attenuated forskolin-induced phosphorylation of vasodilator-stimulated phosphoprotein (VASP), whereas AC2-targeted siRNA had no effect, suggesting that AC6 is primarily involved in DA vasodilation (Online Figure VID).
Figure 8.
The effects of FD1 and FD6 on vasodilation. (A) FD1 and FD6 similarly attenuated indomethacin-induced contraction in DA explants in a dose-dependent manner (n=4-10). (B) The whole body freezing method revealed that the rat DA opens widely after birth and closed 1 h after birth (arrow, n=5). (C) Representative images of rat DAs treated with PGE1 and FD1 using the whole body freezing method. (D) The vasodilating effects of PGE1, FD1 or FD6 were compared (n=5-7). FD1 had a longer action of duration than did PGE1. All rats injected with FD6 died 4h after injection due to apnea. Bars=1 mm. PA: pulmonary artery
Taken together, these results reveal that AC6 play a primary role in hyaluronan-mediated vascular remodeling in the DA through activation of the PKA and MKK3-p38 MAPK pathways and that AC2 has an inhibitory effect on AC6-mediated hyaluronan production and IT.
Discussion
The Effect of AC2, AC5 and AC6 on Vascular Remodeling
The present study demonstrated for the first time that AC plays a significant role in vascular remodeling in the DA. Intimal thickening during development is a critical form of vascular remodeling for postnatal closure of the DA3, 26. We found that hyaluronan induced by PGE-EP4-cAMP signaling is a prominent constituent of the extracellular matrix of IT in the DA3. It seems a worthwhile endeavor to investigate mechanisms leading to an increase in hyaluronan, since insights into its regulatory mechanisms and signaling pathways might eventually lead to ways of controlling hyaluronan-mediated IT in the DA. However, the isoform-selective role of AC in DA vascular remodeling has not previously been reported. Importantly, we found that AC6 is responsible for hyaluronan-mediated IT in the DA via activation of the MKK3-p38 and PKA pathways and that AC2 has an inhibitory effect on AC6-mediated hyaluronan production and DA remodeling. We also demonstrated that simultaneous stimulation of AC2 and AC6 by FD1 produces a longer vasodilatory affect than does PGE1 without inducing hyaluronan-mediated IT.
It is important to identify whether the source of hyaluronan is from SMCs or endothelial cells of the DA. Using cell sorting analysis, we found that the expression levels of HAS1, HAS2 and AC6 mRNAs in CD31-positive/CD45-negative endothelial cells from e21 rat DA were significantly lower than those in CD31-negative/CD45-negative SMCs from e21 rat DA (Online Figure VII). Therefore, we believe that DASMCs are a major source of hyaluronan production.
DA IT in AC6-Deficient Mice
Our results also showed that the DA of AC6KO mice had less fully formed IT during late gestation. These results support the belief that AC6 plays a role in EP4-mediated hyaluronan synthesis. Nevertheless, the DA of AC6KO mice eventually closed after birth, similarly to wild-type (WT) mice, whereas the DA remained open in EP4KO mice. These results suggest that other AC isoforms might compensate for deficiency of AC6. Alternatively, other EP4 signal pathways in addition to the AC-cAMP pathway may be involved in the patency of EP4KO mice. Further study is required to determine how multiple signaling pathways contribute to yield IT in the DA. We assume that the closure of the DA in AC6KO mice may be delayed after birth due to insufficient IT, which will also be addressed in our future studies.
Interaction of AC2 and AC6 Signaling in Hyaluronan-Mediated IT
AC isoforms have specific patterns of regulation by G proteins, protein kinases and calcium/calmodulin. For example, cAMP production by AC2 is stimulated by several signals including Gs-α and βγ-subunits, and by protein kinase C. In contrast, cAMP production by AC6 is only stimulated by Gs-α and inhibited by Gi-α, PKA, and low concentrations of Ca2+ 4, 27. Moreover, different AC isoforms have different effects on cAMP-mediated responses independent of their rate of synthesis of cAMP28. To the best of our knowledge, this is the first study to show how the effect of an AC isoform counteracts the effect of another isoform independent of cAMP production. We demonstrated that overexpression of AC2 by itself has little effect on hyaluronan production and appears to have an inhibitory effect on AC6-induced hyaluronan and IT. These data are consistent with the other experiments in which activation of both AC2 and AC6 by FD1 did not induce hyaluronan and IT in DASMCs and DA explants. The response of FD6 to hyaluronan production was much greater than that of FD1, even though FD6 produced less cAMP than FD1 at the same concentrations, suggesting that this process is not simply dependent on the amount of intracellular cAMP.
The next important question is the mechanism how AC2 and AC6 differentially regulate vascular remodeling of the DA. Our results demonstrated that AC6 induced hyaluronan production via the MKK3-p38 MAPK and PKA pathways and that AC2 inhibited the MKK3-p38 pathway, resulting in inhibition of AC6-induced hyaluronan production. PKC, PLC, PI3 kinase, Epac, and other MAPK pathways including ERK and JNK were not involved in AC6-induced hyaluronan production. In addition, we also found that AC2 is primarily localized in the caveolae fraction, whereas AC5/6 localized in the caveolae and the non-caveolae fractions (Online Figure VIII). This differential localization may change the effect of AC2 and AC6 on the downstream signal pathways. Identification of the upstream target linking AC6 and the MKK3-p38 pathways will be addressed in future studies.
Clinical Implications of Using AC Isoform Selective Modulations
The manipulation of the contractile state of the DA is important for patients with patent DA and complicated congenital heart diseases. All current pharmacological therapies rely on synthetic PGE1 to dilate the DA and prostaglandin H synthase inhibitors to close it. Since these therapies basically change the plasma and/or local concentration of PGE, they broadly affect the contractile state and the cellular responses in other types of smooth muscle and tissues, resulting in severe adverse effects on systemic organs. Moreover, PGE1 has a short duration of action and induces hyaluronan-mediated IT in the DA. In cases of DA-dependent congenital heart diseases, opening of the DA without induction of IT is of particular necessity until the hemodynamics can be improved through surgery. Although differential regulation of vasodilation and IT is preferable for treatment of patients with DA-dependent congenital cardiac malformations, such a treatment is not currently available. Therefore, selective manipulation should be a desirable direction for novel therapeutic strategies. In the present study, we demonstrated that AC isoform-selective activators differentially regulated vascular tone and remodeling in the DA. Our data imply that AC2/6-selective manipulation could be a novel means of achieving DA vasodilation with only minimal effects on the pulmonary arteries and aorta. Moreover, the AC2/6 activator, FD1 has longer pharmacological effects than does PGE1. Recent studies from other authors have indicated that specific agonists/antagonists for EP4 specifically regulate ductal prostaglandin signals29, which could potentially yield a DA-selective vasodilator or vasoconstrictor. However, it should be noted that the EP4 receptor underwent short term agonist-induced desensitization30 which is a common biological phenomenon involving reduction of responsiveness despite continuous agonist induction. Direct activation of AC may overcome the disadvantages of agonist-induced desensitization and FD1 may be beneficial for patients with DA-dependent congenital heart diseases.
In conclusion, we have shown that AC2 and AC6 exert distinct regulation of vascular tone and play an important role in DA remodeling. AC isoform-selective pharmacotherapy using FD1 may serve as a novel therapeutic strategy for patients with DA-dependent congenital heart diseases and as an alternative to current PGE therapy.
Novelty and Significance.
What is known?
Prostaglandin E (PGE)-adenylyl cyclase (AC)-cyclic AMP (cAMP) signaling opens the ductus arteriosus (DA) by vasodilation and closes it by hyaluronan-mediated intimal thickening.
-
Differential regulation of vasodilation and remodeling of the DA is required for patients with DA-dependent congenital heart diseases after birth.
F
What new information does this article contribute?
Adenylyl cyclase type 6 (AC6) is involved in vasodilation and hyaluronan-mediated intimal thickening.
Adenylyl cyclase type 2 (AC2) inhibits AC6-induced intimal thickening.
Stimulation of both AC2 and AC6 by the new forskolin derivative FD1 induced long-lasting vasodilation without intimal thickening in the DA.
PGE plays two opposing roles in the DA: it induces opening of the DA by vasodilation, and closure by hyaluronan-mediated intimal thickening. Dilation of the DA, but not intimal thickening, is necessary in patients with DA-dependent congenital heart diseases after birth. However, the current PGE therapy is not able to differentially regulate vasodilation and intimal thickening in the DA. Our results suggest that AC6 plays a primary role in hyaluronan-mediated vascular remodeling and vasodilation in the DA and that AC2 has an inhibitory effect on AC6-mediated vascular remodeling. We found that stimulation of both AC2 and AC6 by the forskolin derivative FD1 induced long-lasting vasodilation without intimal thickening in the DA. For the first time, we demonstrated that AC2 and AC6 exert distinct regulation of vascular tone and remodeling. In particular, our identification of the interaction of two signaling pathways of AC isoforms in vascular remodeling is novel. AC isoform-selective pharmacotherapy using FD1 may yield a new therapeutic strategy for patients with DA-dependent congenital heart diseases who require DA opening, but not DA closure, through hyaluronan-mediated neointimal thickening. This may become an alternative to the current PGE therapy.
Supplementary Material
Online Figure I (A) Expression of AC2 mRNA was silenced by AC2-targeted siRNA in DASMCs. AC2 mRNA expression was increased by AC6-targeted siRNA (n=6). (B) AC5 mRNA expression was significantly decreased by AC5-targeted siRNA, but not by AC2-and AC6-targeted siRNA (n=6). (C) AC6 mRNA was decreased by AC6-targeted siRNA, but not by AC2-and AC5-targeted siRNA (n=6). *P<0.05, **P<0.01 and ***P<0.001 compared to si-Neg. Data are from at least three independent experiments.
Online Figure II (A-C) Expression of AC3, AC4 or AC7 mRNA was silenced by AC3-, AC4-or AC7-targeted siRNA in DASMCs. (n=4). (D) AC3-, AC4-or AC7-targeted siRNA did not attenuate PGE1-induced hyaluronan (HA) production (n=6). **P<0.001. Data ware from three independent experiments.
Online Figure III (A, B and C) Effect of overexpression of AC2 on mRNA expression of AC2, AC5 and AC6. Overexpression of AC2 significantly increased AC2 mRNA, but not AC5 and AC6 (n=6). (D, E and F) Overexpression of AC6 significantly increased AC6 mRNA, but not AC2 and AC5 (n=6). ***P<0.001 compared to Adv.LacZ. Data are from at least three independent experiments.
Online Figure IV (A) PMA (phorbol-12-myristate-13-acetate, PKC activator, 10−6mol/L) and bis (bisindolylmaleimide I, PKC inhibitor, 10−6mol/L) did not affect hyaluronan (HA) production under basal or FD1/FD6 treatment. (n=6). (B) Wortmanin (wor, PI3K inhibitor) or LY294002 (PI3K inhibitor) did not affect FD6-induced hyaluronan production. (n=6) (C) IP3 receptor agonist (D-IP3, 10−5mol/L) did not increase hyaluronan production compared to an inactive IP3 receptor agosnit (L-IP3, 10−5mol/L). Neomycin sulfate (PLC inhibitor,10−5mol/L) and U73122 (PLC inhibitor, 10−5mol/L) did not affect FD6-induced hyaluronan production. (n=6) (D) FD1 and FD6 increased PKA activity in a dose-dependent manner. PKA was activated in accordance with cAMP produced by FD1 or FD6, respectively (n=8-10). (E) There was not different in GTP-Rap1 protein expression between DASMCs treated with FD1 (10−5.5mol/L) and FD6 (10−5mol/L). Me-cAMP: 8-p-methoxyphenylthon-2′-O-methyl-cAMP, 5X10−5mol/L. ***P<0.001. Data are from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 compared to si-Neg.
Online Figure V (A) DNA synthesis was measured in DASMCs treated with FD1, FD6, forskolin (Fsk) or PGE1 for 4 h. (n=6) (B) Forskolin-indueced supression of DNA synthesis was enhanced in DASMCs transfected with Adv.AC2 or Adv.AC6 (n=6). *P<0.01 compared to CTRL or Adv.LacZ control. Data are from three independent experiments.
Online Figure VI (A) Representative images of the rat aorta and pulmonary arteries treated with FD1 or FD6. (B and C) The diameter of rat ascending aorta was not changed by FD1 or FD6, whereas that of main portion of pulmonary arteries were increased by FD6, but not by FD1 (n=5-7). *P<0.05 compared to before injection. Scale bars, 1 mm. (D) Representative images of phophorylation of VASP (Vasodilator-stimulated phosphoprotein) induced by forskolin (Fsk,10−5mol/L) in DASMCs treated with si-Neg, si-AC2 or si-AC6. Data are from three independent experiments.
Online Figure VII (A) Flow cytometric analysis using FITC-conjugated anti-CD31 and APC/Cy7-conjugated anti-CD45 antibodies in the rat e21 DA tissue. The gates R1 and R2 represent CD31-/CD45- SMCs and CD31+/CD45- endothelial cells, respectively. (B and C) Total number of CD31+ cells in the rat e21 DA tissue. Negative staining without a primary antibody was shown in B. (D and E) AC2 and AC6 mRNA expression in SMCs or endotherial cells of the rat e21 DA or aorta tissue (n=4). (F and G) HAS1 and HAS2 mRNA expression in SMCs or endotherial cells of the rat e21 DA or aorta tissue (n=4). **P<0.01 and ***P<0.001.
Online Figure VIII Representative images of AC2, AC5/6 and caveolin1 protein expression separated by a sucrose gradient method. Fraction number 4-5 and 9-13 indicates buoyant and heavy fractions, respectively. Data are from three independent experiments.
Online Table I Oligonucleotides used for RT-PCR
Online Table II Oligonucleotides used for siRNA
Acknowledgment
We thank Dr. Kazuo Momma and Drs. Hideki Taniguchi, Yun-Wen Zheng, and Atsushi Tanaka for assistance of a whole body freezing method and FACS analysis, respectively. We thank Dr. Koji Otsu for isolation of caveolae fraction.
Funding Sources. This work was supported by grants from NIH (5P01HL066941, HL081741 and HL088426) (H.K.H.), the Ministry of Health Labor and Welfare (Y.I.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (Y.I., S.M.), the Yokohama Foundation for Advanced Medical Science (U.Y., S.M.), the “High-Tech Research Center” Project for Private Universities: MEXT (S.M.), Waseda University Grant for Special Research Projects (S.M.), the Vehicle Racing Commemorative Foundation (S.M.), Miyata Cardiology Research Promotion Founds (U.Y., S.M.), Takeda Science Foundation (Y.I., U.Y., S.M.), , the Japan Heart Foundation Research Grant (U.Y.), the Kowa Life Science Foundation (U.Y.), the Sumitomo Foundation (U.Y), the Cosmetology Research Foundation (Y.I.), Japan Cardiovascular Research Foundation (S.M.), the Uehara Memorial Foundation (U.Y.), the Kitsuen Research Foundation (Y.I.), the Japan Space Forum (Y.I.), the American Heart Association (SDG 0835596D) (K.I.), the American Heart Association Western Affiliate (T.T.), the Department of Veterans Affairs (H.K.H.) and the Foundation of UMDNJ “High-Impact Collaboration Grant” (K. I.).
Non-standard Abbreviations and Acronyms
- AC
adenylyl cyclase
- Adv
adenovirus-mediated gene transfer
- cAMP
cyclic AMP (cAMP)
- DA
ductus arteriosus
- ERK
extracellular signal-related kinase
- FD1
6-[N-(2-isothiocyanatoethyl) aminocarbonyl] forskolin
- FD6
6-[3-(dimethylamino)propionyl]-14 15-dihydroforskolin
- HAS2
hyaluronan synthase type 2
- IT
intimal thickening
- JNK
c-Jun N-terminal kinase
- KO
knockout
- MAPK
mitogen-activated protein kinase
- PGE
Prostaglandin E
- PKC
protein kinase C
- PLC
phospholipase C
- SMCs
smooth muscle cells
- VASP
vasodilator-stimulated phosphoprotein
- WT
wild-type
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith GC. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- 2.Waleh N, Kajino H, Marrache AM, Ginzinger D, Roman C, Seidner SR, Moss TJ, Fouron JC, Vazquez-Tello A, Chemtob S, Clyman RI. Prostaglandin E2--mediated relaxation of the ductus arteriosus: effects of gestational age on g protein-coupled receptor expression, signaling, and vasomotor control. Circulation. 2004;110:2326–2332. doi: 10.1161/01.CIR.0000145159.16637.5D. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 5.Tang WJ, Gilman AG. Adenylyl cyclases. Cell. 1992;70:869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa Y. Isoform-targeted regulation of cardiac adenylyl cyclase. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S1–4. [PubMed] [Google Scholar]
- 7.Bulin C, Albrecht U, Bode JG, Weber AA, Schror K, Levkau B, Fischer JW. Differential effects of vasodilatory prostaglandins on focal adhesions, cytoskeletal architecture, and migration in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:84–89. doi: 10.1161/01.ATV.0000146814.81581.68. [DOI] [PubMed] [Google Scholar]
- 8.Fujino T, Yuhki K, Yamada T, Hara A, Takahata O, Okada Y, Xiao CY, Ma H, Karibe H, Iwashima Y, Fukuzawa J, Hasebe N, Kikuchi K, Narumiya S, Ushikubi F. Effects of the prostanoids on the proliferation or hypertrophy of cultured murine aortic smooth muscle cells. Br J Pharmacol. 2002;136:530–539. doi: 10.1038/sj.bjp.0704749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med. 1997;3:775–779. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Aronson I, Takuma S, Homma S, Naka Y, Alshafie T, Brovkovych V, Malinski T, Oz MC, Pinsky DJ. cAMP pulse during preservation inhibits the late development of cardiac isograft and allograft vasculopathy. Circ Res. 2000;86:982–988. doi: 10.1161/01.res.86.9.982. [DOI] [PubMed] [Google Scholar]
- 11.Slomp J, van Munsteren JC, Poelmann RE, de Reeder EG, Bogers AJ, Gittenberger-de Groot AC. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis. 1992;93:25–39. doi: 10.1016/0021-9150(92)90197-o. [DOI] [PubMed] [Google Scholar]
- 12.Pasricha PJ, Hassoun PM, Teufel E, Landman MJ, Fanburg BL. Prostaglandins E1 and E2 stimulate the proliferation of pulmonary artery smooth muscle cells. Prostaglandins. 1992;43:5–19. doi: 10.1016/0090-6980(92)90060-7. [DOI] [PubMed] [Google Scholar]
- 13.Yau L, Zahradka P. PGE(2) stimulates vascular smooth muscle cell proliferation via the EP2 receptor. Mol Cell Endocrinol. 2003;203:77–90. doi: 10.1016/s0303-7207(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 14.Onda T, Hashimoto Y, Nagai M, Kuramochi H, Saito S, Yamazaki H, Toya Y, Sakai I, Homcy CJ, Nishikawa K, Ishikawa Y. Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem. 2001;276:47785–47793. doi: 10.1074/jbc.M107233200. [DOI] [PubMed] [Google Scholar]
- 15.Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, Tomlinson JE, Umemura S, Scarborough RM, Levy DE, Ishikawa Y. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem. 2004;279:40938–40945. doi: 10.1074/jbc.M314238200. [DOI] [PubMed] [Google Scholar]
- 16.Sutkowski EM, Robbins JD, Tang WJ, Seamon KB. Irreversible inhibition of forskolin interactions with type I adenylyl cyclase by a 6-isothiocyanate derivative of forskolin. Mol Pharmacol. 1996;50:299–305. [PubMed] [Google Scholar]
- 17.Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funakoshi K, Iwamoto M, Nakagome M, Uemura N, Hori H, Yokota S, Ishikawa Y. Multiple transcripts of Ca2+ channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol. 2006;290:H1660–1670. doi: 10.1152/ajpheart.00100.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1998;95:1038–1043. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama U, Minamisawa S, Quan H, Akaike T, Suzuki S, Jin M, Jiao Q, Watanabe M, Otsu K, Iwasaki S, Nishimaki S, Sato M, Ishikawa Y. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J Biol Chem. 2008;283:28702–28709. doi: 10.1074/jbc.M804223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaike T, Jin MH, Yokoyama U, Izumi-Nakaseko H, Jiao Q, Iwasaki S, Iwamoto M, Nishimaki S, Sato M, Yokota S, Kamiya Y, Adachi-Akahane S, Ishikawa Y, Minamisawa S. T-type Ca2+ channels promote oxygenation-induced closure of the rat ductus arteriosus not only by vasoconstriction but also by neointima formation. J Biol Chem. 2009;284:24025–24034. doi: 10.1074/jbc.M109.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momma K, Toyoshima K, Takeuchi D, Imamura S, Nakanishi T. In vivo reopening of the neonatal ductus arteriosus by a prostanoid EP4-receptor agonist in the rat. Prostaglandins Other Lipid Mediat. 2005;78:117–128. doi: 10.1016/j.prostaglandins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci U S A. 2008;105:6386–6391. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovitch M, Beharry S, Bothwell T, Jackowski G. Qualitative and quantitative differences in protein synthesis comparing fetal lamb ductus arteriosus endothelium and smooth muscle with cells from adjacent vascular sites. Dev Biol. 1988;130:250–258. doi: 10.1016/0012-1606(88)90431-9. [DOI] [PubMed] [Google Scholar]
- 27.Wong ST, Baker LP, Trinh K, Hetman M, Suzuki LA, Storm DR, Bornfeldt KE. Adenylyl cyclase 3 mediates prostaglandin E2-induced growth inhibition in arterial smooth muscle cells. J Biol Chem. 2001;276:34206–34212. doi: 10.1074/jbc.M103923200. [DOI] [PubMed] [Google Scholar]
- 28.Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res. 2006;99:845–852. doi: 10.1161/01.RES.0000245189.21703.c0. [DOI] [PubMed] [Google Scholar]
- 29.Kajino H, Taniguchi T, Fujieda K, Ushikubi F, Muramatsu I. An EP4 receptor agonist prevents indomethacin-induced closure of rat ductus arteriosus in vivo. Pediatr Res. 2004;56:586–590. doi: 10.1203/01.PDR.0000139409.25014.35. [DOI] [PubMed] [Google Scholar]
- 30.Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–1037. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure I (A) Expression of AC2 mRNA was silenced by AC2-targeted siRNA in DASMCs. AC2 mRNA expression was increased by AC6-targeted siRNA (n=6). (B) AC5 mRNA expression was significantly decreased by AC5-targeted siRNA, but not by AC2-and AC6-targeted siRNA (n=6). (C) AC6 mRNA was decreased by AC6-targeted siRNA, but not by AC2-and AC5-targeted siRNA (n=6). *P<0.05, **P<0.01 and ***P<0.001 compared to si-Neg. Data are from at least three independent experiments.
Online Figure II (A-C) Expression of AC3, AC4 or AC7 mRNA was silenced by AC3-, AC4-or AC7-targeted siRNA in DASMCs. (n=4). (D) AC3-, AC4-or AC7-targeted siRNA did not attenuate PGE1-induced hyaluronan (HA) production (n=6). **P<0.001. Data ware from three independent experiments.
Online Figure III (A, B and C) Effect of overexpression of AC2 on mRNA expression of AC2, AC5 and AC6. Overexpression of AC2 significantly increased AC2 mRNA, but not AC5 and AC6 (n=6). (D, E and F) Overexpression of AC6 significantly increased AC6 mRNA, but not AC2 and AC5 (n=6). ***P<0.001 compared to Adv.LacZ. Data are from at least three independent experiments.
Online Figure IV (A) PMA (phorbol-12-myristate-13-acetate, PKC activator, 10−6mol/L) and bis (bisindolylmaleimide I, PKC inhibitor, 10−6mol/L) did not affect hyaluronan (HA) production under basal or FD1/FD6 treatment. (n=6). (B) Wortmanin (wor, PI3K inhibitor) or LY294002 (PI3K inhibitor) did not affect FD6-induced hyaluronan production. (n=6) (C) IP3 receptor agonist (D-IP3, 10−5mol/L) did not increase hyaluronan production compared to an inactive IP3 receptor agosnit (L-IP3, 10−5mol/L). Neomycin sulfate (PLC inhibitor,10−5mol/L) and U73122 (PLC inhibitor, 10−5mol/L) did not affect FD6-induced hyaluronan production. (n=6) (D) FD1 and FD6 increased PKA activity in a dose-dependent manner. PKA was activated in accordance with cAMP produced by FD1 or FD6, respectively (n=8-10). (E) There was not different in GTP-Rap1 protein expression between DASMCs treated with FD1 (10−5.5mol/L) and FD6 (10−5mol/L). Me-cAMP: 8-p-methoxyphenylthon-2′-O-methyl-cAMP, 5X10−5mol/L. ***P<0.001. Data are from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 compared to si-Neg.
Online Figure V (A) DNA synthesis was measured in DASMCs treated with FD1, FD6, forskolin (Fsk) or PGE1 for 4 h. (n=6) (B) Forskolin-indueced supression of DNA synthesis was enhanced in DASMCs transfected with Adv.AC2 or Adv.AC6 (n=6). *P<0.01 compared to CTRL or Adv.LacZ control. Data are from three independent experiments.
Online Figure VI (A) Representative images of the rat aorta and pulmonary arteries treated with FD1 or FD6. (B and C) The diameter of rat ascending aorta was not changed by FD1 or FD6, whereas that of main portion of pulmonary arteries were increased by FD6, but not by FD1 (n=5-7). *P<0.05 compared to before injection. Scale bars, 1 mm. (D) Representative images of phophorylation of VASP (Vasodilator-stimulated phosphoprotein) induced by forskolin (Fsk,10−5mol/L) in DASMCs treated with si-Neg, si-AC2 or si-AC6. Data are from three independent experiments.
Online Figure VII (A) Flow cytometric analysis using FITC-conjugated anti-CD31 and APC/Cy7-conjugated anti-CD45 antibodies in the rat e21 DA tissue. The gates R1 and R2 represent CD31-/CD45- SMCs and CD31+/CD45- endothelial cells, respectively. (B and C) Total number of CD31+ cells in the rat e21 DA tissue. Negative staining without a primary antibody was shown in B. (D and E) AC2 and AC6 mRNA expression in SMCs or endotherial cells of the rat e21 DA or aorta tissue (n=4). (F and G) HAS1 and HAS2 mRNA expression in SMCs or endotherial cells of the rat e21 DA or aorta tissue (n=4). **P<0.01 and ***P<0.001.
Online Figure VIII Representative images of AC2, AC5/6 and caveolin1 protein expression separated by a sucrose gradient method. Fraction number 4-5 and 9-13 indicates buoyant and heavy fractions, respectively. Data are from three independent experiments.
Online Table I Oligonucleotides used for RT-PCR
Online Table II Oligonucleotides used for siRNA