Abstract
Inadequate apoptosis contributes to synovial hyperplasia in rheumatoid arthritis (RA). Recent study shows that low expression of Puma might be partially responsible for the decreased apoptosis of fibroblast-like synoviocytes (FLS). Slug, a highly conserved zinc finger transcriptional repressor, is known to antagonize apoptosis of hematopoietic progenitor cells by repressing Puma transactivation. In this study, we examined the expression and function of Slug in RA FLS. Slug mRNA expression was measured in the synovial tissue (ST) and FLS obtained from RA and osteoarthritis patients. Slug and Puma mRNA expression in FLS by apoptotic stimuli were measured by real-time PCR analysis. FLS were transfected with control siRNA or Slug siRNA. Apoptosis was quantified by trypan blue exclusion, DNA fragmentation and caspase-3 assay. RA ST expressed higher level of Slug mRNA compared with osteoarthritis ST. Slug was significantly induced by hydrogen peroxide (H2O2) but not by exogenous p53 in RA FLS. Puma induction by H2O2 stimulation was significantly higher in Slug siRNA-transfected FLS compared with control siRNA-transfected FLS. After H2O2 stimulation, viable cell number was significantly lower in Slug siRNA-transfected FLS compared with control siRNA-transfected FLS. Apoptosis enhancing effect of Slug siRNA was further confirmed by ELISA that detects cytoplasmic histone-associated DNA fragments and caspase-3 assay. These data demonstrate that Slug is overexpressed in RA ST and that suppression of Slug gene facilitates apoptosis of FLS by increasing Puma transactivation. Slug may therefore represent a potential therapeutic target in RA.
Keywords: apoptosis, Puma, rheumatoid arthritis, snail family transcription factors, synovial membrane
Introduction
Rheumatoid arthritis (RA) is a chronic joint disorder characterized by inflammation and proliferation of synovial tissue (ST) resulting in bone and cartilage destruction. Hyperplasia of the synovial intimal lining results from marked increase in macrophage-like and fibroblast-like synoviocytes (FLS) (Firestein, 2003). One explanation for the synovial proliferation is an imbalance between cell proliferation and apoptosis or programmed cell death. The amount of DNA fragmentation is significantly increased in rheumatoid synovium (Firestein et al., 1995; Nakajima et al., 1995), which is presumably due to the oxidative stress generated by chronic inflammation (Tak et al., 2000). However, only low numbers of apoptotic cells are present in rheumatoid synovium (Nakajima et al., 1995; Matsumoto et al., 1996; Sugiyama et al., 1996; Ceponis et al., 1999), which suggests the discrepancy between apoptotic stimuli and real apoptotic cell death occurring in RA synovium. Therefore, impaired apoptosis may play an important role in the pathogenesis of RA.
Slug, which belongs to highly conserved Slug/Snail family, is a zinc finger transcriptional repressor. Slug/Snail protein family was found in diverse species from C. elegans to humans. In vertebrates, Snail and Slug transcription factors have been proposed to participate in mesoderm formation and neural crest cell migration (Hemavathy et al., 2000). These proteins have also been implicated in carcinogenesis, invasiveness and metastasis in various cancers including ovarian cancer, breast cancer and melanoma (Gupta et al., 2005; Kurrey et al., 2005; Martin et al., 2005; Perez-Mancera et al., 2005). Hematopoietic stem cells are resistant to ionizing radiation-induced apoptosis. Wu et al. demonstrated that Slug is transcriptionally induced by p53 upon irradiation and then protects the damaged hematopoietic progenitor cells from apoptosis by directly repressing p53-mediated transcription of Puma (Wu et al., 2005).
Puma is a Bcl-2 homology 3 (BH3)-only proapoptotic Bcl-2 family member and mediates p53-dependent and -independent apoptosis (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001, 2003). The interaction of Puma with anti-apoptotic Bcl-2 protein family members such as Bcl-xL induces mitochondrial dysfunction through Bax (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001). Puma is detected in both RA and osteoarthritis ST, but interestingly, its expression is low in RA synovial intimal lining, which is a major site of apoptotic stimuli and p53 expression. Exogenous p53 overexpression induced p21, but not Puma in FLS and this was associated with resistance of FLS to apoptosis. However, transfection of plasmid encoding Puma expression vector lead to rapid and profound apoptosis with caspase-3 activation in RA FLS (Cha et al., 2006). Tissue- and cell type-specific selectivity of p53 in transactivation of downstream genes has been reported (Fei et al., 2002). However, the mechanism in which p53 overexpression does not lead to Puma induction or apoptosis in FLS is unknown. Slug, which has been reported to repress Puma gene transcription in hematopoietic progenitor cells, might play a role in the down-regulation of Puma and impaired apoptosis of RA FLS. In this study, we investigated the expression and function of Slug in ST and cultured FLS in order to evaluate a potential strategy to induce apoptosis in synoviocytes. We now demonstrate that Slug gene is overexpressed in RA ST and that suppression of Slug facilitates apoptosis of RA FLS by increasing Puma gene expression.
Results
Slug expression in RA ST and FLS
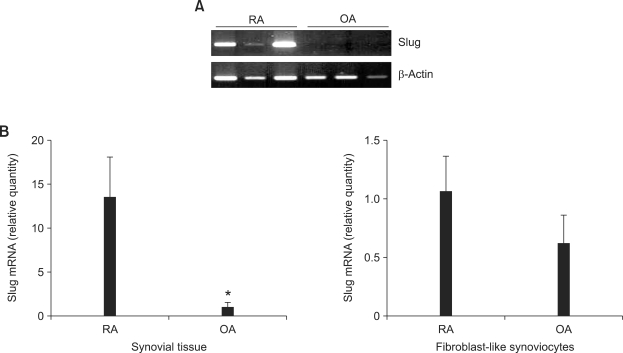
Initially, we performed studies to determine whether Slug is expressed in RA ST and FLS. As shown in Figure 1A, Slug mRNA was detected in RA ST but not in osteoarthritis ST on gel PCR (n = 3 each). Quantitative real-time PCR also showed significantly elevated Slug mRNA level in RA ST compared to osteoarthritis ST (n = 6 each, P < 0.005) (Figure 1B). Slug mRNA was also detected in FLS with a tendency of increased expression in RA FLS compared to osteoarthritis FLS (n = 3 each), but did not reach statistical significance (P > 0.10) (Figure 1B).
Figure 1.
Slug is overexpressed in RA ST. (A) Total RNA was extracted from RA (n = 3) and osteoarthritis (OA) (n = 3) ST, and RT-PCR was performed to detect Slug mRNA expression. (B) Quantitative real-time PCR analysis was performed to quantify Slug mRNA expression in RA and osteoarthritis ST (n = 6 each) and FLS (n = 3 each). Data are mRNA level normalized to β-actin expression and shown as relative cell equivalents. *P < 0.005 compared with RA ST.
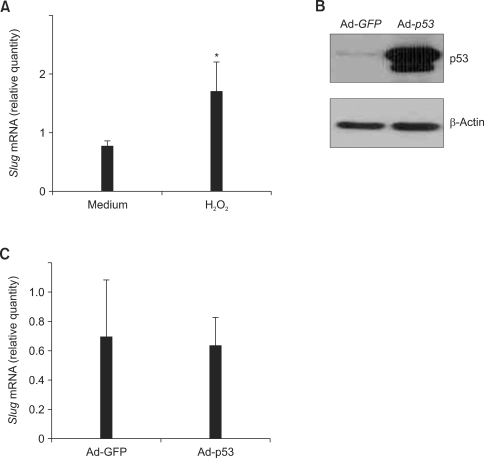
Next, we tried to examine whether apoptotic stimuli could induce Slug gene expression. Exposure of RA FLS to 1 mM H2O2 for 6 h resulted in significant increase of Slug mRNA (n = 3, 3.0 ± 0.8 fold induction, P < 0.05) (Figure 2A). To see the effect of p53 on Slug expression in FLS, Ad-p53 or Ad-GFP were infected for 24 h in RA FLS and marked increase in p53 protein expression in Ad-p53-infected FLS was confirmed on Western blot analysis (Figure 2B). However, as shown in Figure 2C, Ad-p53-infected FLS showed no increase of Slug mRNA compared to Ad-GFP-infected FLS (n = 6, P > 0.10).
Figure 2.
Slug is induced by H2O2 but not by p53 in RA FLS. (A) Total RNA was isolated from RA FLS (n = 3) after 6 h of medium or H2O2 (1 mM) treatment and quantitative real-time PCR was performed to measure Slug mRNA expression. Data are mRNA level normalized to β-actin expression and shown as relative cell equivalents. *P < 0.05 compared with medium-treated cells. (B) Ad-p53 or Ad-GFP was infected to RA FLS for 24 h. The overexpression of p53 protein in Ad-p53-infected FLS was confirmed by Western blot analysis (representative blot). (C) RA FLS (n = 6) were infected with Ad-p53 or Ad-GFP for 24 h, and quantitative real-time PCR was performed to measure Slug mRNA expression.
Effect of Slug suppression on Puma expression in RA FLS
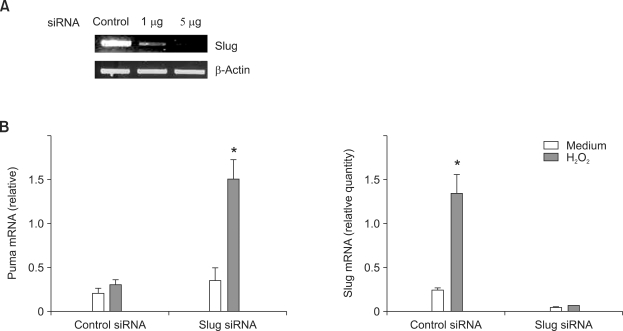
Because Slug is known as a transcriptional repressor of Puma, we next investigated the effect of Slug suppression on Puma expression in RA FLS. RA FLS were transfected with control or Slug siRNA for 3 days and the knocked-down effect was confirmed by RT-PCR (Figure 3A). H2O2 stimulation (1 mM for 6 h) did not significantly increase Puma expression in control siRNA-transfected FLS (n = 3, 1.6 ± 0.2 fold increase, P > 0.10). However, in Slug siRNA-transfected FLS, H2O2 stimulation significantly induced Puma mRNA expression on quantitative real-time PCR experiments (n = 3, 4.7 ± 1.6 fold increase, P < 0.05)(Figure 3B). Without H2O2 treatment, increase of Puma mRNA by Slug siRNA transfection was minimal. In the same samples, we could find that the effect of significant induction of Slug by H2O2 in control siRNA-transfected FLS disappeared in Slug siRNA-transfected FLS with suppressed expression of Slug mRNA irrespective of H2O2 stimulation (Figure 3B).
Figure 3.
Slug knockdown increases H2O2-induced Puma expression in RA FLS. (A) Cultured FLS were transfected with 1 or 5 µg of Slug siRNA or scrambled nonsilence control siRNA as described in Methods. FLS were then incubated for 3 days, and RT-PCR was performed. Maximum knockdown effect was observed with 5 µg of Slug siRNA transfection. (B) RA FLS (n = 3) were transfected with 5 µg of control or Slug siRNA for 3 days, and then treated with medium or 1 mM H2O2 for 6 h. Total RNA was collected, and Puma mRNA was measured by quantitative real-time PCR. Slug mRNA level was measured in the same RNA samples using quantitative real-time PCR. Note the Slug suppression effect of Slug siRNA in both medium and H2O2-treated cells. *P < 0.05 compared with medium-treated cells.
Effect of Slug suppression on FLS apoptosis
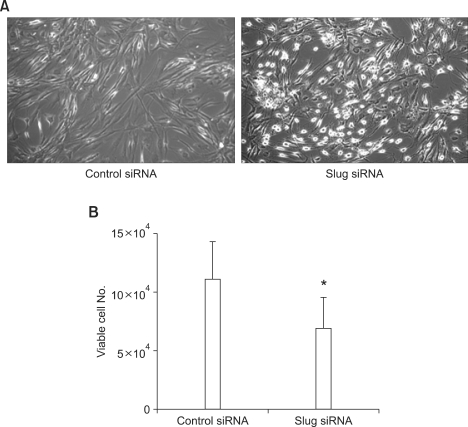
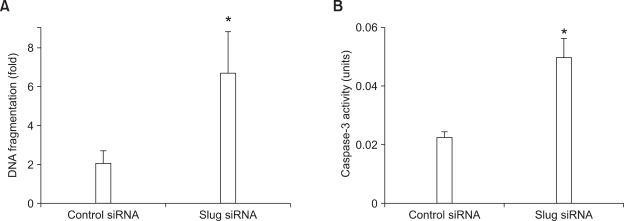
Because we found that Slug suppression increased Puma expression in RA FLS, we next tried to examine whether Slug suppression could induce apoptosis of RA FLS. RA FLS were transfected with control or Slug siRNA for 3 days and then stimulated with 0.5 mM H2O2 for 6 h. Cell morphology was observed on phase-contrast microscopy. As shown in Figure 4A, dead cells, which are characterized by nonadherent round appearance, were abundant in Slug siRNA-transfected FLS compared to control siRNA-transfected FLS. We next evaluated the effect of Slug suppression on cell death using trypan blue exclusion (n = 4). Slug siRNA transfection resulted in significantly decreased viable cell number after 9 h of 0.5 mM H2O2 stimulation compared to control siRNA transfection (6.9 × 104 ± 2.6 × 104 vs 11.1 × 104 ± 3.1 × 104, P < 0.05) (Figure 4B). Enhanced apoptosis of RA FLS by Slug suppression was confirmed by ELISA detecting fragmented DNA in cytoplasm. RA FLS transfected with Slug siRNA showed 6.7 ± 2.1 fold induction of DNA fragmentation compared to 2.1 ± 0.6 fold induction of DNA fragmentation in control siRNA-transfected FLS by H2O2 (0.5 mM for 6 h)(n = 5, P < 0.05) (Figure 5A). To confirm the activation of apoptosis pathway by Slug suppression, caspase-3 activity was measured after treatment of 0.5 mM H2O2 for 6 h in Slug siRNA- and control siRNA-transfected FLS. As shown in Figure 5B, caspase-3 activity in response to H2O2 treatment was significantly elevated in Slug siRNA-transfected FLS compared to control siRNA-transfected FLS (n = 3, P < 0.05).
Figure 4.
Slug knockdown facilitates H2O2-induced FLS death. (A) RA FLS were transfected with control or Slug siRNA for 3 days and then stimulated with 0.5 mM H2O2. After 6 h, cell morphology was observed under phase-contrast microscopy (magnification 50×). (B) RA FLS were transfected with control or Slug siRNA for 3 days and then treated with 0.5 mM H2O2. After 9 h, viable cell number was counted by trypan blue exclusion as described in Methods. *P < 0.05 compared with control siRNA-transfected cells.
Figure 5.
Slug knockdown enhances H2O2-induced FLS apoptosis and caspase-3 activation. (A) RA FLS (n = 5) were transfected with control or Slug siRNA for 3 days and then stimulated with medium or 0.5 mM H2O2. After 6 h, DNA fragmentation was measured by ELISA. The magnitude of induction of DNA fragmentation in H2O2-treated FLS is shown relative to control value (medium-treated FLS). *P < 0.05 compared with control siRNA-transfected cells. (B) RA FLS (n = 3) transfected with control or Slug siRNA were treated with 0.5 mM H2O2 for 6 h. Caspase-3 activity was measured using ApoAlert Caspase-3 assay. *P < 0.05 compared with control siRNA-transfected cells.
Discussion
Tumor-like proliferation of synoviocytes plays a key role in the progression of RA, and inadequate apoptosis of FLS contributes to this process (Firestein, 2003). Therefore, facilitating apoptosis in these cells is a reasonable strategy in the treatment of RA. In this report, we demonstrated for the first time that suppressing Slug could efficiently enhance apoptosis in RA FLS.
In the present study, Slug was detected in ST and FLS, and was transcriptionally more activated in RA ST compared to osteoarthritis ST. This result suggests that increased activation of Slug in RA synovium might contribute to impaired apoptosis. Slug was first identified as a transcription factor whose expression during embryonic development has been associated with mesoderm and migratory neural crest cells undergoing epithelial mesenchymal transformation (Hemavathy et al., 2000). Subsequently, evidence is accumulating that Slug regulates tumor carcinogenesis, metastasis and invasiveness in adult tissues. For example, Slug-overexpressing mice developed mesenchymal cancers, which were mainly leukemias (Perez-Mancera et al., 2005). Aberrant expression of Slug in breast adenocarcinomas protected against apoptosis induced by genotoxic stress and resulted in phenotypic alterations, including loss of cell adhesion and acquisition of invasive growth (Kajita et al., 2004). Induction of Slug by autocrine production of stem cell factor and c-Kit activation resulted in multidrug resistance in malignant mesothelioma cells (Catalano et al., 2004). Slug down-regulation by RNA interference facilitated apoptosis and inhibited invasive growth in neuroblastoma cells (Vitali et al., 2008). Slug is also known to be expressed and induced in nonmalignant cells such as keratinocytes by ultraviolet radiation (Hudson et al., 2007). Our study further demonstrated that Slug is inducible by genotoxic stimuli in RA FLS. Slug suppression by RNA interference markedly enhanced H2O2-induced apoptosis in RA FLS, and this was mediated by increased caspase-3 activation. H2O2-induced cell necrosis could also have contributed to FLS death in our experiment (Byun et al., 2008), but obvious influence of Slug suppression on FLS apoptosis has been demonstrated by apoptosis specific ELISA and caspase-3 activation assay. Because experiments comparing RA FLS with osteoarthritis FLS were not performed, we don't know whether these results are specific to RA FLS and it is unclear whether these Slug mediated apoptotic regulation is also operating in osteoarthritis or normal FLS. However, these results suggest that Slug plays an important role in the regulation of FLS apoptosis.
In response to DNA damage, the tumor suppressor p53 can either elicit apoptosis via Puma transactivation or activate p21 pathway, resulting in cell-cycle arrest and DNA repair (Vousden and Lu, 2002). However, how this critical step is differentially regulated in specific cell types remains largely unknown. In FLS, overexpression of p53 did not induce apoptosis via Puma transactivation, but induced p21 (Cha et al., 2006), and the mechanism for this selective transactivation of downstream molecules could not be explained. Slug was proposed to regulate the activation of p53 downstream molecules in hematopoietic progenitor cells. Once activated by DNA damage, p53 induces molecules including Puma and Slug. Slug then shifts the fate of damaged myeloid progenitor cells toward arrest and survival by selectively repressing Puma gene transactivation (Wu et al., 2005; Zilfou et al., 2005). Our present findings also demonstrate that Slug is involved in Puma gene regulation. Puma transactivation by H2O2 was minimal in control FLS, and this was compatible with the previous findings (Cha et al., 2006). On the other hand, we found that when Slug was knocked-down by RNA interference, Puma gene transactivation was significantly increased by H2O2 stimulation. Without H2O2 treatment, Puma induction by Slug knock-down was minimal, which suggests that influence of Slug on Puma expression is restricted on the condition of the presence of extracellular apoptotic stimuli such as H2O2. Contrary to previous findings, however, Slug was not induced by p53 in FLS in our study. Exogenous overexpression of p53 did not increase transcriptional activation of Slug in FLS, and this finding was discordant with the results observed in hematopoietic progenitor cells as mentioned above (Wu et al., 2005). These results imply that, in FLS, Slug can be induced by genotoxic stimuli such as H2O2 via p53-independent mechanism, which then leads to the suppression of proapoptotic gene transcription.
Although Slug has been observed to be induced by p53 in hematopoietic stem cells, this finding has not been reproduced in other cell types. In early vertebrate mesoderm, Slug was reported to be induced by NF-κB, which, in turn, was activated by Bcl-xL (Zhang et al., 2006). Slug was induced by stem cell factor/c-Kit pathway in multidrug-resistant malignant mesothelioma cells (Catalano et al., 2004). Ultraviolet radiation-induced Slug elevation in keratinocytes was mediated, at least in part, through the ERK and p38 MAPK cascades (Hudson et al., 2007). The mechanism in which Slug is induced by H2O2 in FLS remains to be illuminated in the future, but p53 does not seem to be an important mediator from our result. Despite the fact that p53 has been reported to be overexpressed in RA synovium, the role of p53 in RA FLS still remains uncertain. In fact, p53 may not play an important role in FLS apoptosis. Adenoviral gene transfer of p53 did not induce apoptosis of FLS (Cha et al., 2006). A recent study indicated that p53 not only was found to be important in inducing Puma in nucleus, but also was found to be necessary in cytoplasm to mediate the downstream apoptotic effect of Puma (Chipuk et al., 2005). However, in FLS, p53 was not required in Puma-mediated apoptosis (You et al., 2006). Puma efficiently induced apoptosis in both control and p53-deficient FLS (You et al., 2006). Furthermore, adenoviral gene transfer of Puma caused massive apoptosis of malignant glioma cells regardless of p53 status (Ito et al., 2005). These inconsistent findings imply that apoptosis regulating molecules including p53, Slug, and Puma may be interacting differentially according to tissue-specific cell types. The FLS-specific apoptotic pathway needs to be elucidated in order to establish apoptosis as a therapeutic target in RA.
In conclusion, the present study demonstrated that Slug was expressed and induced by genotoxic stimuli in RA FLS. Suppression of Slug augmented H2O2-induced Puma gene transactivation and facilitated apoptosis of FLS. Slug may therefore represent a novel therapeutic target in RA.
Methods
ST samples and primary synoviocytes culture
The experimental protocol was approved by the local ethics committee and a signed consent form was obtained from each patient. ST samples were obtained from patients with osteoarthritis and RA at the time of joint replacement. Patients with RA were diagnosed according to the standards of the American College of Rheumatology (Arnett et al., 1988). The ST samples were either processed for cell culture or snap frozen and stored at -80℃. ST was minced, digested overnight with 5 mg/ml type IV collagenase (Sigma, St. Louis, MO) and 150 µg/ml type I DNase (Sigma), and separated from undigested tissue by unit gravity sedimentation. After collecting the suspended cells into fresh tubes, the cells were harvested by centrifugation at 500 × g for 10 min. The pellet was washed twice with DMEM (Life Technologies, NY) containing 10% FCS. Resuspended cells were plated at a concentration of 2 × 106/ml in a total volume of 1 ml/200 mm2 into T-25 culture flasks. After overnight incubation, non-adherent cells were removed by replacing fresh culture medium, and attached cells were cultured in DMEM with 10% FCS and 50 units/ml penicillin, 50 µg/ml streptomycin, and 0.025 µg/ml amphotericin B until 90% confluent growth. Primary cultured cells were passaged three to four times over several weeks for subsequent experiments.
RNA isolation and RT-PCR
Total RNA was isolated from ST or cultured FLS using TRIZOL reagent that contained phenol and guanidine isothionate in a monophase solution following the manufacturer's recommendations (Invitrogen Carlsbad, CA). 1 µg of pooled RNA was reverse transcribed in a total reaction volume of 20 µl containing 0.5 µg random hexamer primer, 20 units RNasin® ribonuclease inhibitor and superscript™ II reverse transcriptase (Invitrogen, CA). The PCR amplification of the cDNA aliquots was performed by adding 2.5 mM deoxyribonucleoside triphosphate (dNTPs), 2.5 U Taq DNA polymerase (Boehringer Mannheim, Germany) and 0.25 µM each of the sense and anti-sense primers. The following sense and anti-sense primers for Slug and β-actin were used (all written in 5'→3' direction): Slug sense TCCTCCATCTGACACCTCCTC, anti-sense CCCCCGTGTGAGTTCTAATGT; β-actin sense TACGTTGCTATCCAGGCTGTG, anti-sense AGTACTTGCGCTCAGGAGGAG. The levels of Slug, Puma, and β-actin mRNA expression were measured by real-time RT-PCR using an ABI PRISM 7000 sequence detector system (Applied Biosystems, Foster City, CA). The real-time RT-PCR amplification was performed using the pre-developed Assay-on-demand gene expression set for Slug gene (Assay ID Hs00161904_m1, Gene bank accession number NM_003068, Applied Biosystems), Puma gene (Assay ID Hs00248075_m1, Gene bank accession number NM_014417, Applied Biosystems) and human ACTB (β-actin) for the endogenous control (VIC/MGB Probe, Applied Biosystems) in combination with the TaqMan® Universal PCR Master Mix. Quantitation of Slug and Puma mRNA expression was calculated using the absolute method provided by the manufacturer (Applied Biosystems). Analysis was performed using the ABI PRIMS 7000 Sequence Detection software (Applied Biosystems, Foster City, CA). The expression level of selected genes in unknown samples was calculated as ratio of selected gene mRNA versus β-actin. Quantitation of selected gene mRNA and β-actin were performed using a standard curves made from known serial dilution of rheumatoid FLS. A negative control without template was included in each experiment.
Synoviocytes transfections
Recombinant adenovirus expressing human wild-type p53 gene (Ad-p53) and recombinant adenovirus expressing green fluorescent protein (Ad-GFP) were purchased from VECTOR BIOLABS (Philadelphia,PA). Cells cultured in DMEM supplemented with 1% FCS for 24 h were infected with Ad-p53 or Ad-GFP at an approximate multiplicity of infection of 100 for 24 h. Small interference RNA (siRNA) for Slug and control siRNA were purchased from Dharmacon (Lafayette, CO). Transient transfection of FLS was performed using the Amaxa Human Dermal Fibroblast Nucleofector™ kit (NHDF-adult; Amaxa Biosystems, Gaithersburg, MD), according to the manufacturer's protocols. Briefly, siRNA (1 µg) was added to 5 × 105 FLS suspended in 100 µl of Nucleofector™ solution. The mixture was then transferred into the electroporation cuvette and subjected to electroporation using the U-23 program, according to the manufacturer's instructions. Immediately after electroporation, the cells were suspended in 500 µl of cell culture medium, and transferred into culture dishes or plates. The transfected cells were then grown until the knocked-down effect of Slug siRNA was evident by RT-PCR.
Western blot analysis
Whole cell lysates (WCL) were prepared using RIPA buffer that contained 50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 1% NP40, 0.1% SDS, and 10 mM sodium deoxycholate. The concentration of WCL was determined using a Bio-RAD protein assay kit (Bio-Rad, Richmond, CA). Next, 15 µl of WCL were separated by electrophoresis on a 10% acrylamide gel using a constant voltage of 80 V for 2 h. The separated proteins were transferred electrophoretically onto nitrocellulose membranes that had been preblocked for 4 h at room temperature in TTBS (20 mM Tris-Cl, pH 8.0, 500 mM NaCl, 0.05% (v/v) Tween-20) containing 1% skim milk powder. The membranes were incubated overnight at 4℃ with 1:1000-diluted antibodies to p53 (mouse monoclonal anti-p53 antibody, Santacruz, CA) and β-actin (mouse monoclonal anti-β-actin antibody, Sigma, St. Louis, MO) in TTBS. The membranes were washed with TTBS three times for 15min, and then incubated for 1 h with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit antibody (1:5000, Jackson Immunoresearch, West Grove, PA). Proteins were detected using an ECL™ Western blot detection system (Amersham Life Science, Buckinghamshire, UK).
Cell viability and apoptosis assays
Three days after siRNA transfection, FLS were stimulated with 0.5 mM H2O2 for 9 h. Both nonadherent and adherent FLS were suspended in 0.4% trypan blue in PBS (pH 7.4), and counted using Hemacytometer (Horsham, PA). The cells that excluded the blue dye and had a well-defined cellular outline were scored as live. Total number of live cells was calculated and averaged over at least three times. FLS transfected with siRNA were plated in 96-well plastic plates (2 × 104 cells/well) and incubated with media or 0.5 mM H2O2 for 6 h. After incubation, apoptosis was determined using a Cell Death Detection ELISAPLUS kit (Roche, Mannheim, Germany) according to the manufacturer's instruction. It is based on a quantitative sandwich-enzyme immunoassay using mouse monoclonal antibodies against DNA and histones that allow for the specific, quantitative determination of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes). Each measurement was done in duplicate, and the results are presented as the fold induction compared with control samples (media-treated cells).
Caspase-3 activation assay
FLS transfected with control or Slug siRNA were plated in six-well plastic plates (2 × 105 cells/well) and incubated with media or 0.5 mM H2O2 for 6 h. Caspase-3 activation was measured using the ApoAlert Caspase-3 Colorimetric Assay kit (Clontech, CA, US) according to the manufacturer's instructions.
Statistical analysis
Data are expressed as mean ± SEM. The statistical significance of experimental results was calculated by paired Student's t-test or Mann-Whitney U-test. P values less than 0.05 were considered significant.
Acknowledgements
This research was supported by the Samsung Biomedical Research Institute grant, #SBRI C-A7-224 and by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0073632).
Abbreviations
- Ad-GFP
recombinant adenovirus expressing green fluorescent protein
- Ad-p53
recombinant adenovirus expressing human wild-type p53 gene
- FLS
fibroblast-like synoviocytes
- RA
rheumatoid arthritis
- ST
synovial tissue
- WCL
whole cell lysates
References
- 1.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 2.Byun HS, Won M, Park KA, Kim YR, Choi BL, Lee H, Hong JH, Piao L, Park J, Kim JM, et al. Prevention of TNF-induced necrotic cell death by rottlerin through a Nox1 NADPH oxidase. Exp Mol Med. 2008;40:186–195. doi: 10.3858/emm.2008.40.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano A, Rodilossi S, Rippo MR, Caprari P, Procopio A. Induction of stem cell factor/c-Kit/slug signal transduction in multidrug-resistant malignant mesothelioma cells. J Biol Chem. 2004;279:46706–46714. doi: 10.1074/jbc.M406696200. [DOI] [PubMed] [Google Scholar]
- 4.Ceponis A, Hietanen J, Tamulaitiene M, Partsch G, Patiala H, Konttinen YT. A comparative quantitative morphometric study of cell apoptosis in synovial membranes in psoriatic, reactive and rheumatoid arthritis. Rheumatology (Oxford) 1999;38:431–440. doi: 10.1093/rheumatology/38.5.431. [DOI] [PubMed] [Google Scholar]
- 5.Cha HS, Rosengren S, Boyle DL, Firestein GS. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:587–592. doi: 10.1002/art.21631. [DOI] [PubMed] [Google Scholar]
- 6.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 7.Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002;62:7316–7327. [PubMed] [Google Scholar]
- 8.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 9.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, Zhu L, Chittenden T. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 13.Hudson LG, Choi C, Newkirk KM, Parkhani J, Cooper KL, Lu P, Kusewitt DF. Ultraviolet radiation stimulates expression of Snail family transcription factors in keratinocytes. Mol Carcinog. 2007;46:257–268. doi: 10.1002/mc.20257. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Kanzawa T, Miyoshi T, Hirohata S, Kyo S, Iwamaru A, Aoki H, Kondo Y, Kondo S. Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status. Hum Gene Ther. 2005;16:685–698. doi: 10.1089/hum.2005.16.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Muller-Ladner U, Gay RE, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996;23:1345–1352. [PubMed] [Google Scholar]
- 19.Nakajima T, Aono H, Hasunuma T, Yamamoto K, Shirai T, Hirohata K, Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- 20.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Mancera PA, Gonzalez-Herrero I, Perez-Caro M, Gutierrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Sanchez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene. 2005;24:3073–3082. doi: 10.1038/sj.onc.1208505. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama M, Tsukazaki T, Yonekura A, Matsuzaki S, Yamashita S, Iwasaki K. Localisation of apoptosis and expression of apoptosis related proteins in the synovium of patients with rheumatoid arthritis. Ann Rheum Dis. 1996;55:442–449. doi: 10.1136/ard.55.7.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- 24.Vitali R, Mancini C, Cesi V, Tanno B, Mancuso M, Bossi G, Zhang Y, Martinez RV, Calabretta B, Dominici C, Raschella G. Slug (SNAI2) down-regulation by RNA interference facilitates apoptosis and inhibits invasive growth in neuroblastoma preclinical models. Clin Cancer Res. 2008;14:4622–4630. doi: 10.1158/1078-0432.CCR-07-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 26.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 27.You X, Boyle DL, Hammaker D, Firestein GS. PUMA-mediated apoptosis in fibroblast-like synoviocytes does not require p53. Arthritis Res Ther. 2006;8:R157. doi: 10.1186/ar2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Carl TF, Trudeau ED, Simmet T, Klymkowsky MW. An NF-kappaB and slug regulatory loop active in early vertebrate mesoderm. PLoS ONE. 2006;1:e106. doi: 10.1371/journal.pone.0000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zilfou JT, Spector MS, Lowe SW. Slugging it out: fine tuning the p53-PUMA death connection. Cell. 2005;123:545–548. doi: 10.1016/j.cell.2005.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]