Abstract
Artemesinin-combination therapies (ACT) for falciparum malaria reduce gametocyte carriage, and therefore reduce transmission. Artemisinin derivatives will act against only young gametocytes whereas primaquine acts on mature gametocytes which are present usually in the circulation at the time when the patient presents for treatment. Both artemisinin derivatives and primaquine have short half-lives, less than 1 hr and 7 hr, respectively. Therefore, asexual parasites or young gametocytes remain after completed ACT. A single dose of primaquine (0.50-0.75 mg base/kg) at the end of ACT can kill only mature gametocytes but cannot kill young gametocytes (if present). Remaining asexual forms after completion of ACT course, e.g., artesunate-mefloquine for 3 days, may develop to mature gametocytes 7-15 days later. Thus, an additional dose of primaquine (0.50-0.75 mg base/kg) given 2 weeks after ACT completion may be beneficial for killing remaining mature gametocytes and contribute to more interruption of Plasmodium falciparum transmission than giving only 1 single dose of primaquine just after completing ACT.
Keywords: Plasmodium falciparum, artemisinin-combination therapy, primaquine, malaria transmission blocking
A single dose of 45 mg primaquine is routinely given for Plasmodium falciparum malaria in endemic areas to reduce the risk of transmission. Primaquine markedly reduces circulating gametocytes and sterilizes those remaining [1]. Effective blood schizontocide treatment may leave surviving gametocytes [2]. When slow-acting antimalarials are used, brief duration of primaquine gametocidal activity precedes elimination of trophozoites which may differentiate to gametocytes. A single 45-mg dose of primaquine adjunctive to therapy for P. falciparum infection may not completely block transmission [3].
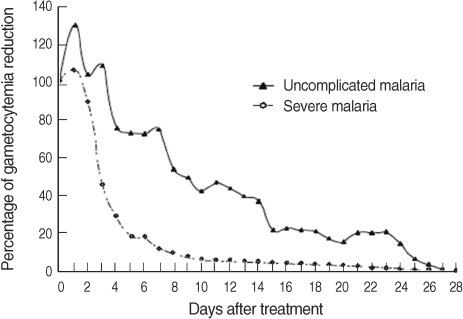
WHO (2006) mentioned that artemisinin-combination therapies (ACT) reduce gametocyte carriage markedly, and therefore reduce transmission since artemisinin derivatives have effects on young gametocytes [4]. Tangpukdee et al. [5] studied the gametocyte clearance after ACT in uncomplicated malaria and severe malaria (Fig. 1). All patients had gametocytes on day 0 of admission. Oral artesunate plus mefloquine for 3 days to uncomplicated malaria patients and intravenous artesunate for 5 days followed by oral mefloquine to severe malaria patients were given, respectively. Primaquine was not administered. It was shown that 41.5%, 13.1%, 3.8%, 2%, and 2% of uncomplicated malaria patients had gametocytemia on day 3, 7, 14, 21, and 28 of treatment, respectively, whereas 33.6%, 8.2%, 2.7%, 0.9%, and 0.9% of severe malaria patients had gametocytemia on day 3, 7, 14, 21, and 28 of treatment, respectively [5]. It was confirmed that artesunate could not kill all gametocytes after completion of the artesunate treatment course [5].
Fig. 1.
Post-treatment percentage reduction in sexual parasitemia (adopted from Tangpukdee et al. [5], Korean J Parasitol 2008; 46: 65-70 with permission).
If the patients entered to endemic areas after completion of 3-day ACT course for uncomplicated malaria or after 5 days' intravenous artesunate treatment in severe malaria, some patients still have gametocytemia and could transmit gametocytes to mosquitoes. Piyaphanee et al. [6] studied emergence and clearance of uncomplicated P. falciparum malaria with different regimens of ACT (dihydroartemisinin plus mefloquine, dihydroartemisinin-piperaquine-trimethoprim, artesunate plus mefloquine, dihydroartemisinin-piperaquine) in either 2 or 3 days. Primaquine was not given. The study included the patients with and without gametocytemia on day 0 of admission [6]. On day 0, 72.5% of the patients had gametocytemia. On day 7 and 14, 10% and < 4% of the patients had gametocytemia. First appearance of parasitemia at 4, 4.5, and 8 days of admission was found in 0.8%, 0.4%, and 0.4% respectively [6]. Therefore, if those patients entered malaria endemic areas after completion of the treatment courses, some patients still have gametocytemia and might cause gametocyte transmission to mosquitoes.
According to these studies [5,6], primaquine seems to be beneficial after ACT completion for gametocyte blocking as an additional benefit. Although WHO (2006) recommended that where an ACT is not used, a single oral dose of primaquine of 0.75 mg base/kg (45 mg base maximal for adults) combined with a fully effective blood schizontocide could be used to reduce transmission if it is possible to achieve high coverage of population infected with malaria [4]. However, according to both studies, gametocytemia still presented in some patients even with completion of ACT; therefore, primaquine might be beneficial to give to those ACT-patients. Primaquine and transmission blocking agents have been widely used after falciparum malaria treatment in Southeast Asia and South America. The National Antimalarial Drug Policy of Thailand has recommended giving primaquine 30 mg in a single dose after artesunate and mefloquine treatment for uncomplicated malaria regardless of presence or absence of gametocytemia in blood smears as the routine additional treatment after ACT completion.
Primaquine at a dose of 30-45 mg has been shown to be very effective against gametocytes of P. falciparum. In children, primaquine 0.50-0.75 mg base/kg single dose may be used [7]. Single primaquine dose was well tolerated and prior testing for G6PD deficiency was not required [4]. However, there is no experience with its use in Africa where G6PD deficiency is the highest in the world.
Many studies earlier have addressed the effects of antimalarial drug treatment on gametocytes and most of these studies used microscopy for detection and quantification of gametocytes, but it has been shown that patients without microscopically detectable gametocytes can infect mosquitoes [8] and submicroscopic gametocytemia can be common [9]. Detailed quantitative studies on submicroscopic gametocytes are now possible with the recently developed gametocyte-specific Pfs25 quantitative nucleic acid sequence-based amplification (QT-NASBA) which can detect gametocyte densities above 20 gametocytes per ml of blood [10]. Schneider et al. [11] showed that the potential of P. falciparum malaria transmission remains high even after treatment with ACT, although the prevalence and density of gametocytes is lower after artesunate and sulfadoxine-pyrimethamine treatment in Kenya patients. In their study, the gametocyte prevalence was 86% by a quantitative method compared with 22% by microscopy [11]. Gametocytemia is detectable by microscopy down to densities of approximately 10-20/µl. At least 1 male gametocyte's progeny (8 microgametes) and 1 female macrogametocyte are required in a mosquito blood meal (approximately 2-3 µl) for infection to occur. Gametocyte densities of 1/µl can theoretically infect mosquitoes and this is below the density which can be detected by routine microscopy [12]. Thus, gametocytemia after ACT treatment as in Tungpukdee and Piyaphanee's studies [5,6] were meaningful that gametocytes might transmit after completed ACT without primaquine treatment.
Merozoites emerging from a single schizont developed either into further asexual stages or into gametocytes [13,14]. Gametocytemia arises 7-15 days after the initial asexual wave [15]. This maturation period has long been compared to that of the other human malaria species (1-3 days) [16]. The ratio of gametocytes to asexual stages in P. falciparum is less than 1:10; a recent study showed a much lower ratio (1 : 156) [15]. The half-life of mature gametocyte in the blood is generally reported to be 2.4 days [17]. However, mean circulation time of 6.4 days which is about twice the expected 3.4 days deduced from a 2.4 half-life was reported [15]. Some gametocytes have been found to have the longevity of up to 4 weeks in blood circulation [18]. Field and Shute [19] first described 5 different maturation stages of P. falciparum gametocytes which were further characterized by light microscopy and later by electron microscopy [19]. Young gametocytes may be sequestered in tissues and they take 8-10 days to become mature gametocytes in the peripheral circulation which can be infective to mosquitoes for 10-14 days [4]. P. falciparum differs from the other human malarias in 2 important respects; first, the gametocyte formation is delayed with respect to the peak production of asexual stages, and second, mature gametocytes are resistant to most of the antimalarial drugs, which affect asexual stages [12].
In infections with Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale, the asexual and sexual stages appear almost together, and in contrast to P. falciparum, they are sensitive to all drugs which kill the asexual stages [12,20]. Artemisinin is a sesquiterpenre lactone peroxide. The derivatives of artesunate and artemether are hydrolyzed to the active metabolite dihydroartemisinin (DHA) which has an elimination half-life of approximately 45 min. Artemisinin derivatives and to a far lesser extent, chloroquine will act against young gametocytes. Artemisinin derivatives greatly reduce young sequestered gametocytes and have "little" effects to mature circulating gametocytes and unknown sporontocidal effects of gametocytes to mosquitoes. They destroy immature gametocytes, preventing new infective gametocytes from entering the circulation, but their effects on mature gametocytes are less and so they will not affect the infectivity of those already present in the circulation at the time a patient presents for treatment.
Primaquine is an 8-aminoquinoline and has actions greatly against mature gametocytes of P. falciparum but has unknown gametocytocidal effects and sporontocidal effects to young sequestered gametocytes [4]. It is well absorbed and is cleared by hepatic transformation to more polar metabolite carboxy-primaquine with an elimination half-life of 7 hr. Primaquine is the only drug known to act on mature infective gametocytes in circulation and accelerates gametocyte clearance, as opposed to artemisinins which mainly inhibit gametocyte development. If a single 45-mg dose of primaquine is added to therapy for P. falciparum infection at the end of ACT, gametocytemia (if present) may be 2 possible outcomes:
1. After completion of ACT and the treatment outcome is cure and no asexual parasite or young gametocytes are found, primaquine will kill all mature gametocytes (if present) and the patient will have no gametocytemia in blood circulation later.
2. After completion of ACT some asexual parasites or young gametocytes may remain.
Regarding the short half-life of artemisinin derivatives, after ACT completion, there will be no remaining DHA in circulation to kill young gametocytes. A single dose of primaquine given at the end of ACT will kill only mature gametocytes but not young gametocytes (if present), and the patient will have mature gametocytemia in blood circulation 7-15 days later (from young gametocyte development). In this situation, if another 45-mg dose of primaquine is given 15 days or 2 weeks after ACT, primaquine will kill mature gametocytes which will develop 7-15 days later. In the patients with retreatment after primary treatment failure, double doses of 45 mg of primaquine may be given; the first dose just after completion of ACT and the second dose at 2 weeks later to ensure eradication of mature gametocytes.
In the future, if tafenoquine (slowly eliminated 8-aminoquinoline) can be used in the clinical practice, it can be given only once after asexual parasite treatment and it is not necessary to be followed by another dose 2 weeks later since tafenoquine has a terminal elimination half-life of 2 weeks. The drug is more efficacious than primaquine.
In conclusion, to interrupt transmission of falciparum malaria in the population in hypoendemic areas, at the end of a fully effective blood schizontocide ACT, primaquine 0.50-0.75 mg/kg (30-45 mg base maximal for adults) may be given twice; at the end of ACT and again 2 weeks later. At present, it is unknown whether the use of single or double doses of primaquine after ACT completion would result in a further suppression of infectivity. However, it appears possible in principle, since artemisinin derivatives and primaquine act on different stages of gametocytes. Further studies to prove this hypothesis are warranted. Adherence to the second primaquine dose, if used, needs good health services and good patients' collaboration.
References
- 1.Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD. Gametocidal and sporontocidal effects of primaquine and of sulfadoxine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bull WHO. 1968;38:625–632. [PMC free article] [PubMed] [Google Scholar]
- 2.Lawpoolsri S, Klein EY, Singhasivanon P, Yimsamran S, Thanyavanich N, Maneeboonyang W, Hungerford LL, Maguire JH, Smith DL. Optimal timing of primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malaria J. 2009;8:159. doi: 10.1186/1475-2875-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for the treatment of malaria. 1st ed. Geneva, Switzerland: WHO; 2006. pp. 71pp. 133–143. [Google Scholar]
- 5.Tangpukdee N, Krudsood S, Srivilairit S, Phophak N, Chonsawat P, Yanpanich W, Kano S, Wilairatana P. Gametocyte clearance in uncomplicated and severe Plasmodium falciparum malaria after astesunate-mefloquine treatment in Thailand. Korean J Parasitol. 2008;46:65–70. doi: 10.3347/kjp.2008.46.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piyaphanee W, Krudsood S, Tangpukdee N, Thanachartwet W, Silachamroon U, Phophak N, Duangdee C, Haoharn O, Faithong S, Wilairatana P, Leowattana W, Looareesuwan S. Emergence and clearance of gametocytes in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;74:432–435. [PubMed] [Google Scholar]
- 7.Na-Bangchang K, Karbwang J. Clinical pharmacology of primaquine. In: Karbwang J, Wernsdorfer W, editors. Clinical Pharmacology of Antimalarials. 1st ed. Bangkok, Thailand: Clinical Pharmacology Unit, Faculty of Tropical Medicine, Mahidol University; 1993. p. 316. [Google Scholar]
- 8.Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH. The reservoir of Plasmodium falciparum malaria in a holoendmic area of western Kenya. Trans R Soc Trop Med Hyg. 1992;86:355–358. doi: 10.1016/0035-9203(92)90216-y. [DOI] [PubMed] [Google Scholar]
- 9.Nassir E, Abdel-Muhsin AM, Suliaman S, Kenyon F, Kheir A, Geha H, Ferguson HM, Walliker D, Babiker HA. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol. 2005;35:49–55. doi: 10.1016/j.ijpara.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Schoone G, Schallig H, Verhage D, Telgt D, Eling W, Sauerwein R. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol. 2004;137:35–41. doi: 10.1016/j.molbiopara.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Schneider P, Bousema T, Omar S, Gouagna L, Sawa P, Schallig H, Sauerwein R. (Sub)microscopic Plasmodium falciparum gametocytemia in Kenyan children after treatment with sulfadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol. 2006;36:403–408. doi: 10.1016/j.ijpara.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.White NJ. The role of anti-malarial drugs in eliminating malaria. Malaria J. 2008;7(suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talman AM, Paul RE, Sokhna CS, Domarle O, Ariey F, Trape JF, Robert V. Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. Am J Trop Med Hyg. 2004;71:739–744. [PubMed] [Google Scholar]
- 14.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genetics, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans R Soc Trop Med Hyg. 2001;95:497–501. doi: 10.1016/s0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- 16.Robert V, Boudin C. Biologie de la transmission homme-moustique du Plasmodium. Bull Soc Pathol Exot. 2003;96:6–20. [PubMed] [Google Scholar]
- 17.Sinden RE. Sexual development of malaria parasite. Adv Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 18.Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74:1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- 19.Field JW, Shute PG. A Morphological Study of the Erythrocytic Parasites. Kuala Lumpur, Malaysia: Government Press; 1956. The microscopic diagnostic of human malaria; p. 142. [Google Scholar]
- 20.Pukrittayakamee S, Imwong M, Stepniewska K, Day NPJ, White NJ. The effects of different antimalarial drugs on gametocyte carriage in Plasmodium vivax malaria. Am J Trop Med Hyg. 2008;79:378–384. [PubMed] [Google Scholar]