Abstract
Hematologic malignancies account for a substantial percentage of cancers worldwide, and the heterogeneity and biological characteristics of leukemias and lymphomas present unique therapeutic challenges. Although treatment options exist for most of these diseases, many types remain incurable and the emergence of drug resistance is pervasive. Thus, novel treatment approaches are essential to improve outcome. Nearly half of the agents used in cancer therapy today are either natural products or derivatives of natural products. The enormous chemical diversity in nature, coupled with millennia of biological selection, has generated a vast and underexplored reservoir of unique chemical structures with biologic activity. This review will describe the investigation and application of natural products derived from higher plants in the treatment of leukemia and lymphoma and the rationale behind these efforts. In addition to the approved vinca alkaloids and the epipodophyllotoxin derivatives, a number of other plant compounds have shown promise in clinical trials and in preclinical investigations. In particular, we will focus on the discovery and biological evaluation of the plant-derived agent silvestrol, which shows potential for additional development as a new therapeutic agent for B-cell malignancies including chronic lymphocytic leukemia.
Keywords: Epipodophylloxin derivatives, flavopiridol, hematological malignancies, leukemia, lymphoma, natural products, silvestrol, vinca alkaloids
1. INTRODUCTION
Approximately 10% of all cancers in the United States are hematologic in origin [1]. This category of diseases can involve nearly any cellular component of the immune system, but the most commonly diagnosed are multiple myeloma, B-cell chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), and the broad category of non-Hodgkin lymphoma (NHL)[1]. In the past decade, aggressive research in this area has generated a variety of new therapeutic approaches, many of which have led to viable treatment options for patients with leukemias and lymphomas. These recent advances can be credited not only to significant improvements in our understanding of tumor cell biology, but also to high-throughput screening and medicinal chemistry strategies to identify and produce diverse new agents for preclinical investigation. Additionally, enormous effort has been put into defining patient subsets within each disease using genetic or cell-surface markers and studying how these different subsets respond to treatment. While this approach is intended to “personalize” medicine and provide the most effective treatment for each patient, it also has the significant benefit of enhancing our understanding of the underlying biology of cancer. Each molecular characteristic of aggressive disease that is identified and validated can expose a new therapeutic target for investigation and intervention.
Despite these hopeful steps, considerable challenges remain. Successful treatment of patients with hematologic malignancies varies widely not just by disease category but by subset (genetic or otherwise) within each disease, and reasons for this are generally unclear. Second, scientific advances made in recent years have a long trek to clinical application, and improving testing strategies to streamline the process and eliminate poor drug candidates early will be invaluable. Third, the dissemination of tumor cells in hematologic malignancies precludes localized surgery and radiation approaches, and the sheltered microenvironment of bone marrow limits the efficacy of many treatments. Advances in understanding the protective role of the microenvironment will be essential for successful treatment of leukemias and lymphomas. Finally, novel and potent agents must be identified that target tumor cell survival pathways while sparing normal cells. This is a significant challenge, as there are undoubtedly targetable survival mechanisms yet to be discovered. To make advances toward curative therapy, we must continue to identify new tumor survival pathways and protective mechanisms; we cannot rely only on what is currently known.
This last challenge highlights a key strength of natural product investigation. Using cytotoxic activity-guided screening strategies, agents with potent anti-tumor activity can be identified in an unbiased fashion, increasing the likelihood of uncovering novel tumoricidal pathways and survival mechanisms. Once an effective molecule is identified, the mode of action can be investigated and additional or alternative agents that impact the target can be synthesized. Thus even when an active natural product does not reach clinical use, its investigation can provide critical clues for the development of new targeted cancer drugs.
Natural products, obtained to date mainly from fungi, higher plants, and soil microorganisms, have a long history of beneficial use by mankind for the treatment of disease. These substances may be useful in their structurally unmodified form or may be derivatized by chemical synthesis to enhance potency or pharmacologic properties such as water-solubility or thermostability [2]. The term “natural product” is generally taken to mean a compound that has no known primary biochemical role in an organism. Such compounds are also called “secondary metabolites”, and apparently are produced by the organism for ecological or defensive purposes, thus promoting its survival [3]. The value of natural products in the arsenal of leukemia therapies is demonstrated by such agents as L-asparaginase, daunorubicin, and the anthracyclines.
In the present review, we highlight several plant-derived compounds that have established clinical uses in treating hematological malignancies. Following this, examples of plant compounds in clinical trials and agents under preclinical development will be provided. Silvestrol, an oxygen heterocyclic belonging to the rocaglate compound type, is of special interest and provides an example of a plant natural product in the process of development toward clinical testing. It is hoped that as a result of this review, readers will have a greater awareness of the excellent promise that plant-derived secondary metabolites and their derivatives show for use in the therapy of leukemia and lymphoma.
2. PLANT-DERIVED NATURAL PRODUCTS IN CURRENT USE FOR HEMATOLOGICAL MALIGNANCIES
Vinca alkaloids
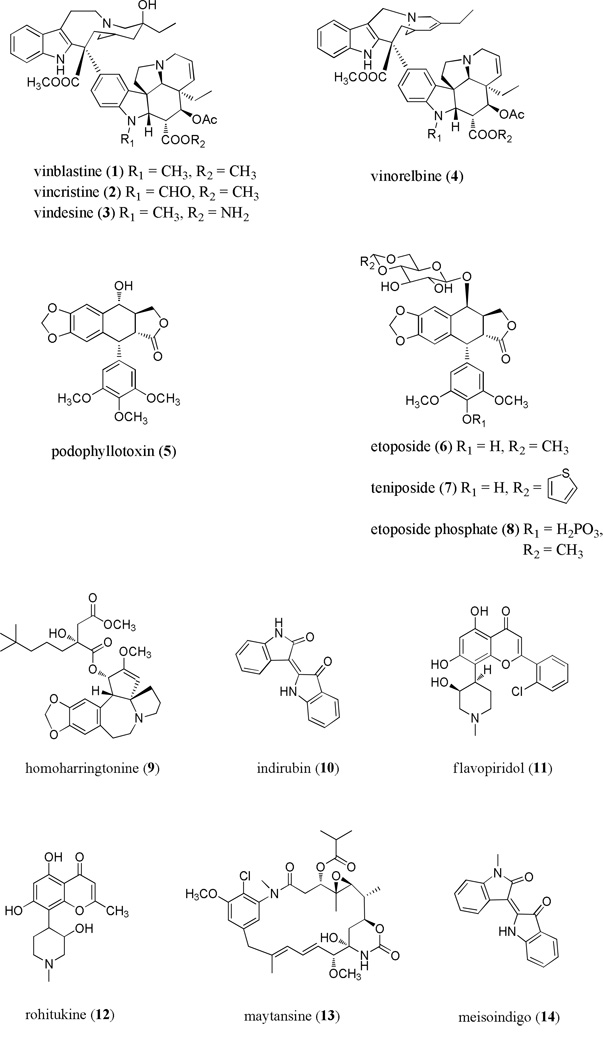
The vinca alkaloid, vinblastine (1), was first isolated some 50 years ago from the Madagascar periwinkle Catharanthus roseus (formerly Vinca rosea) by two independent groups. The potential anticancer activity of the alkaloid constituents of this species were discovered by serendipity when researchers at the University of Western Ontario noticed a reduction in white blood cell count and destruction of bone marrow in rats that were injected with C. roseus extract as part of a search for new diabetes agents [4]. Noble, Beer and Cutts isolated an alkaloid responsible for the observed activity, and named it vincaleukoblastine, which was later shortened to vinblastine [4, 5]. Svoboda and colleagues at Eli Lilly & Co., Indianapolis Indiana, also used C. roseus as a source to isolate vinblastine as well as the related compound leurocristine, later renamed vincristine [6–8]. The cytotoxic activity of the vinca alkaloids is due to their ability to bind tubulin, thus blocking the process of mitosis and causing metaphase arrest [9]. This crucial discovery prompted aggressive efforts to identify additional tubulin-interacting compounds for use in medicine. In 1962, the structures of vincaleukoblastine (vinblastine) and leurocristine (vincristine) (2) were established by interpretation of 1H NMR spectra [10].
Vincristine and vinblastine are structurally similar bisindole alkaloids containing a catharanthine and vindoline unit, with the latter differing only in the presence of one substituent group. Vinblastine contains a N-methyl group, in contrast to a N-formyl group in vincristine [10]. In vivo studies using mice engrafted with L1210 and P1534 leukemia cells and Ehrlich ascites tumor cells, performed shortly after the discovery of vinblastine, demonstrated its antineoplastic activity [11]. Early clinical trials with vincristine sulfate resulted in complete remission for the majority of the patients with acute leukemia [12]. Currently, vinblastine is primarily used for the treatment of lymphomas including Hodgkin’s disease. The activity of the vinca alkaloids is due to their ability to bind tubulin, thus blocking cell mitosis causing metaphase arrest [9, 13]. Vincristine sulfate (Oncovin®) and vinblastine sulfate (Velban®) were the first plant-derived anticancer agents to be approved by the U.S. FDA, in 1963 and 1965, respectively [14]. Vinblastine sulfate is used for the treatment of lymphomas inclusive of Hodgkin’s disease, and is also employed in the therapy of bladder and breast cancers. Vincristine sulfate is utilized for acute lymphoblastic leukemias and lymphomas in combination chemotherapy [13, 14].
Vindesine (3) and vinorelbine (4) are semisynthetic derivatives of vinblastine with current or potential use in treating hematological malignancies. Vindesine differs from vinblastine in possessing an amino ester group in the vindoline unit. Vinorelbine contains an additional unsaturation and the absence of a hydroxy group in the catharanthine unit, in comparison to vinblastine [14]. A Phase II investigation of vindesine in Hodgkin and non-Hodgkin lymphomas indicated efficacy, and this agent (Eldisine®) is used in France, the UK, and other countries to treat drug-resistant acute lymphoid leukemia [15], although it is currently not approved for marketing in the United States. Phase II clinical trials of vinorelbine demonstrated its potential activity in Hodgkin’s disease [16, 17]. Vinorelbine tartrate (Navelbine®) is used mainly as monotherapy or in combination therapy to treat non-small cell lung carcinoma (NSCLC) or advanced breast cancer [14].
Podophyllotoxin derivatives
Etoposide (6) and teniposide (7) are semi-synthetic derivatives of podophyllotoxin (5), a lignan isolated initially from Podophyllum peltatum (American mandrake; Berberidaceae) [18]. Although podophyllotoxin was first isolated in 1880, its structure was not determined until 1951 by Hartwell and Schrecker of the U.S. National Cancer Institute [19]. Podophyllotoxin is also obtained from Indian podophyllum, Podophyllum emodi [18]. Despite its discernible antineoplastic activity in tumored mice in the laboratory setting, clinical use of podophyllotoxin was found to be not feasible due to toxicity. For this reason, researchers pursued less toxic analogs of podophyllotoxin, leading to the preparation of several epipodophyllotoxin glycosides. These glycosides exhibit decreased potency when compared to podophyllotoxin, and further modifications were performed to improve activity. This resulted in the development of the active compounds etoposide and teniposide [20]. These agents are topoisomerase II inhibitors that act by interfering with topoisomerase-mediated re-annealing of single- and double-strand DNA breaks, particularly in the S and G2 phases of the cell cycle [20]. The result of this inhibition is an accumulation of DNA damage and potent induction of caspase-dependent apoptosis.
Clinical trials of etoposide and teniposide in the 1970s indicated the potential usefulness of these drugs in the treatment of acute myeloid leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphoma [21]. While etoposide is used most widely to treat lung cancer and testicular cancer, it is also effective for different types of leukemias and lymphomas. Teniposide also has activity against various types of hematological malignancies, either alone or in combination chemotherapy [21, 22]. Etoposide (VePesid®) received FDA approval in 1983, and a water-soluble prodrug of etoposide, etoposide phosphate (Etopophos®) was approved by the FDA in 1996. Etoposide phosphate has similar pharmacological and toxicological properties to the parent drug, and is preferred for intravenous administration. Teniposide (Vumon®) was approved in 1993 [21, 22].
3. PLANT NATURAL PRODUCTS IN CLINICAL TRIALS: EXPERIMENTAL THERAPY
Homoharringtonine
The Cephalotaxus alkaloid, homoharringtonine (9) shows promise for treating several hematological malignancies, and the development of this agent has been reviewed [23, 24]. The medicinal uses of plants in the genus Cephalotaxus (family Cephalotaxaceae), the source of homoharringtonine, are well known in traditional Chinese medicine. Homoharringtonine was isolated in the early 1970s from Cephalotaxus harringtonia by Powell and colleagues, along with three structurally similar, naturally occurring cephalotaxine esters [25]. The parent compound, cephalotaxine, was first reported by Paudler and associates [26], and its structure was revised by Abraham et al. as a result of the application of single-crystal X-ray crystallography on cephalotaxine methiodide [27]. Of the four analogs isolated by Powell et al., homoharringtonine most effectively prolonged survival in a P388 murine lymphocytic leukemia model.
Homoharringtonine is a protein synthesis inhibitor, originally thought to inhibit the initiation of polypeptide synthesis [28]. Further studies showed that Cephalotaxus alkaloids block peptide bond formation and aminoacyl tRNA binding, thus inhibiting translation at the elongation, rather than initiation, phase [29]. The resulting loss of protein synthesis has a variety of effects including cell cycle inhibition and induction of apoptosis through depletion of pro-survival proteins [30]. Homoharringtonine, alone or in combination with IFN-α and cytosine arabinoside (Ara-C), induced apoptosis in human normal and chronic myelogenous leukemia (CML) hematopoietic progenitor cells at concentrations as low as 100 ng/ml [30, 31]. Testing of homoharringtonine for growth-inhibitory effects against ten human leukemia and lymphoma cell lines revealed the highest activity against human promyelocytic cells (HL-60), and the lowest activity against DND-41 acute promyelocytic leukemia cells, with a 70-fold difference in activity [31]. It was concluded that the degree of cellular uptake of this agent gives rise to varying cell line sensitivity, and that the exposure time to this alkaloid has a greater influence than concentration. Using the HL-60 cell line, it was also shown that the antileukemic effect of homoharringtonine is due to diversion of proliferating blast cells into a differentiation pathway, resulting in arrest of cellular proliferation [32]. Another study investigating the effect on clonal proliferation and differentiation found that homoharringtonine inhibited colony formation of myeloid and lymphoid cell lines and fresh leukemic cells [33]. In vivo testing of homoharringtonine in mice with engrafted murine L1210 cells showed inhibition of neoplastic proliferation at doses of 0.5 – 4.0 mg/kg as measured by the mitotic index of leukemic cells [25], and mice treated with homoharringtonine showed survival rates of 142% versus control animals [34].
Following reports of clinical efficacy in China, particularly in acute myeloid leukemia, multiple clinical trials involving homoharringtonine were initiated in the United States. O’Brien and colleagues conducted a trial in late-stage (more than one year from diagnosis) chronic myelogenous leukemia (CML). Seventy-two percent of the 58 evaluable patients exhibited a complete hematologic response, which was prolonged in some patients [35]. A subsequent trial by the same group investigated six courses of homoharringtonine followed by interferon alpha (IFN-α) in early phase CML (less than one year from diagnosis). The results were compared with those obtained from a prior group of patients treated with IFN-α alone. The trial showed 92% of patients achieved a complete hematological response, a significant improvement over historical populations treated only with interferon [36]. Due to these encouraging results, homoharringtonine continues to be investigated in CML patients who do not respond to therapy with imatinib or other tyrosine kinase inhibitors. Interestingly, extended dosing of this agent is required for optimal efficacy, consistent with in vitro observations.
Indirubin
Indirubin (10) is a 3,2′-bisindole isomer of indigo, a well-known natural dye, which has ethnomedicinal use in traditional Chinese medicine and is used to treat chronic myelogenous leukemia [37, 38]. Mechanistic studies have shown that indirubin and derivatives act by inhibiting cyclin dependent kinases (CDKs) [39]. Indirubin possesses a flat heterocyclic ring system that fits into the ATP binding side of the kinase catalytic site, competing with ATP [37], with a resultant block of cell cycle progression. Indirubin has demonstrated in vivo effects in a mouse leukemia L7212 model [40]. Clinical efficacy of indirubin was observed some decades ago in the People’s Republic of China. Indirubin administered orally at a dose of 150–450 mg/day to patients with CML produced complete remissions in 82 of 314 cases (26%), with partial remission in 105 cases (33%), and the drug was generally well tolerated [41].
Flavopiridol
Flavopiridol (11) is a semi-synthetic flavone based on the alkaloid rohitukine (12), isolated in the late 1970s from the Indian plant Dysoxylum binectariferum [42, 43]. Flavopiridol is regarded as a “pan-CDK” inhibitor, and inhibits multiple cyclin-dependent kinases with subsequent cell cycle arrest. Despite this, this compound appears to be most potent against CDK9 [44]. However, it is still unclear which of the CDK’s, if any, are involved in the sometimes dramatic anti-tumor effects seen in patients treated with flavopiridol. In the multitude of reports investigating this agent, it is evident that there are at least several distinct mechanisms of action that likely differ by cell type. Flavopiridol has shown low nanomolar efficacy in a wide variety of tumor cell lines and patient samples. Specific to leukemia patient cells, flavopiridol was shown to downregulate inducible nitric oxide synthase and the endogenous CDK inhibitor p27kip1 in CLL tumor cells [45]. Our group also showed in primary CLL cells that flavopiridol down-regulates the mitochrondrial protein Mcl-1 and has an early impact on mitochondrial membrane integrity, and that these effects occur prior to cell death [46]. Flavopiridol has also been investigated using in vivo tumor models. When treated with flavopiridol at 7.5 mg/kg, mice bearing human acute myeloblastic leukemia (HL-60) cell line xenografts underwent complete remission, and remained disease-free several months after one course of treatment. In the same study, immunodeficient mice bearing disseminated human acute lymphoblastic leukemia Nalm/6 cells showed a 15-day prolongation in survival [47].
Initial clinical trials using flavopiridol in hematologic malignancies were disappointing [48, 49]. However, Byrd and Grever found that the results could, at least in part, be explained by the large difference in protein binding of flavopiridol between human plasma and fetal bovine serum as typically used in cell culture. Using this observation and data from completed trials, a schedule of flavopiridol administration was designed with the goal of achieving brief high levels of free drug. Using this new schedule, this group showed that flavopiridol has potent activity in patients with advanced, drug-refractory CLL [50], with the unusual effect in some patients of acute tumor lysis syndrome due to rapid tumor cell death. Because of this, flavopiridol is not administered to CLL patients with circulating tumor cell counts greater than 150,000/µL and all patients are closely monitored. In another phase I trial, flavopiridol followed by 1-β-d-arabinofuranosylcytosine (ara-C) and mitoxantrone was administered to patients with relapsed and refractory acute myelogenous and lymphoblastic leukemias (AML; ALL) [51]. Overall response rates in this heterogeneous population were 31% and 12.5%, respectively. A subsequent Phase II study by the same group produced 75% complete remissions in newly diagnosed secondary AML and first-relapse AML, although results for patients with multiple-relapse or refractory AML were poor [52]. As of this writing, flavopiridol is being evaluated in a multi-center Phase II trial in relapsed/refractory CLL and continues to be investigated in other hematologic malignancies as well [53].
Maytansinoids
Maytansinoids are tubulin-binding cytotoxic agents that have been isolated from various plants, mosses and an actinomycete, Actinosynnema pretiosum [54]. The parent nitrogen-containing macrocylic substance, maytansine (13), was originally isolated in 1972 by Kupchan and colleagues from the Ethiopian shrub Maytenus serrata [55]. Similar to the vinca alkaloids, maytansinoids show antimitotic activity due to tubulin binding, resulting in inhibition of microtubule assembly [56, 57]. Binding to tubulin occurs at a site overlapping the vinca alkaloid binding site, and is different from the colchicine binding site [58, 59]. Maytansinoids showed inhibitory activity against the Lewis lung carcinoma B-16 melanocarcinoma murine solid tumor test system, and antileukeumic activity against P-388 lymphocytic leukemia over a 50–100 fold dosage range at the µg/kg level [60, 61]. Unfortunately, clinical trials with maytansine, both alone and as a monoclonal antibody conjugate, showed toxicity as well as low response rates in adults with advanced cancer [62–64]. Therefore, metabolism studies involving maytansine have been carried out so that analogs with better clinical potential might be generated [65]. The extremely high in vitro potency of the maytansinoids has resulted in a strong continuing interest in structure-activity relationship studies, analog development, total synthesis, and preclinical studies [54].
Meisoindigo
Meisoindigo (14), or N-methylisoindigotin, is a semi-synthetic derivative of indirubin (10) generated to improve on the poor water solubility of the parent compound and to reduce its potential for gastrointestinal toxicity [66]. In vitro studies showed anti-proliferative effects on the myelocytic leukemia cell lines HL-60 and NB4, albeit at elevated concentrations [67, 68]. A 1997 phase II clinical trial conducted in the People’s Republic of China assessed the effectiveness of meisoindigo in the treatment of chronic myelogenous leukemia (CML). Approximately 400 cases of CML were treated with meisoindigo alone at a dosage of 75–150 mg per day. The response rate was reported at 90% and the complete and partial remission rate was 81%; effects were comparable to approved agents hydroxyurea and busulfan, and in fact showed even greater effects when combined with hydroxyurea. Side effects included bone, joint and muscle pain of varying degree, but none of the patients developed severe myelosuppression [38, 69]. These results support the therapeutic potential of meisoindigo and its derivatives, and investigation of this class of agents continues.
4. PLANT NATURAL PRODUCTS IN PRECLINICAL INVESTIGATIONS FOR THE TREATMENT OF HEMATOLOGIC MALIGNANCIES
Noscapine
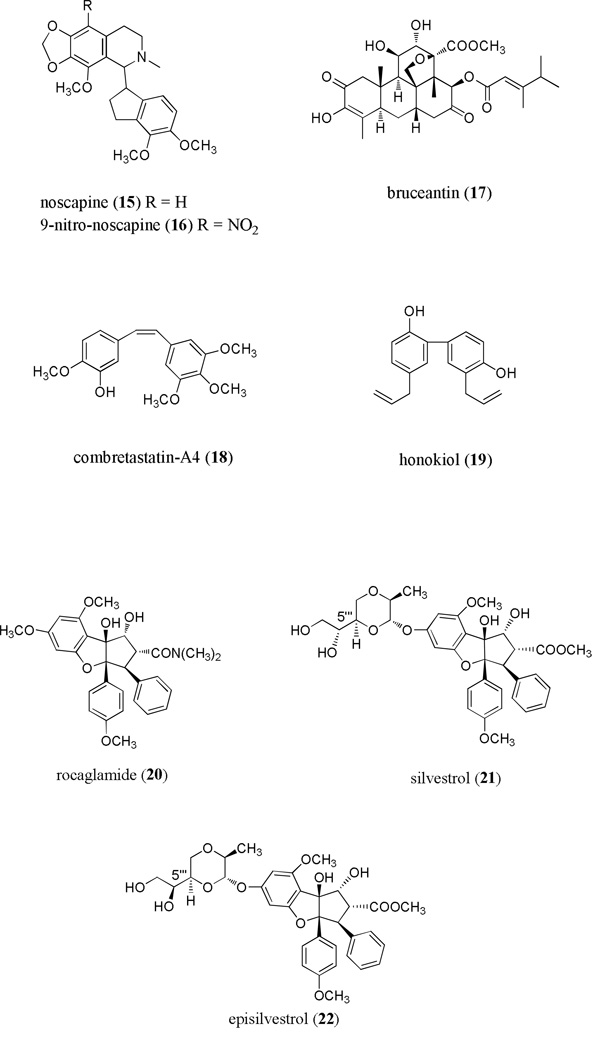
Noscapine (15) is a well-known phthalideisoquinoline alkaloid found in opium poppy, obtained from the capsules of Papaver somniferum. Noscapine comprises approximately 10% w/w of the total alkaloid content in opium, and has long been used as an antitussive [70]. In vitro assays using lymphoma cells resulted in 50% growth inhibition with 10 µM noscapine with little evidence of toxicity to normal cells [71]. The 9-nitro derivative of noscapine (9-nitro-noscapine) (16) caused apoptosis in a T-cell lymphoma multidrug resistant cell line without affecting normal cells [72]. Like many of the agents discussed here, noscapine acts by binding to tubulin and causing mitotic arrest at the metaphase stage of the cell cycle [73]. Noscapine produced significant tumor shrinkage in mice bearing lymphoid tumors, demonstrating in vivo efficacy. Furthermore, noscapine was found to be safe when administered orally to the mice, as demonstrated by a lack of mortality in the in vivo assay [74]. Additional in vivo experiments with noscapine reported efficacy in a mouse model of prostate cancer [75]. At the time of this writing, noscapine is being evaluated in a Phase I trial for patients with relapsed or refractory multiple myeloma [64].
Bruceantin
Bruceantin (17), a quassinoid (nortriterpenoid), was initially isolated from Brucea antidysenterica by the Kupchan group, and was observed to exhibit potent antileukemic in vitro and in vivo activity [76, 77]. Bruceantin was evaluated in Phase I trials in several malignancies, but unsatisfactory results due to apparent inactivity of the drug led to discontinuation of the investigations [78]. Renewed interest was aroused when it was noted that bruceantin down-regulates the c-myc oncogene, an important factor associated with the development of leukemia and lymphoma [43]. In an in vitro study with a panel of leukemic cells, bruceantin showed potent down-regulation of c-myc oncoproteins associated with mitotic arrest at the G1 or S phases of the cell cycle [79]. Bruceantin inhibited tumor proliferation and induced apoptosis in mice inoculated with RPMI 8226 multiple myeloma cells without any observed toxicity, suggesting that bruceantin should be reinvestigated for the treatment of selected hematological malignancies [80].
Combretastatins
Pettit and colleagues isolated several bioactive stilbenoid compounds from the South African tree Combretum caffrum, including the parent compound, (−)-combretastatin [81, 82]. The combretastatins comprise several series of related compounds, with the A series being cis-stilbenes active in vitro against the leukemic P388 and L1210 cell lines. Combretastatin-A4 (18; CA4P) showed the greatest potency in the P388 and L1210 cell lines [83]. In vitro assays with acute myeloid leukemia cells showed that this compound is cytotoxic at extremely low doses (1 nM). Treatment of mice that were subcutaneously injected or systemically inoculated with HL-60 cells demonstrated the in vivo effectiveness of MZ3, a synthetic analog of CA4P, in removing circulating and vascular attached leukemic cells without significant toxicity. This agent also provided a survival benefit in this model [84]. Again, this agent is purported to act by interaction with tubulin, although additional mechanisms of action are postulated including disruption in mitochondrial membrane potential, down-regulation of anti-apoptotic proteins such as XIAP, and DNA fragmentation [84, 85].
Honokiol
Honokiol (19) is a simple phenolic derivative originally named after “Hōnoki”, the Japanese name for its plant of origin, Magnolia obovata[86]. The genus Magnolia has widely recognized use in traditional Chinese and Japanese medicine. Anti-proliferative and anti-angiogenic activities were noted in vitro using the SVR angiosarcoma cell line [87]. Interestingly, honokiol showed selective apoptosis induction in primary CLL tumor cells relative to normal controls. This effect was observed together with up-regulation of the pro-apoptotic Bcl-2 family protein Bax and down-regulation of the anti-apoptotic protein Mcl-1 [88]. Honokiol was also cytotoxic for both human multiple myeloma cell lines and tumor cells from patients having relapsed multiple myeloma, and this compound induced apoptosis in the experimental systems used through both caspase-dependent and caspase-independent pathways [89]. While honokiol has shown activity in a variety of systems, its relatively low potency prevents development of the parent molecule. Therefore, active efforts to generate more potent derivatives are underway [90].
5. SILVESTROL AS A POTENTIAL THERAPY FOR B-CELL MALIGNANCIES
Silvestrol Discovery and Characterization
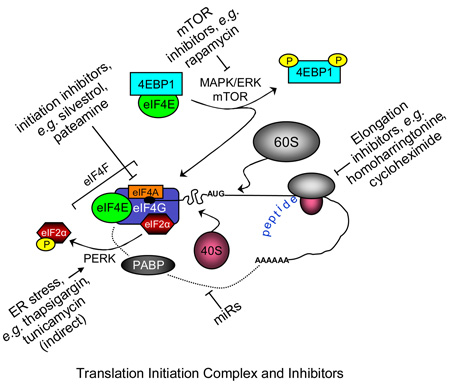
Cyclopenta[b] benzofuran constituents of plants from the genus Aglaia were first discovered in 1982, and have been of considerable interest since one such molecule, rocaglamide (20), was found to exhibit anti-leukemic activity in the P388 lymphocytic leukemic murine model [91, 92]. Subsequently, several groups showed anti-proliferative activity of members of the cyclopenta[b] benzofuran class using human tumor cell lines [93–96] (reviewed in Kim et al. [97]), and recently, primary human tumor cells [98]. Silvestrol, a member of this class, was identified by our group as part of an NCI-funded project to identify structurally unique cytotoxic molecules from tropical plants by activity-guided chromatographic fractionation. The identification of cytotoxic extracts from Aglaia foveolata led to the purification of silvestrol (21) as well as (+)-episilvestrol (22), its C-5’’’ S epimer at the diol side chain of the dioxanyl ring. We then characterized the structure and absolute configuration of silvestrol, using detailed NMR studies and single-crystal X-ray diffraction [99]. Silvestrol is a unique member of the cyclopenta[b] benzofuran class, and bears a bulky dioxanyl group unprecedented in nature. Preliminary structure-activity relationship studies suggest that the dioxanyl side chain is important for cytotoxicity, as silvestrol is much more potent than rocaglamide in vitro, and acetylation of the two dioxanyloxy hydroxyl groups causes a ten-fold reduction in potency [99]. Because of its unique structure and high potency, silvestrol has attracted the attention of synthetic organic chemists. Two groups have now reported total syntheses of (−)-silvestrol [100, 101], and in so doing confirmed the structure and stereochemistry reported earlier. Additionally, the Rizzacasa group further refined their synthesis of silvestrol and generated additional bioactive derivatives [102]. Importantly, this work indicated that stereochemistry of the cyclopentabenzofuran backbone was critical for the anti-cancer activity of silvestrol. Structure-activity relationship studies by Cencic et al. [103] confirm the importance of both the cyclopenta[b] benzofuran core and sidechain for optimal potency. While silvestrol was originally isolated from fruits and twigs of A. foveolata, our more recent work shows it can be successfully extracted from the leaves and stems of this plant [104]. The occurrence of this compound in several plant parts including leaves might enable silvestrol to be produced in large quantities as a “renewable resource” that will not sacrifice the plant of origin.
The mechanism and targets of cyclopenta[b] benzofurans in general, and silvestrol in particular, are incompletely understood. Previous work with members of this class suggest that their targets may include inhibition of nucleotide and/or protein synthesis [93, 94] or inhibition of transcription factors NF-κB [105] or NF-AT [106]. Zhu et al. recently described a potential mechanism for rocaglamide-mediated cytotoxicity in which activation of p38 MAPK, together with suppression of ERK, leads to apoptosis involving activation of caspases 8 and 9 [100]. Specific to silvestrol, Swanson and colleagues showed that this agent produces a p53-independent cell cycle blockade at the G2/M checkpoint in the LNCaP human prostate cancer cell line [107], consistent with an effect on cyclins. Additional work by this group demonstrated that silvestrol activates an unusual pathway of apoptosis in LNCaP cells, as indicated by the involvement of caspases 2, 9 and 10, but not 3 and 7 [108]. We demonstrated that silvestrol treatment of B leukemia cells results in loss of the pro-survival protein Mcl-1 by selective inhibition of translation [109]. Together, it is evident that this family of agents may have multiple targets that vary not only by agent but by cell type. More recently, extensive and elegant work by Pelletier and colleagues has defined the inhibition of translation initiation as a key mechanism in the cytotoxic activity of silvestrol [103, 110].
Silvestrol Mechanism and Activity in Leukemia
Silvestrol was shown to have potent in vitro cytotoxicity against lung, breast, and prostate cancer cell lines, with ED50 in the nanomolar range [95, 99]. We investigated silvestrol both in leukemia cell lines and in primary tumor cells from patients with CLL. In our studies, silvestrol exhibited an LC50 (concentration lethal to 50%) of just 7 nM in CLL cells, and similar potency was observed in a series of B-lymphoma cell lines. Importantly, we showed that silvestrol is significantly more active against B-cells than against T-cells [109]. We noted this intriguing quality not just in cell lines, but in isolated peripheral blood cells, whole blood, and in vivo using the Eμ-Tcl1 murine model of CLL [109, 111]. This finding is significant, as currently available drugs for CLL and other B-leukemias also severely impact the T-cell compartment, leaving patients at risk of potentially lethal viral reactivation and opportunistic infections.
As noted above, we demonstrated that silvestrol mediates translation inhibition, with subsequent loss of the key survival protein Mcl-1 and resultant loss of mitochondrial integrity [109]. In our CLL studies this effect was rapid; Mcl-1 levels were notably reduced as soon as 4 hours after silvestrol addition. Pelletier’s group demonstrated that silvestrol blocks translation at the initiation step by interfering with assembly of the eIF4F translation complex. More specifically, they demonstrated using cell-free systems that silvestrol promotes an abnormal interaction of eIF4A with capped mRNA, effectively depleting this key RNA helicase from the eIF4F complex and thus preventing ribosomal assembly and subsequent translation. The result of inhibiting translation initiation is the selective loss of proteins with rapid turnover such as Mcl-1 as noted in CLL cells. In the MDA-MB-231 human breast cancer cell line, silvestrol treatment resulted in the depletion not only of Mcl-1, but also of cyclin D1, Bcl-2, survivin and c-myc [103]. Taken together, the likely mechanism of silvestrol-mediated killing in leukemia cells is the selective loss of short half-life proteins including (but not limited to) those listed above. We previously reported that siRNA-induced depletion of Mcl-1 was by itself sufficient to induce apoptosis in CLL patient cells [112], demonstrating the importance of this protein in CLL cell survival and its usefulness as a therapeutic target. However it is very probable that additional factors are involved, and further research is needed not just to understand the cytotoxic activity of silvestrol but to learn how to potentiate its killing of leukemia and lymphoma cells.
Silvestrol In Vivo Studies
Silvestrol inhibited the growth of PC-3 human prostate cancer cells in a murine xenograft study [113]. In vivo activity was further demonstrated by our group using the A2780S human ovarian carcinoma xenograft model (unpublished data) and in the hollow fiber assay [114] with colon, epithelial or prostate cancer cell lines. In these assays, silvestrol at 2.5 mg/kg daily for 5 days produced over 50% reduction in tumor growth relative to control without toxicity as assessed by weight loss. In a leukemia model using P388 cells injected intraperitoneally [92], silvestrol at 2.5 mg/kg daily for 5 days demonstrated promising anti-tumor activity (150% survival, treated/control) without weight loss [99]. Pelletier and colleagues used a murine model of Eμ-myc-driven lymphoma either with deficient PTEN or elevated eIF4E to assess in vivo activity of silvestrol [110]. In these models, silvestrol had no efficacy as a single agent but significantly potentiated the anti-tumor activity of doxorubicin. In a follow-up manuscript [103], this group went on to show that silvestrol shows single-agent activity in breast and prostate cancer xenograft models, although the reason for the different responses among these tumor models is not yet understood. In our work using a xenograft model of aggressive acute lymphoblastic leukemia, silvestrol showed a moderate but highly significant improvement in survival using a three-times weekly dosing schedule [109]. It is important to note that pharmacokinetic data with silvestrol indicates a short in vivo half-life and poor bioavailability with standard method of administration used by all groups to date. Thus, the already notable in vivo activity of silvestrol is likely to be dramatically improved with optimization of dosing schedule and through use of an improved formulation that takes into account the complex amphipathic nature of the drug.
6. FUTURE PROSPECTS
There remains a great interest in the search for natural products from terrestrial and marine animals, microorganisms and plants as potential drug chemical leads for the treatment of a wide range of disease conditions [62, 115–119]. There is an especially strong interest in developing new natural product anticancer agents from natural sources, because of the many past successes in this endeavor [120–122]. However, screening efforts of many natural products laboratories have concentrated more recently on attempts to find cures for the most common forms of cancer (e.g. breast, colorectal, lung, prostate) rather than rarer acute and chronic forms of leukemia. In a recent review article, Cragg, Newman, and Yang from the National Cancer Institute noted that as a result of a human cancer cell-based evaluation of 90,000 terrestrial and marine organisms, a large number of leads were obtained with activity against leukemia cell lines, most of which have not yet been followed up by activity-guided fractionation to characterize the key constituent(s) [123]. Clearly, there is ample scope for new discoveries to be made of natural products from plants and other organisms with promising activities against hematological malignancies. In addition to random screening in this regard, it is likely that the targeted investigation of plants used in systems of traditional medicine may be particularly beneficial. The value of collaboration between laboratories specializing in natural products isolation and hematology in this type of oncology drug discovery must be emphasized.
Acknowledgments
In connection with the silvestrol studies described in this review, the authors wish to acknowledge support of National Cancer Institute grants U19 CA52956 and P01 CA125066 to ADK as well as P01 CA081534 to MRG/TJ Kipps and P50 CA140158 to MRG/JC Byrd. This work also received support from the American Cancer Society and the Samuel Waxman Cancer Research Foundation.
Footnotes
Conflict of interest statement The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kinghorn AD. In: Foye’s Principles of Medicinal Chemistry. Lemke T, Williams DA, editors. Baltimore: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp. 12–25. [Google Scholar]
- 3.Williams DH, Stone MJ, Hauck PR, Rahman SK. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989;52:1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- 4.Noble RL. Vincaleukoblastine. Can Med Assoc J. 1961;85:610–611. [PMC free article] [PubMed] [Google Scholar]
- 5.Noble RL, Beer CT, Cutts JH. Further biological studies of vincaleukoblastine – an alkaloid isolated from Vinca rosea (L.) Biochem. Pharmacol. 1958;1:347–348. [Google Scholar]
- 6.Svoboda GH, Neuss N, Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J Am Pharm Assoc. 1959;48:659–666. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 7.Johnson IS, Wright HF, Svoboda GH, Vlantis J. Antitumor principles derived from Vinca rosea Linn. I. Vincaleukoblastine and leurosine. Cancer Res. 1960;20:1016–1022. [PubMed] [Google Scholar]
- 8.Svoboda GH. Alkaloids of Vinca rosea (Cathanthus roseus) V. Preparation and characterization of alkaloids. Lloydia. 1961;24:173–180. [Google Scholar]
- 9.Cutts JH. The effect of vincaleukoblastine on dividing cells in vivo. Cancer Res. 1961;21:168–172. [PubMed] [Google Scholar]
- 10.Neuss N, Gorman M, Boaz HE, Cone NJ. Vinca alkaloids. XI. Structures of leurocristine and vincaleukoblastine. J. Am. Chem. Soc. 1962;84:1509–1510. [Google Scholar]
- 11.Cutts JH, Beer CT, Noble RL. Biological properties of vincaleukoblastine, an alkaloid in Vinca rosea Linn. with reference to its antitumor action. Cancer Res. 1960;20:1023–1031. [PubMed] [Google Scholar]
- 12.Karon M, Freireich EJ, Frei E, et al. The role of vincristine in the treatment of childhood acute leukemia. Clin Pharmacol Ther. 1966;7:332–339. doi: 10.1002/cpt196673332. [DOI] [PubMed] [Google Scholar]
- 13.Chabner BA, Amrein PC, Druker BJ, et al. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th Edition. Brunton LL, Lazo JS, Parker KL, editors. New York: McGraw-Hill; 2006. pp. 1257–1262. [Google Scholar]
- 14.Guérritte F, Fahy J. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC/Taylor & Francis; 2005. pp. 123–135. [Google Scholar]
- 15.Dancey J, Steward WP. The role of vindesine in oncology--recommendations after 10 years' experience. Anticancer Drugs. 1995;6:625–636. doi: 10.1097/00001813-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Devizzi L, Santoro A, Bonfante V, et al. Vinorelbine: an active drug for the management of patients with heavily pretreated Hodgkin's disease. Ann Oncol. 1994;5:817–820. doi: 10.1093/oxfordjournals.annonc.a059010. [DOI] [PubMed] [Google Scholar]
- 17.Diehl V, Re D, Harris NL, P.M. M. In: Cancer: Principles and Practice of Oncology. 8th Ed. DeVita VT, Lawrence TS, Rosenberg SA, editors. Philadelphia: Williams & Wilkins; 2008. pp. 2167–2220. [Google Scholar]
- 18.Canel C, Moraes RM, Dayan FE, Ferreira D. Podophyllotoxin. Phytochemistry. 2000;54:115–120. doi: 10.1016/s0031-9422(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 19.Hartwell JL, Schrecker AW. Components of podophyllin. V. The constitution of podophyllotoxin. J. Am. Chem. Soc. 1951;73:2909–2916. [Google Scholar]
- 20.van Maanen JMS, Retèl J, de Vries J, Pinedo HM. Mechanism of action of the antitumor drug etoposide: a review. J. Nat. Cancer Inst. 1988;80:1526–1533. doi: 10.1093/jnci/80.19.1526. [DOI] [PubMed] [Google Scholar]
- 21.Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee K-H, Xiao Z. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC/Taylor & Francis; 2005. pp. 71–87. [Google Scholar]
- 23.Itokawa H, Wang X, Lee K-H. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC/Taylor & Francis; 2005. pp. 47–70. [Google Scholar]
- 24.Kantarjian HM, Talpaz M, Santini V, et al. Homoharringtonine: history, current research, and future direction. Cancer. 2001;92:1591–1605. doi: 10.1002/1097-0142(20010915)92:6<1591::aid-cncr1485>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Powell RG, Weisleder D, Smith CR., Jr Antitumor alkaloids from Cephalotaxus harringtonia: structure and activity. J Pharm Sci. 1972;61:1227–1230. doi: 10.1002/jps.2600610812. [DOI] [PubMed] [Google Scholar]
- 26.Paudler WW, Kerley GI, McKay J. Alkaloids of Cephalotaxus drupacea and Cephalotaxus fortunei. J. Org. Chem. 1963;28:2194–2197. [Google Scholar]
- 27.Abraham DJ, Rosenstein RD, McGandy EL. Structure of cephalotaxine and related alkaloids. Tetrahedron Lett. 1969:4085–4086. [Google Scholar]
- 28.Baaske DM, Heinstein P. Cytotoxicity and cell cycle specificity of homoharringtonine. Antimicrob Agents Chemother. 1977;12:298–300. doi: 10.1128/aac.12.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fresno M, Jimenez A, Vazquez D. Inhibition of translation in eukaryotic systems by harringtonine. Eur J Biochem. 1977;72:323–330. doi: 10.1111/j.1432-1033.1977.tb11256.x. [DOI] [PubMed] [Google Scholar]
- 30.Visani G, Russo D, Ottaviani E, et al. Effects of homoharringtonine alone and in combination with alpha interferon and cytosine arabinoside on in vitro growth and induction of apoptosis in chronic myeloid leukemia and normal hematopoietic progenitors. Leukemia. 1997;11:624–628. doi: 10.1038/sj.leu.2400608. [DOI] [PubMed] [Google Scholar]
- 31.Takemura Y, Ohnuma T, Chou TC, Okano T, Holland JF. Biologic and pharmacologic effects of harringtonine on human leukemia-lymphoma cells. Cancer Chemother Pharmacol. 1985;14:206–210. doi: 10.1007/BF00258117. [DOI] [PubMed] [Google Scholar]
- 32.Boyd AW, Sullivan JR. Leukemic cell differentiation in vivo and in vitro: arrest of proliferation parallels the differentiation induced by the antileukemic drug harringtonine. Blood. 1984;63:384–392. [PubMed] [Google Scholar]
- 33.Zhou JY, Chen DL, Shen ZS, Koeffler HP. Effect of homoharringtonine on proliferation and differentiation of human leukemic cells in vitro. Cancer Res. 1990;50:2031–2035. [PubMed] [Google Scholar]
- 34.Grem JL, Cheson BD, King SA, Leyland-Jones B, Suffness M. Cephalotaxine esters: antileukemic advance or therapeutic failure? J Natl Cancer Inst. 1988;80:1095–1103. doi: 10.1093/jnci/80.14.1095. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien S, Kantarjian H, Keating M, et al. Homoharringtonine therapy induces responses in patients with chronic myelogenous leukemia in late chronic phase. Blood. 1995;86:3322–3326. [PubMed] [Google Scholar]
- 36.O'Brien S, Kantarjian H, Koller C, et al. Sequential homoharringtonine and interferon-alpha in the treatment of early chronic phase chronic myelogenous leukemia. Blood. 1999;93:4149–4153. [PubMed] [Google Scholar]
- 37.Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J Cancer Res Clin Oncol. 2004;130:627–635. doi: 10.1007/s00432-004-0579-2. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Z, Hao Y, Liu B, Qian L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk Lymphoma. 2002;43:1763–1768. doi: 10.1080/1042819021000006295. [DOI] [PubMed] [Google Scholar]
- 39.Marko D, Schatzle S, Friedel A, et al. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer. 2001;84:283–289. doi: 10.1054/bjoc.2000.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du D, Ceng Q. Effect of indirubicin on the incorporation of isotope related precursors into nucleic acid and protein of tumor tissues. Zhong Cao Yao. 1981;12:406–409. [Google Scholar]
- 41.Gan WJ, Yang T, Wen S, et al. Studies on the mechanism of indirubicin action in treatment of chronic myelocytic leukemia (CML). II. 5'-Nucleotidase in the peripheral white blood cells of CML. Chin. Acad. Med. Sci. Beijing. 1985;6:611–613. [Google Scholar]
- 42.Harmon AD, Weiss U, Silverton JV. The structure of rohitukine, the main alkaloid of Amoora rohituka (syn. Aphanamixus polystachya) (Meliaceae) Tetrahedron Lett. 1979:721–724. [Google Scholar]
- 43.Newman DJ, Cragg GM. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC Taylor & Francis; 2005. pp. 553–571. [Google Scholar]
- 44.de Azevedo WF, Jr, Canduri F, da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293:566–571. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 45.Billard C, Kern C, Tang R, Ajchenbaum-Cymbalista F, Kolb JP. Flavopiridol downregulates the expression of both the inducible NO synthase and p27(kip1) in malignant cells from B-cell chronic lymphocytic leukemia. Leukemia. 2003;17:2435–2443. doi: 10.1038/sj.leu.2403139. [DOI] [PubMed] [Google Scholar]
- 46.Hussain SR, Lucas DM, Johnson AJ, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arguello F, Alexander M, Sterry JA, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity in vivo against human leukemia and lymphoma xenografts. Blood. 1998;91:2482–2490. [PubMed] [Google Scholar]
- 48.Byrd JC, Peterson BL, Gabrilove J, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Clin Cancer Res. 2005;11:4176–4181. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 49.Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29:1253–1257. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karp JE, Passaniti A, Gojo I, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-d-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res. 2005;11:8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 52.Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–4473. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 53.Fathi AT, Karp JE. New agents in acute myeloid leukemia: beyond cytarabine and anthracyclines. Curr Oncol Rep. 2009;11:346–352. doi: 10.1007/s11912-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassady JM, Chan KK, Floss HG, Leistner E. Recent developments in the maytansinoid antitumor agents. Chem. Pharm. Bull. 2004;52:1–26. doi: 10.1248/cpb.52.1. [DOI] [PubMed] [Google Scholar]
- 55.Kupchan SM, Komoda Y, Court WA, et al. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc. 1972;94:1354–1356. doi: 10.1021/ja00759a054. [DOI] [PubMed] [Google Scholar]
- 56.Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189:1002–1005. doi: 10.1126/science.1241159. [DOI] [PubMed] [Google Scholar]
- 57.Wolpert-Defilippes MK, Adamson RH, Cysyk RL, Johns DG. Initial studies on the cytotoxic action of maytansine, a novel ansa macrolide. Biochem Pharmacol. 1975;24:751–754. doi: 10.1016/0006-2952(75)90257-9. [DOI] [PubMed] [Google Scholar]
- 58.Hamel E. Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol Ther. 1992;55:31–51. doi: 10.1016/0163-7258(92)90028-x. [DOI] [PubMed] [Google Scholar]
- 59.Rai SS, Wolff J. Localization of the vinblastine-binding site on beta-tubulin. J Biol Chem. 1996;271:14707–14711. doi: 10.1074/jbc.271.25.14707. [DOI] [PubMed] [Google Scholar]
- 60.Kupchan SM, Komoda Y, Branfman AR, Dailey RG., Jr Tumor inhibitors. 96. Novel maytansinoids. Structural interrelationships and requirements for antitumor activity. J. Am. Chem. Soc. 1974;96:3706–3708. [Google Scholar]
- 61.Kupchan SM, Komoda Y, Thomas GJ, Hintz HPJ. Maytanprine and maytanbutine, new antileukemic ansa macrolides from Maytenus buchananii. J. Chem. Soc. Chem. Commun. 1972:1065. [Google Scholar]
- 62.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 63.Ravry MJ, Omura GA, Birch R. Phase II evaluation of maytansine (NSC 153858) in advanced cancer. A Southeastern Cancer Study Group trial. Am J Clin Oncol. 1985;8:148–150. doi: 10.1097/00000421-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 64. [Accessed October 2009]; www.clinicaltrials.gov.
- 65.Liu Z, Floss HG, Cassady JM, Chan KK. Metabolism studies of the anti-tumor agent maytansine and its analog ansamitocin P-3 using liquid chromatography/tandem mass spectrometry. J Mass Spectrom. 2005;40:389–399. doi: 10.1002/jms.800. [DOI] [PubMed] [Google Scholar]
- 66.Jautelat R, Brumby T, Schafer M, et al. From the insoluble dye indirubin towards highly active, soluble CDK2-inhibitors. Chembiochem. 2005;6:531–540. doi: 10.1002/cbic.200400108. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Zhu X, Xiao Z, et al. Inducing effect of mesindigo on apoptosis of leukemia cell line HL-60 and its mechanisms. Aizheng. 2005;24:1464–1468. [PubMed] [Google Scholar]
- 68.Weng Y, Liu B, Peng Z, Xiao Z. Effects of mesindigo on acute promyelocytic leukemia cell line NB4 cells in vitro Baixuebing Linbaliu. 2005;14:136–139. [Google Scholar]
- 69.Cooperative Study of Phase III Clinical Trial on Meisoindigo, Tianjin, People’s Republic of China. Zhonghua Xueyexue Zazhi. 1997;18:69–72. [PubMed] [Google Scholar]
- 70.Robbers JE, Speedie MK, Tyler VE. Pharmacognosy and Pharmacobiotechnology. Baltimore MD: Williams & Wilkins; 1996. [Google Scholar]
- 71.Ke Y, Ye K, Grossniklaus HE, et al. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother. 2000;49:217–225. doi: 10.1007/s002620000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye K, Ke Y, Keshava N, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landen JW, Lang R, McMahon SJ, et al. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res. 2002;62:4109–4114. [PubMed] [Google Scholar]
- 74.Aneja R, Vangapandu SN, Lopus M, et al. Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol. 2006;69:1801–1809. doi: 10.1124/mol.105.021899. [DOI] [PubMed] [Google Scholar]
- 75.Barken I, Geller J, Rogosnitzky M. Noscapine inhibits human prostate cancer progression and metastasis in a mouse model. Anticancer Res. 2008;28:3701–3704. [PubMed] [Google Scholar]
- 76.Kupchan SM, Britton RW, Lacadie JA, Ziegler MF, Sigel CW. The isolation and structural elucidation of bruceantin and bruceantinol, new potent antileukemic quassinoids from Brucea antidysenterica. J Org Chem. 1975;40:648–654. doi: 10.1021/jo00893a023. [DOI] [PubMed] [Google Scholar]
- 77.Kupchan SM, Britton RW, Ziegler MF, Sigel CW. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J Org Chem. 1973;38:178–179. doi: 10.1021/jo00941a049. [DOI] [PubMed] [Google Scholar]
- 78.Cuendet M, Pezzuto JM. Antitumor activity of bruceantin: an old drug with new promise. J Nat Prod. 2004;67:269–272. doi: 10.1021/np030304+. [DOI] [PubMed] [Google Scholar]
- 79.Mata-Greenwood E, Cuendet M, Sher D, et al. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc. Leukemia. 2002;16:2275–2284. doi: 10.1038/sj.leu.2402696. [DOI] [PubMed] [Google Scholar]
- 80.Cuendet M, Christov K, Lantvit DD, et al. Multiple myeloma regression mediated by bruceantin. Clin Cancer Res. 2004;10:1170–1179. doi: 10.1158/1078-0432.ccr-0362-3. [DOI] [PubMed] [Google Scholar]
- 81.Pettit GR, Cragg GM, Herald DL, Schmidt JM, Lohavanijaya P. Antineoplastic agents. 84. Isolation and structure of combretastatin. Can. J. Chem. 1982;60:1374–1376. [Google Scholar]
- 82.Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 83.Pinney KG, Jelinek C, Edvardsen K, Chaplin DJ, Pettit GR. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton, FL: CRC Taylor & Francis; 2005. pp. 23–46. [Google Scholar]
- 84.Fang L, He Q, Hu Y, Yang B. MZ3 induces apoptosis in human leukemia cells. Cancer Chemother Pharmacol. 2007;59:397–405. doi: 10.1007/s00280-006-0294-6. [DOI] [PubMed] [Google Scholar]
- 85.Petit I, Karajannis MA, Vincent L, et al. The microtubule-targeting agent CA4P regresses leukemic xenografts by disrupting interaction with vascular cells and mitochondrial-dependent cell death. Blood. 2008;111:1951–1961. doi: 10.1182/blood-2007-05-089219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujita M, Itokawa H, Sashida Y. Honokiol, a new phenolic compound isolated from the bark of Magnolia obovata. Chem. Pharm. Bull. 1972;20:212–213. [Google Scholar]
- 87.Bai X, Cerimele F, Ushio-Fukai M, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 88.Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–697. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- 89.Ishitsuka K, Hideshima T, Hamasaki M, et al. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106:1794–1800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo Y, Xu Y, Chen L, et al. Semi-synthesis and anti-proliferative activity evaluation of novel analogues of honokiol. Bioorg Med Chem Lett. 2009;19:4702–4705. doi: 10.1016/j.bmcl.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 91.King ML, Chiang CC, Ling HC, et al. X-ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J. Chem. Soc. Chem. Commun. 1982:1150–1151. [Google Scholar]
- 92.Rose WC, Schurig JE, Meeker JB. Correlation of in vitro cytotoxicity with preclinical in vivo antitumor activity. Anticancer Res. 1988;8:355–367. [PubMed] [Google Scholar]
- 93.Bohnenstengel FI, Steube KG, Meyer C, et al. Structure activity relationships of antiproliferative rocaglamide derivatives from Aglaia species (Meliaceae) Z Naturforsch [C] 1999;54:55–60. [PubMed] [Google Scholar]
- 94.Bohnenstengel FI, Steube KG, Meyer C, et al. 1H-cyclopenta[b]benzofuran lignans from Aglaia species inhibit cell proliferation and alter cell cycle distribution in human monocytic leukemia cell lines. Z Naturforsch [C] 1999;54:1075–1083. doi: 10.1515/znc-1999-1212. [DOI] [PubMed] [Google Scholar]
- 95.Su BN, Chai H, Mi Q, et al. Activity-guided isolation of cytotoxic constituents from the bark of Aglaia crassinervia collected in Indonesia. Bioorg Med Chem. 2006;14:960–972. doi: 10.1016/j.bmc.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Wu TS, Liou MJ, Kuoh CS, et al. Cytotoxic and antiplatelet aggregation principles from Aglaia elliptifolia. J Nat Prod. 1997;60:606–608. doi: 10.1021/np970163+. [DOI] [PubMed] [Google Scholar]
- 97.Kim S, Salim AA, Swanson SM, Kinghorn AD. Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anticancer Agents Med Chem. 2006;6:319–345. doi: 10.2174/187152006777698123. [DOI] [PubMed] [Google Scholar]
- 98.Zhu JY, Lavrik IN, Mahlknecht U, et al. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int J Cancer. 2007;121:1839–1846. doi: 10.1002/ijc.22883. [DOI] [PubMed] [Google Scholar]
- 99.Hwang BY, Su BN, Chai H, et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. ibid. 6156. [DOI] [PubMed] [Google Scholar]
- 100.El Sous M, Khoo M, Holloway G, et al. Total synthesis of (−)-episilvestrol and (−)-silvestrol. Angew. Chemie Int. Ed. 2007;46:7835–7838. doi: 10.1002/anie.200702700. [DOI] [PubMed] [Google Scholar]
- 101.Gerard B, Cencic R, Pelletier J, Porco J. Enantioselective synthesis of the complex rocaglate (−)-silvestrol. Angew. Chemie Int. Ed. 2007;46:7831–7834. doi: 10.1002/anie.200702707. [DOI] [PubMed] [Google Scholar]
- 102.Adams TE, El Sous M, Hawkins BC, et al. Total synthesis of the potent anticancer Aglaia metabolites (−)-silvestrol and (−)-episilvestrol and the active analogue (−)-4'-desmethoxyepisilvestrol. J Am Chem Soc. 2009;131:1607–1616. doi: 10.1021/ja808402e. [DOI] [PubMed] [Google Scholar]
- 103.Cencic R, Carrier M, Galicia-Vazquez G, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS One. 2009;4:e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salim AA, Chai HB, Rachman I, et al. Constituents of the leaves and stem bark of Aglaia foveolata. Tetrahedron. 2007;63:7926–7934. doi: 10.1016/j.tet.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baumann B, Bohnenstengel F, Siegmund D, et al. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J Biol Chem. 2002;277:44791–44800. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- 106.Proksch P, Giaisi M, Treiber MK, et al. Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J Immunol. 2005;174:7075–7084. doi: 10.4049/jimmunol.174.11.7075. [DOI] [PubMed] [Google Scholar]
- 107.Mi Q, Kim S, Hwang BY, et al. Silvestrol regulates G2/M checkpoint genes independent of p53 activity. Anticancer Res. 2006;26:3349–3356. [PubMed] [Google Scholar]
- 108.Kim S, Hwang BY, Su BN, et al. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer Res. 2007;27:2175–2183. [PMC free article] [PubMed] [Google Scholar]
- 109.Lucas DM, Edwards RB, Lozanski G, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bordeleau ME, Robert F, Gerard B, et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J Clin Invest. 2008;118:1–11. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bichi R, Shinton SA, Martin ES, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hussain SR, Cheney CM, Johnson AJ, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13:2144–2150. doi: 10.1158/1078-0432.CCR-06-2294. [DOI] [PubMed] [Google Scholar]
- 113.Meurer-Grimes BM, Yu J, Vario GL. Therapeutic compounds and methods. US6710075 B2. U.S. Patent. 2004
- 114.Hollingshead M, Roberson J, Decker W, et al. In vivo drug screening applications of HIV-infected cells cultivated within hollow fibers in two physiologic compartments of mice. Antiviral Res. 1995;28:265–279. doi: 10.1016/0166-3542(95)00055-q. [DOI] [PubMed] [Google Scholar]
- 115.Baker DD, Chu M, Oza U, Rajgarhia V. The value of natural products to future pharmaceutical discovery. Nat Prod Rep. 2007;24:1225–1244. doi: 10.1039/b602241n. [DOI] [PubMed] [Google Scholar]
- 116.Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 118.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 119.Miller SJ, Clardy J. Natural products: Beyond grind and find. Nature Chem. 2009;1:261–263. doi: 10.1038/nchem.269. [DOI] [PubMed] [Google Scholar]
- 120.Cragg GM, Kingston DGI, Newman DJ. Anticancer Agents from Natural Products. Boca Raton, FL: CRC Taylor & Francis; 2005. [Google Scholar]
- 121.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 122.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 123.Cragg GM, Newman DJ, Yang SS. Natural product extracts of plant and marine origin having antileukemia potential. The NCI experience. J Nat Prod. 2006;69:488–498. doi: 10.1021/np0581216. [DOI] [PubMed] [Google Scholar]