Abstract
The anti-tumorigenic effects of Toxoplasma gondii (RH) antigens were studied in a murine sarcoma-180 tumor model. To determine the anti-tumor effects, the reduction in tumor size and expression of CD31 (an angiogenesis marker in the tumor tissue) were examined after injection of BALB/c mice with T. gondii lysate antigen (TLA) or formalin-fixed, proliferation-inhibited, T. gondii tachyzoites. Tumors were successfully produced by an intradermal injection of sarcoma-180 cells with plain Matrigel in the mid-backs of mice. After injection with TLA or formalin-fixed T. gondii tachyzoites, the increase in tumor size and weight nearly stopped while tumor growth continued in control mice that were injected with PBS. CD31 expression in TLA-treated or formalin-fixed T. gondii-injected mice was lower than the control mice. Accordingly, the present study shows that the treatment of mice with formalin-fixed T. gondii or TLA in the murine sarcoma-180 tumor model results in a decrease of both tumor size and CD31 expression.
Keywords: Toxoplasma gondii, lysate antigen, CD31, sarcoma-180, anti-tumorigenic effect
Toxoplasma gondii is a protozoan parasite that is ubiquitous in nature and infects a variety of mammals and birds [1]. There are 3 infectious stages completing the life cycle of T. gondii, including tachyzoites (trophozoites), bradyzoites (tissue cysts), and oocysts containing sporozoites [2]. T. gondii infection has been reported all over the world. In the United States, National Health and Nutrition Examination Survey (NHANES) reported that 10.8% of the general population (6-49 years of age) and 11.0% of women (15-44 years of age) have Toxoplasma-specific IgG antibodies [3]. Toxoplasmosis is not a severe or critical infectious disease in healthy adults [1]. However, toxoplasmosis is sometimes fatal in immunosuppressed conditions, such as AIDS [4,5] and transplantation patients as well as pregnant women [6,7]. Toxoplasmosis in AIDS patients mainly affects the brain and is frequently fatal [8].
Most previous studies have focused on the pathogenesis, clinical, and epidemiologic aspects of toxoplasmosis. However, several recent studies are highlighted by the duality of parasitic infections, i.e., parasites are occasionally helpful rather than harmful for the prognosis of some other diseases. For instance, T. gondii has been suggested as a protozoan parasite for immunotherapy of other diseases. The growth of Lewis lung carcinoma is inhibited following an oral infection with T. gondii ME49 strain [9]. Mice injected with both T. gondii and cancer cells showed an increase in CD8+ T-cells, IFN-γ mRNA levels, and serum IgG2a titers. In addition, hemoglobin concentration inside the Matrigel plug inserted into mice was decreased by T. gondii infection, and a decrease in the tumor mass by inhibition of angiogenesis was suggested [9]. IFN-γ, TNFRp55, and iNOS are important for the inflammatory lesion and tissue parasitism after T. gondii infection [10]. IFN-γ, TNF family ligands, and iNOS are also known to be associated with anti-tumorigenic mechanisms by macrophages and NK cells [11-13]. However, the mechanism is not yet fully elucidated and the possibility of direct immuno-modulation by antigenic proteins of T. gondii has not been determined.
The present study investigated the effects of T. gondii lysate antigens (TLA) on tumorigenesis in BALB/c mice. For this purpose, a murine lymphoma cell line, sarcoma-180 (S-180) cells, was used for tumor generation and successfully created solid tumors (S-180 tumors). The anti-tumor effects were determined by measuring the tumor size and weight, and the expression of CD31 in the tumor tissue. The present study was performed to observe the changing patterns of the tumor size and CD31 expression in the S-180 tumor mass in mice injected with TLA or formalin-fixed T. gondii tachyzoites.
Female BALB/c mice were purchased from ORIENT BIO Animal Center (Seongnam, Gyeonggi-do, Korea). Mice were housed at room temperature (20-23℃) on a 12-hr light and 12 hr-dark cycle, with unlimited supplies with food and water sterilized by irradiation and autoclaving. All experiments using mice were performed according to the ethical standards formulated from the IACUC (SNU-090102-3) in the Seoul National University. TLA were prepared according to the procedure described previously, with a slight modification [14]. Briefly, tachyzoites of the virulent T. gondii RH strain were obtained from the peritoneal exudate of intraperitoneally-infected mice. The exudate was passed twice through a 25-gauge needle and followed by passing through filter membranes of 5 µm pore size to remove debris and host cells. The isolated parasites were washed, resuspended in phosphate-buffered saline (pH 7.4), and sonicated on ice. The supernatant (TLA) was filter-sterilized through a 0.22 µm pore size membrane. The protein concentration of TLA was determined by the BCA™ Protein Assay Kit (Thermo Scientific, Rockford, Michigan, USA) and stored at -70℃ until used. Tumors were generated by subcutaneous injection of a mixture of 1.0 × 107 S-180 cells (KCLB 40066, Korean Cell Line Bank, Seoul, Korea) and 500 µl of growth factor-reduced BD Matrigel™(BD Science, Miami, Florida, USA) at the mid-backs of 6-week-old BALB/c mice [9,15].
When tumors were grown to an average volume of approximately 40 mm3 on day 14 after the injection of S-180 cells, mice were randomly divided into the following 3 experimental groups (N = 3 for each group); TLA, formalin-fixed T. gondii, and PBS. After fixation of tachyzoites with formalin for 20 min, tachyzoites were centrifuged at 3,000 rpm, 4℃ for 10 min and washed 5 times to remove formalin completely. These formalin-fixed tachyzoites were injected into the tumor mass of tumor-bearing mice. The residual formalin in the last washing solution was analyzed by high performance liquid chromatography (HPLC) to prove the safety of the injection into mice; 10 µl of the last washing solution was applied to HPLC (Gilson, Middleton, Wisconsin, USA). In this experiment, 5 × 105 formalin-fixed tachyzoites were injected into the tumor mass once during the experimental period. However, TLA diluted in PBS was injected with 50 µg/100 µl twice weekly. The control group was injected with 100 µl PBS into the tumor mass. An Ultra-Fine II Insulin Syringe (BD Science) was used to minimize the tissue damage by twice weekly TLA or PBS injections. The tumor size was measured using a caliper and calculated by the following formula: length × width × height × 0.5 [16]. The tumor weight was also measured once after the mice were sacrificed (data not shown). Tumor proteins were prepared in RIPA buffer (1 × PBS, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) supplemented with 1 × Roche complete Mini protease inhibitor cocktail (Roche, Indianapolis, Indianapolis, USA). The protein concentration was measured using the BCA method (Thermo Scientific). The statistics programs used were SPSS 12.0K and SigmaPlot 10.0. A P-value < 0.05 was regarded as significant.
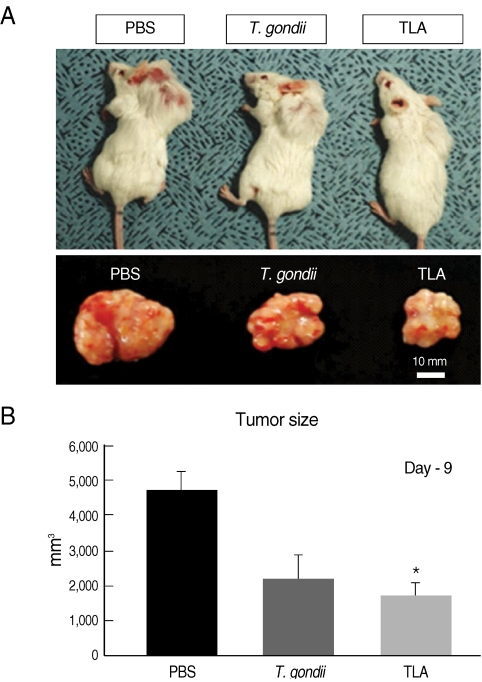
Tumor sizes were measured on day 9 post-injection of TLA or formalin-fixed T. gondii, then the tumor masses were isolated from the mice after sacrifice (Fig. 1). The tumor sizes of TLA (1,731 ± 366.5 mm3) or T. gondii-injected (2,200 ± 669.6 mm3) groups were smaller than those of PBS-injected control group (4,667 ± 549.4 mm3). These observations were in agreement with the results of different tumors used in T. gondii infections, including Lewis lung carcinoma [9] and WEHI-164 fibrosarcoma [17]. The TLA-treated group was significantly smaller than the control group in the tumor size (P = 0.00153). Both formalin-fixed T. gondii-injection and TLA-treatment showed the same effects on the inhibition of tumor growth and the effect would be augmented by a repeated TLA injection. CD31 is expressed on platelets and endothelial cells, involved in cell recognition, motility, and signaling in the biological process, and suggested as an important marker of angiogenesis in tumors [18]. Expression of CD31 (PECAM-1) was investigated by immunohistochemical staining of the sectioned tumor mass and the results showed a decrease in spots stained with anti-CD31 mAb in TLA- or T. gondii-injected tumors (data not shown). The CD31 protein levels were determined in the tumor mass and appeared to be decreased in the TLA- and T. gondii-treatment groups compared to the control group on Western blots (Fig. 2). Because CD31 is highly expressed on tumor tissues in a general state of angiogenesis [18], our results may be correlated with anti-angiogenesis (Figs. 1B, 2). Although it is known that immune responses after T. gondii infection are related with anticancer effects in tumor-bearing mice [9,18], further studies are needed using TLA to better elucidate anti-angiogenesis.
Fig. 1.
Comparison of tumor sizes among PBS-, formalin-fixed T. gondii tachyzoites-, and T. gondii lysate antigen (TLA)-group. (A) Tumor sizes of T. gondii-injected and TLA-treated mice were smaller than them of control mice. (B) Tumor masses were isolated from mice at day 9 post-PBS-, -T. gondii-, or -TLA-injection. Tumors of TLA group were significantly smaller than control group (P=0.00153).
Fig. 2.
Western blot analysis of the tumor tissues using CD31 antibody. Expressions of CD31 in T. gondii- and TLA-injected group were reduced compared to PBS-injected control group.
Our study focused on the effects of T. gondii-antigenic proteins, as well as T. gondii injection, on anti-tumorigenic effects. The results showed a decrease in CD31, a marker of angiogenesis, after TLA injection into the tumor mass. This result demonstrates the anti-tumorigenic effect of a protozoan parasite, T. gondii, may be induced by the intervention of molecular mechanisms inhibiting vascular formation by T. gondii lysate proteins. However, the relationship between the immune response induced by TLA- or formalin-fixed T. gondii-injection and the suppression of tumor growth must not be overlooked. In this regard, we observed the increase of IL-12 in TLA-injected tumor-bearing mice (data not shown). IL-12 is related to suppression of angiogenesis when tumors are produced [19,20]. IL-12 has also been shown to be induced by T. gondii antigens (TLA) in promonocytic cell line cultures (THP-1 cells) [21]. Considering the possible mechanisms by which TLA exerts an effect (a direct anti-tumorigenic effect, or indirect immune response), this study supports the results of previous studies reporting anti-angiogenesis effects of T. gondii infection.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-E00020). This work was also supported by 02-2009-037 from the SNUBH research fund. This study was supported by BK21 Human Life Sciences, Ministry of Education, Republic of Korea.
References
- 1.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- 4.Lago EG, Conrado GS, Piccoli CS, Carvalho RL, Bender AL. Toxoplasma gondii antibody profile in HIV-infected pregnant women and the risk of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2009;28:345–351. doi: 10.1007/s10096-008-0631-2. [DOI] [PubMed] [Google Scholar]
- 5.Sukthana Y, Chintana T, Lekkla A. Toxoplasma gondii antibody in HIV-infected persons. J Med Assoc Thai. 2000;83:681–684. [PubMed] [Google Scholar]
- 6.Lopes FM, Goncalves DD, Mitsuka-Bregano R, Freire RL, Navarro IT. Toxoplasma gondii infection in pregnancy. Braz J Infect Dis. 2007;11:496–506. doi: 10.1590/s1413-86702007000500011. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo Y, Takeishi S, Miyamoto T, Nonami A, Kikushige Y, Kunisaki Y, Kamezaki K, Tu L, Hisaeda H, Takenaka K, Harada N, Kamimura T, Ohno Y, Eto T, Teshima T, Gondo H, Harada M, Nagafuji K. Toxoplasmosis encephalitis following severe graft-vs.-host disease after allogeneic hematopoietic stem cell transplantation: 17 yr experience in Fukuoka BMT group. Eur J Haematol. 2007;79:317–321. doi: 10.1111/j.1600-0609.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 8.Cingolani A, Luca AD, Ammassari A, Murri R, Linzalone A, Grillo R, Antinori A. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. Molecular Diagnosis. 1996;45:472–476. doi: 10.1099/00222615-45-6-472. [DOI] [PubMed] [Google Scholar]
- 9.Kim JO, Jung SS, Kim SY, Kim TY, Shin DW, Lee JH, Lee YH. Inhibition of Lewis lung carcinoma growth by Toxoplasma gondii through induction of Th1 immune responses and inhibition of angiogenesis. J Korean Med Sci. 2007;22(Suppl):S38–S46. doi: 10.3346/jkms.2007.22.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva NM, Vieira JC, Carneiro CM, Tafuri WL. Toxoplasma gondii: the role of IFN-gamma, TNFRp55 and iNOS in inflammatory changes during infection. Exp Parasitol. 2009;123:65–72. doi: 10.1016/j.exppara.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Oba M, Yano S, Shuto T, Suico MA, Eguma A, Kai H. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. 2008;32:1317–1324. doi: 10.3892/ijo_32_6_1317. [DOI] [PubMed] [Google Scholar]
- 12.Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358–5366. [PubMed] [Google Scholar]
- 13.Sanzen I, Imanishi N, Takamatsu N, Konosu S, Mantani N, Terasawa K, Tazawa K, Odaira Y, Watanabe M, Takeyama M, Ochiai H. Nitric oxide-mediated antitumor activity induced by the extract from Grifola frondosa (Maitake mushroom) in a macrophage cell line, RAW264.7. J Exp Clin Cancer Res. 2001;20:591–597. [PubMed] [Google Scholar]
- 14.Lee EJ, Heo YM, Choi JH, Song HO, Ryu JS, Ahn MH. Suppressed production of pro-inflammatory cytokines by LPS-activated macrophages after treatment with Toxoplasma gondii lysate. Korean J Parasitol. 2008;46:145–151. doi: 10.3347/kjp.2008.46.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Z, Willingham MC, Hicks AM, Alexander-Miller MA, Howard TD, Hawkins GA, Miller MS, Weir HM, Du W, DeLong CJ. Spontaneous regression of advanced cancer: identification of a unique genetically determined, age-dependent trait in mice. Proc Natl Acad Sci U S A. 2003;100:6682–6687. doi: 10.1073/pnas.1031601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looney WB, Mayo AA, Janners MY, Mellon JG, Allen P, Salak D, Morris HP. Cell proliferation and tumor growth in hepatomas 3924A. Cancer Res. 1971;31:821–825. [PubMed] [Google Scholar]
- 17.Darani HY, Shirzad H, Mansoori F, Zabardast N, Mahmoodzadeh M. Effects of Toxoplasma gondii and Toxocara canis antigens on WEHI-164 fibrosarcoma growth in a mouse model. Korean J Parasitol. 2009;47:175–177. doi: 10.3347/kjp.2009.47.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang SX, Steinberg SM, Nguyen D, Wu TD, Modrusan Z, Swain SM. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin Cancer Res. 2008;14:5893–5899. doi: 10.1158/1078-0432.CCR-07-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang XW, Larue SM, Avery PR, Dewhirst MW, Ullrich RL. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. International Journal of Hyperthermia. 2006;22:587–606. doi: 10.1080/02656730600983063. [DOI] [PubMed] [Google Scholar]
- 20.Sunamura M, Sun L, Lozonschi L, Duda DG, Kodama T, Matsumoto G, Shimamura H, Takeda K, Kobari M, Hamada H, Matsuno S. The antiangiogenesis effect of interleukin 12 during early growth of human pancreatic cancer in SCID mice. Pancreas. 2000;20:227–233. doi: 10.1097/00006676-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Aldebert D, Durand F, Mercier C, Brenier-Pinchart MP, Cesbron-Delauw MF, Pelloux H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the THP-1 human monocytic cell line. Cytokine. 2007;37:206–211. doi: 10.1016/j.cyto.2007.03.012. [DOI] [PubMed] [Google Scholar]