Abstract
Introduction
Most trials evaluating the efficacy of atrial fibrillation ablation report follow-up periods of 1–2 years. Long term results (> 5 years) following segmental pulmonary vein isolation (PVI) for paroxysmal atrial fibrillation (PAF) are largely unreported.
Objective
To evaluate the long-term efficacy of segmental PVI for the treatment of symptomatic, medically refractory, PAF.
Methods
Patients with paroxysmal atrial fibrillation who underwent pulmonary vein isolation at the University of California, San Diego Medical Center were evaluated retrospectively to determine the outcome of the index procedure. Of one hundred and eighteen segmental pulmonary vein isolation procedures preformed at UCSD Medical Center between January 1, 2002 and August 31, 2003, seventy-one patients who had long-term follow-up data were included. The five year outcomes were determined by last clinic encounter and telephone encounter at or after five years from the index procedure. Patients had routine clinic visits with EKGs and underwent cardiac monitoring for any complaints of symptoms suggestive of recurrent arrhythmia.
Results
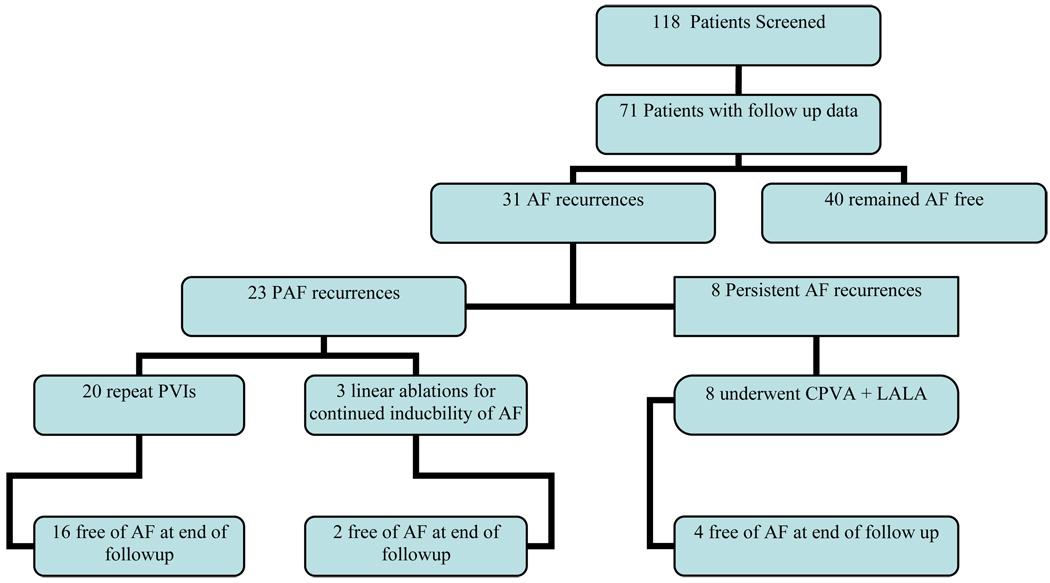
Seventy-one patients (60±10 years, 56 male) were followed for > 5 years. After one procedure, with 12 months of follow up, sixty-one (86%) of patients were free from symptomatic atrial fibrillation (AF). After 24 months, fifty-six (79%) of patients remained free of AF. At the end of a follow up period of 63±5 months, only forty patients (56%) remained free from symptomatic atrial fibrillation after one procedure. In sixteen patients (22.5%), atrial fibrillation recurred after the second year post-ablation, with four patients recurring during the third year, four patients during the fourth year, and eight patients having their first recurrence of atrial fibrillation greater than 48 months after their index ablation procedure.
Thirty-one patients underwent more than one ablation procedure (average 1.9±0.9 procedures per patient). After multiple procedures, sixty patients (84%) were arrhythmia free off medication at 63±5 months after their initial procedure. In patients who underwent more than one ablation procedure, the mean duration of follow up after the last ablation procedure was 7.5±2.1 months.
Conclusions
Overall five-year outcomes after segmental PVI for PAF are consistent with previously reported shorter term follow-up (≤ 2 years); however, late recurrences (>2 years) after a successful initial ablation procedure are not infrequent, and repeat ablation procedures are often required to maintain freedom from symptomatic arrhythmias. Continued long-term follow-up of patients with PAF after initially successful ablation may be warranted, especially in those patients in whom anticoagulation is discontinued.
Introduction
Segmental ablation to achieve pulmonary vein isolation (PVI) is an established therapeutic option for patients with symptomatic paroxysmal atrial fibrillation (PAF) refractory to medical therapy. The procedure results in a 65–85% freedom from recurrent AF over the next 12 months with 30–40% of patients having more than one procedure.(1,2)
The original studies reporting outcomes from AF ablation reported follow up durations of 6–12 months (1,3,4), and even more recent “long term” follow up studies report follow up durations of < 26 months (5–7). Few studies have provided follow up data beyond 2 years, and some concerns have been raised with regard to true long-term success rates, and observations of recurrences more than two years after an initially successful ablation (8,9). The objective of this study is to evaluate the five-year durability of segmental PVI for the treatment of symptomatic, medically refractory paroxysmal atrial fibrillation.
Methods
Patients
Patients with paroxysmal atrial fibrillation who underwent pulmonary vein isolation at the University of California, San Diego Medical Center who had at least five years of follow up data were evaluated retrospectively to determine the outcome from their index ablation procedure. Of one hundred and eighteen PVI procedures preformed at UCSD Medical Center between January 1, 2002 and August 31, 2003, seventy-one patients met criteria and were included in this analysis. The five year outcomes were determined by last clinic encounter and telephone encounter at or after five years from the index procedure. Patients had routine clinic visits with EKGs and underwent cardiac monitoring for any complaints of symptoms suggestive of recurrent arrhythmia.
Electrophysiological Study
Coumadin was held for five days before the ablation procedure, and patients were bridged with Lovenox at 1mg/kg SQ BID until the morning of the ablation procedure. All antiarrhythmics were held for five half lives before the procedure. All catheters were introduced through a femoral or internal jugular vein. A steerible decapolar catheter (Polaris, Boston Scientific or dynamic deca, ) was positioned in the coronary sinus. After introduction of the sheaths and placement of the coronary sinus catheter a transeptal catheterization was preformed and a wire was placed into the left upper pulmonary vein. An 8 mm tip ablation catheter (Blazer) was then tracked along the wire and thus two sheaths were placed into the left atrium (one over the wire, and one over the ablation catheter). The wire was removed and a circular decapolar mapping catheter (Lasso, Biosense Webster, Inc) was placed in the left atrium for all cases. After transeptal access was obtained, systemic anticoagulation was achieved with IV heparin (10000 Unit bolus followed by a continuous infusion) to maintain an ACT between 350 – 400 seconds.
Segmental Pulmonary Vein Isolation
Each of the pulmonary veins was entered using a circular decapolar mapping catheter (Lasso, Biosense Webster, Inc). The ostium of each pulmonary vein was identified by pulling the catheter back under fluoroscopic guidance until the tip entered the cardiac silhouette associated with simultaneous appearance of atrial potentials. With the lasso catheter placed at the PV ostia, each vein was mapped circumferentially to document typical sharp local pulmonary vein potentials during steady state coronary sinus pacing (left pulmonary veins) or sinus rhythm (right pulmonary veins). PV isolation was performed by applying radiofrequency energy at ostial sites at which the earliest bipolar PV potentials were seen.
Radiofrequency energy was delivered with a temperature controlled 8 mm tip ablation catheter (Blazer, …) at an average power of 25–40 Watts, with a target temperature of 48 degrees for up to 30 seconds at each location during the initial ablation in 2002. With repeat ablations preformed after 2004, an average power of 50 Watts, with a target temperature of 52 degrees for up to 30 seconds was used at each location. The end points were the elimination of all ostial PV potentials and complete entrance and exit block into or out of the pulmonary vein. The right lower pulmonary vein was not routinely isolated during PVI procedures performed before 2003. A cavo-tricuspid isthmus line was preformed in all patients with documentation of bidirectional isthmus block.
Circumferential Pulmonary Vein Ablation plus Left Atrial Linear Ablation
In patients who presented for repeat ablation procedures, if they presented with persistent AF, or had inducible AF after repeat PVI, they underwent a circumferential pulmonary vein isolation and/or linear ablation. For circumferential pulmonary vein ablation (CPVA), a 3-dimentional geometry of the left atrium was reconstructed by the use of an electroanatomical mapping system (CARTO, Biosense Webster Inc., or Nav X, St. Jude Medical). Radiofrequency current was applied with an 8 mm tip catheter (Navistar, Biosense-Webster, or Blazer, ) with a maximum temperature of 55 degrees and maximum power of 50 Watts (Stockert 70 RF, Biosense-Webster, or ) to encircle the left and right pulmonary veins. The ablation lines consisted of focal lesions deployed at 10 mm distance from the pulmonary vein ostia. Radiofrequency energy was applied for 20–30 seconds, and until the maximum local electrogram amplitude was < 0.1 mV. A linear line (LALA) was created at the roof of the left atrium connecting the two circles, and a line was drawn from the inferior portion of the left encircling lesion to the lateral mitral valve annulus. Pacing from the left atrial appendage and also from the coronary sinus catheter was preformed to document bidirectional mitral isthmus block in cases preformed after 2004. If block was not achieved, ablation within the coronary sinus was preformed after order to achieve bidirectional mitral isthmus block. Finally, the lasso catheter was placed in the pulmonary vein ostial to confirm pulmonary vein isolation. If the pulmonary veins were not isolated, ablation was continued until there was elimination of all pulmonary vein potentials. At the end of the left atrial procedure, the cavo-tricuspid isthmus was evaluated for persistence of CTI block. If bidirectional block was not present, a repeat CTI ablation was preformed.
Post ablation Care
After the ablation procedure, patients were hospitalized overnight and given SQ Lovenox 1 mg/kg starting six hours after all sheaths were pulled and homeostasis achieved. Patients were discharged on the antiarrhythmic medication that they had been on prior to the ablation procedure (most commonly a class IC antiarrhythmic) and were maintained on this medication for 4 weeks, after which it was discontinued.
Repeat Procedures
A repeat ablation procedure was offered if the patient had symptomatic atrial fibrillation or atrial flutter off of antiarrhythmic medications at a time more than three months after the initial ablation procedure. In addition to repeating PV isolation at the second procedure, if isoproterenol infusion at 20mcg/minute infusion demonstrated ectopic atrial beats, these non-pulmonary vein foci were targeted. Subsequently, if burst pacing from the coronary sinus at a cycle length down to 180 msec induced AF, additional linear lesions usually along the roof between the two upper pulmonary veins and at the mitral isthmus were created. Also if patients presented with recurrent persistent AF, the repeat procedure included circumferential ablation with documentation of PVI and left atrial linear ablation as described above with both a roof and mitral isthmus line.
Follow-Up
After discharge, patients were seen in the outpatient arrhythmia clinic at 1, 3, 6, and 12 months after the ablation procedure and at six month intervals thereafter. Patients were encouraged to maintain personal records with descriptions of any antiarrhythmic symptoms. At clinic visits, patients underwent routine EKGs and were questioned extensively about any symptoms suggestive of recurrent arrhythmia. Mobile outpatient telemetry monitors were preformed for any reported symptoms of recurrent arrhythmia. Telephone visits were also preformed in all patients at the end of a five year follow up period.
Study End Points
The primary end point of the study was freedom from symptomatic atria arrhythmias after a single ablation procedure at 12, 24 and > 60 months. Secondary end points included freedom from symptomatic atrial arrythmias after multiple ablation procedures. All patients who had a repeat ablation procedure were followed for a minimum of 6 months after the last ablation procedure (7.5±2.1 months). Because atrial arrhythmias that occur early after an ablation procedure may be transient in nature, atrial arrhythmias that occurred within the first three months were excluded from this analysis as recommended in the HRS consensus guidelines. (10)
Statistical Analysis
All continuous variables are reported as mean ± standard deviation (SD) and were compared by student’s t-test. Categorical variables were compared by chi-square or Fisher’s exact method, as appropriate. Survival curves were generated by Kaplan-Meier method and outcomes were assessed by Cox regression model to calculate hazard-ratio with 95% confidence intervals. The variables in the multivariant analysis included: age, gender, presence of hypertension, hyperlipidemia, type of procedure: complete (defined as isolation of all pulmonary veins) or incomplete (defined as isolation of all pulmonary veins except right lower pulmonary vein), structural heart disease (defined by prior myocardial infarction or revascularization, moderate left ventricular hypertrophy, advanced left atrial dilation and cardiomyopathies). A two tailed P value < 0.05 was considered to be statistically significant. Statistical analysis was performed using SAS software version 9.1 ( SAS Inc. USA).
Results
Clinical Characteristics of the Patients Enrolled
Of one hundred and eighteen PVI procedures preformed at UCSD Medical Center between January 1, 2002 and August 31, 2003, seventy-one patients who had five year follow up data were included in this evaluation. Fifty-six males (79%) with mean age of 60±10 years and fifteen females (21%) with mean age 61±9 years underwent pulmonary vein isolation for paroxysmal atrial fibrillation with the mean duration of AF of 4.5±1.2 years. Associated medical conditions included hypertension (26 patients, 37%), hyperlipidemia (21 patients, 29%), and hypothyroidism treated with thyroid replacement (4 patients, 6%). Five patients (7%) had structural heart disease. The mean LA diameter was 3.9±1.1 and mean LVEF was 56±14%. Thirty-eight patients (53%) had isolation of all pulmonary veins while in thirty-three (47%) right lower pulmonary vein was not isolated in index procedure. Table-1 summarizes the clinical characteristics of the patients included in this evaluation.
Table-1.
Clinical characteristics of the patients enrolled (N=71)
| Age(yr) | 60±9 |
| Gender (Male/Female) | 55/16 |
| Duration of Atrial Fibrillation (yr) | 4.5±1.2 |
| LA diameter (cm) | 3.9±1.1 |
| LVEF(%) | 56±14% |
| Structural heart disease* | 7 |
| PVI:Complete/Incomplete | 38/33 |
| Diagnosis of Hypertension | 26 |
| Diagnosis of Hyperlipidemia | 21 |
| Thyroid disease | 4 |
structural heart disease (defined by prior myocardial infarction or revascularization, moderate/ advanced left ventricular ypertrophy, advanced left atrial dilation and cadiomyopathies)
Clinical Outcome of the Index Procedure
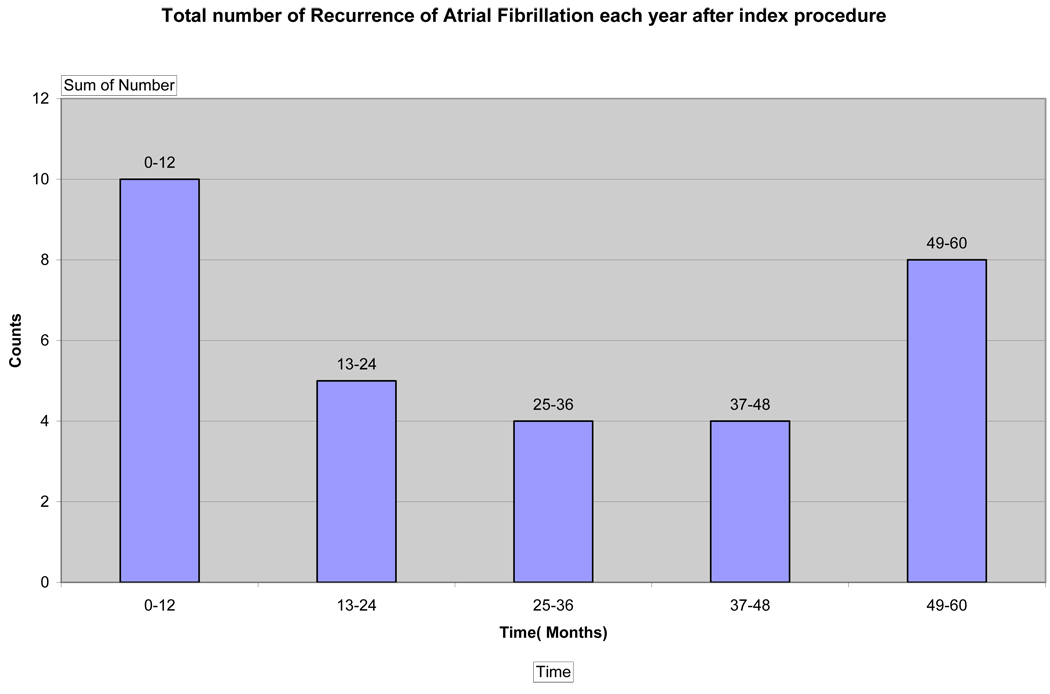
After one procedure, with 12 months of follow up, sixty-one (86%) of patients were free from symptomatic atrial fibrillation. After 24 months, fifty-six (79%) of patients remained free of symptomatic PAF. At the end of a follow up period of 63±5 months, only forty patients (56%) remained free from symptomatic atrial fibrillation after one procedure (Figure 1). In sixteen patients (22.5%), atrial fibrillation recurred after the second year post-ablation, with four patients recurring during the third year, four patients during the fourth year, and eight patients having recurrence of atrial fibrillation greater than 48 months after their index ablation procedure. Between 24 to 48 months, there was a 7.6%/year recurrence rate of AF. After 48 months, there was a 17%/year recurrence rate of AF. Figure-2 demonstrates number of recurrences at each year following initial ablation procedure.
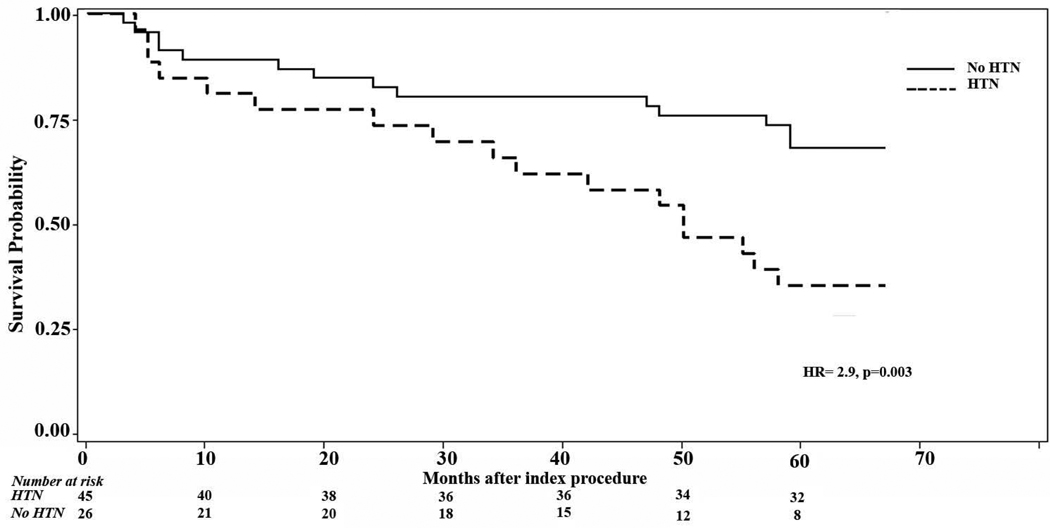
Figure 1.
Kaplan Meyer Showing AF recurrences over 5 years after one procedure
Figure 2.
Outcome After Multiple Procedures
Patients were offered a repeat ablation for recurrent symptomatic AF after a trial of anti-arrhythmic medication for 3 months. Thirty-one patients underwent more than one ablation procedure. Patients who presented with recurrent paroxysmal atrial fibrillation received repeat segmental pulmonary vein isolation (N=20). If after PVI, AF was still inducible with atrial burst pacing at cycle lengths down to 180 msec, additional linear ablation was preformed (N=3). If patients subsequently presented with persistent AF, their repeat ablation procedure consisted of circumferential ablation with additional left atrial linear ablation (N=8). (Figure 3)
Figure 3.
After multiple procedures, sixty patients (84%) were arrhythmia free off of antiarrhythmic medications at 63±5 months after their initial procedure. (Figure 4, not done yet). In patients who underwent more than one ablation procedure, the mean duration of follow up after the last ablation procedure was 7.5±2.1 months.
FIGURE 4.
Type of Recurrent Atrial Fibrillation
In thirty-one patients who developed recurrent AF during the follow up period, twenty three (74%) presented with recurrent PAF. All patients who underwent repeat ablation, including those with late (>24 months from index ablation) recurrences, demonstrated recurrent PV connections in greater than one pulmonary vein. Three of the twenty three patients who underwent repeat ablation for PAF demonstrated inducibility of AF after repeat PVI, and subsequently underwent additional linear ablation with a roof and mitral isthmus line (inducibility was not checked during the index ablation procedure). Eight patients presented with persistent AF (>7 days duration of sustained AF) (10) after initially having an ablation for paroxysmal atrial fibrillation. (Figure 3).
Risk Factors for Developing Recurrent Atrial Fibrillation
Age, gender, type of procedure: complete (defined as isolation of all pulmonary veins) or incomplete (defined as isolation of all pulmonary veins except right lower pulmonary vein), structural heart disease (defined by prior myocardial infarction or revascularization, moderate left ventricular hypertrophy, left atrial dilation > 5.0 cm, and cardiomyopathy) and presence of hypertension and/or hyperlipidemia at the time of the index ablation procedure were included in the analysis (Table 2, not done yet).
Table 2.
Risk Factors for Developing Recurrent Atrial Fibrillation
| Risk Factor | Variable | Adjusted Hazard Ratio (95% CI) |
p-value |
|---|---|---|---|
| Age | For every 10 years | 1.05 (0.64,1.45) | 0.20 |
| Gender | Male vs. Female | 1.13 (0.23,2.23) | 0.87 |
| Pulmonary vein isolation* |
Incomplete vs. Complete |
1.4 (0.89, 1.96) | 0.14 |
| Hypertension† | Hypertensive vs. Normotensive |
2.9 (2.6,3.1) | 0.003 |
| Hyperlipidemia‡ | 0.95 (0.34,1.83) | 0.80 |
Pulmonary vein isolation: Complete = isolation of all 4 pulmonary veins, Incomplete = isolation of all but the right lower pulmonary vein;
BP>140/90 on more than 2 clinic visit or taking antihypertensive medications;
treatment with statins or fibrates
Patients with a diagnosis of hypertension at the time of their index ablation procedure had a significantly higher risk of developing recurrence of atrial fibrillation compared with normotensive patients (HR 2.9, p=0.003). There was no statistically significant difference in risk of recurrence of AF after index procedure within five years post-ablation among patients who had complete PVI comparing those with incomplete PVI ( HR=1.4, p=0.15). Likewise, age, gender, and hyperlipidemia did not predict recurrence of atrial fibrillation, nor did the presence of structural heart disease; however only five patients had structural heart disease in this series.
Complications
There were three complications out of one hundred and two ablation procedures (including repeat procedures) which consisted of two femoral hematomas, and one femoral pseudoaneurysm. All resolved with conservative management. There were no cases of stroke or pericardial tamponade. There were no cases of symptomatic pulmonary vein stenosis, however no attempt was made to evaluate for asymptomatic pulmonary vein stenosis.
Discussion
Radiofrequency catheter ablation for the treatment of supraventricular tachycardias, such as AV node reentry tachycardia and AV reentry tachycardia, has demonstrated durable long term success rates. After a successful ablation procedure for these arrhythmias, patients are felt to be cured of their arrhythmia with an extremely low likelihood of recurrence. Atrial fibrillation ablation is relatively newer, the ablation procedure is more technically complicated, and the mechanisms are less well understood. It is possible that the natural history after catheter based ablation for atrial fibrillation is different from other atrial arrhythmias. Despite a large number of publications on the ablation of atrial fibrillation, there is a paucity of data on the long term efficacy of the procedure. Most studies of “long-term outcome” report an average of 20–26 months of follow up (5–7,11–13). Recently, concerns have been raised about the potential for late recurrences of AF after successful ablation procedure, (6,8,9). And there can be severe clinical consequences if AF recurs, especially if anticoagulation and other medications have been discontinued after an initially successful ablation procedure. Although there is a lack of consensus and guidelines regarding the use of chronic anticoagulation after successful ablation of AF, it is common practice by many electrophysiologist to discontinue coumadin after a successful AF ablation procedure (14).
Our study demonstrates that the five year outcomes after segmental pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation are consistent with the outcomes demonstrated from studies with shorter follow up periods, with 84% of patients being free of symptomatic arrhythmias off of antiarrhythmic medications at 63±5 months of follow-up. However, recurrences are not uncommon, and repeat ablation is often necessary to maintain continued freedom from arrhythmia. Importantly, this study also demonstrates that many patients will develop AF years after an initially successful ablation procedure. In sixteen patients (22.5%), atrial fibrillation recurred after the second year post-ablation, with four patients recurring during the third year, four patients during the fourth year, and eight patients developing their first symptomatic recurrence of atrial fibrillation greater than 48 months after their index ablation procedure. These patients can often be treated successfully with a repeat PVI procedure, but occasionally need more substrate modification. Of patients who recurred with PAF, three patients demonstrated inducibility of atrial fibrillation after repeat pulmonary vein isolation necessitating further substrate modification with linear ablation. Also, eight patients who initially underwent ablation for paroxysmal atrial fibrillation later presented with persistent AF, also indicating more substrate modification would be necessary to achieve durable long-term results (2).
Our study has several unique features. First, it has the longest duration of follow up of any published series of catheter ablation for atrial fibrillation. Second, all patients with recurrent AF underwent repeat electrophysiology study and ablation. Also, it is purely a series of patients with paroxysmal atrial fibrillation, who for the most part did not have structural heart disease. No patients in this evaluation initially had persistent AF, and no patients were maintained on antiarrhythmic drug in order to maintain sinus rhythm. The incidence of late recurrence in this report is a minimal estimate since we identified only symptomatic recurrence of AF, and routine monitoring for asymptomatic arrhythmia recurrence was not preformed outside of routine EKGs at scheduled clinic visits.
Prior relevant studies
To date, there are very few studies that address the issue of durability after initially successful AF ablation; however, our results are similar to those seen in recently published studies of longer duration AF follow up.
Cheema et al. initially reported that 4% of patients developed their first recurrence of AF > 12 months after there initial ablation procedure. (6) More recently, in a series of thirty-nine patients, Katritsis et al. found continued recurrences of AF the longer patients were followed (8). They also demonstrated improved long-term outcomes after multiple procedures. In that series, long term success rates of 66% were achieved after multiple procedures after a follow up period of 42.2±6.0 months, and 56% of recurrences occurred more than 12 months after the initial ablation. The overall lower success rates in that study as compared to ours are likely due to differences in patient populations, differences in ablation techniques, and their more extensive use of transtelephonic monitoring to detect asymptomatic episodes of AF recurrence.
Shah et. al also found a significant late recurrence rate of 8.7% at 34±16 months of follow-up after an initially successful AF ablation procedure. In the patients who were followed to five years, they noted a 25.5% recurrence rate. This is consistent with our study which showed a 17% recurrence of AF between 48 and 60 months after successful ablation. All three of these studies demonstrate that AF ablation may not be a long-term curative procedure, but more of a palliative procedure ameliorating symptoms thereby improving quality of life, and repeat ablations may be necessary to maintain continued freedom from arrhythmia.
Mechanisms for Late Recurrences
The mechanisms of AF are complicated and variable. Late recurrence may due to a variety of factors including resumption of conduction at previously ablated sights, the emergence of non pulmonary vein (PV) triggers, and progression and modification of atrial substrate that promotes AF. Several studies have related AF recurrence to PV conduction (15–18); however, some investigators have suggested that permanent isolation of the pulmonary veins is not always necessary for a successful outcome (19–21). In our study, recurrence of PV conduction in more than one PV was observed in all patients who underwent repeat ablation, including those who underwent repeat ablation > 24 months after their initial procedure. This observation has also been seen in other studies (8,9,12); however when PV conduction recurred in relation to development of recurrent AF is not known.
The importance of PV triggers is evident by the success of repeated PVI in the majority of patients who underwent repeat PVI ablation (16/20, 80%). However, like the study by Shah el al, we also found that some patients with paroxysmal atrial fibrillation needed additional linear ablation due to continued inducibiliy of AF after repeat pulmonary vein isolation. Furthermore, eight patients who initially underwent treatment for PAF, subsequently developed persistent AF, demonstrating that ongoing substrate modification can also lead to recurrences of AF. We found the presence of hypertension to be a risk factor for recurrence of atrial fibrillation; this observation has also been reported by others. (9)
Ectopic activity from the pulmonary veins is likely responsible for the majority of recurrences of AF in patients with PAF and structurally normal hearts; however, the etiology of AF recurrence can be multifactorial with clinical factors such as hypertension contributing to continued modification of the substrate of the left atrium. Further research will have to focus on the mechanisms of late recurrences after an initially successful procedure.
Conclusions
Overall five-year outcomes after PVI for PAF are consistent with previously reported shorter term follow-up (≤2 years); however, late recurrences (>2 years) after an apparently successful initial ablation procedure are not infrequent, and repeat ablations are necessary to maintain continued freedom from arrhythmia. These late recurrences of AF are an important phenomenon that has clinical implications with regard to the management of patients after initially successful radiofrequency catheter ablation. Continued long-term follow-up with periodic surveillance with telemetry monitoring may be warranted, especially if anticoagulation is discontinued after a successful ablation procedure. It remains to be seen if newer ablation strategies, with higher power delivery and the use of irrigated tip catheters, will result in more durable long term result.
Limitations
The technique and experience level with AF ablation has changed significantly since 2002 when these patients first underwent ablation. Although many groups have moved away from segmental PVI to a more circumferential approach to PVI, we have found it to be equally efficacious and with a lower incidence of the subsequent development of left atrial macro-reentrant tachycardia in selected patients with paroxysmal atrial fibrillation. (22) We did not seek to identify non PV triggers or evaluate for continued AF inducibility after pulmonary vein isolation during the initial AF ablation procedure; however, this was done at the time of repeat ablation procedures.
This was a non-randomized population of patients who underwent ablation and long-term follow-up at UCSD Medical Center. Patients did not undergo routine cardiac monitoring to evaluate for the occurrence of asymptomatic atrial arrhythmias, other than routine EKGs at clinic visits, so the recurrence rates of atrial fibrillation are likely underreported. Our study is also limited by its relatively small sample size. Still, this is the only series with an average follow up period exceeding five years after the initial atrial fibrillation ablation procedure, and it demonstrates that patients initially felt to be “cured” of atrial fibrillation can present with very late recurrences, and warrant continued long term follow-up and periodic telemetry monitoring.
Acknowledgments
Funding support: None
Abbreviations
- AF
atrial fibrillation
- CPVA
circumferential pulmonary vein ablation
- LALA
left atrial linear ablation
- PAF
paroxysmal atrial fibrillation
- PV
pulmonary vein
- PVI
pulmonary vein isolation
Footnotes
Conflicts of Interest:
N. Sawhney: None relevant to this manuscript
References
- 1.Haissaguerre M, Shah DC, Jais P, et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;102:2463–2465. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 5.Bertaglia E, Stabile G, Senatore G, et al. Long-term outcome of right and left atrial radiofrequency ablation in patients with persistent atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:153–158. doi: 10.1111/j.1540-8159.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheema A, Vasamreddy CR, Dalal D, et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–155. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 7.Solheim E, Hoff PI, Off MK, Ohm OJ, Chen J. Significance of late recurrence of atrial fibrillation during long-term follow-up after pulmonary vein isolation. Pacing Clin Electrophysiol. 2007;30 Suppl 1:S108–S111. doi: 10.1111/j.1540-8159.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 8.Katritsis D, Wood MA, Giazitzoglou E, Shepard RK, Kourlaba G, Ellenbogen KA. Long-term follow-up after radiofrequency catheter ablation for atrial fibrillation. Europace. 2008;10:419–424. doi: 10.1093/europace/eun018. [DOI] [PubMed] [Google Scholar]
- 9.Shah AN, Mittal S, Sichrovsky TC, et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol. 2008;19:661–667. doi: 10.1111/j.1540-8167.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Brugada J, Packer DL, et al. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh MH, Tai CT, Lee SH, et al. The different mechanisms between late and very late recurrences of atrial fibrillation in patients undergoing a repeated catheter ablation. J Cardiovasc Electrophysiol. 2006;17:231–235. doi: 10.1111/j.1540-8167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 12.Mainigi SK, Sauer WH, Cooper JM, et al. Incidence and predictors of very late recurrence of atrial fibrillation after ablation. J Cardiovasc Electrophysiol. 2007;18:69–74. doi: 10.1111/j.1540-8167.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanders P, Hocini M, Jais P, et al. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation. Long-term clinical outcome. Eur Heart J. 2007;28:1862–1871. doi: 10.1093/eurheartj/ehl548. [DOI] [PubMed] [Google Scholar]
- 14.Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114:759–765. doi: 10.1161/CIRCULATIONAHA.106.641225. [DOI] [PubMed] [Google Scholar]
- 15.Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–1604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 16.Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226–1229. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 18.Stabile G, Turco P, La Rocca V, Nocerino P, Stabile E, De Simone A. Is pulmonary vein isolation necessary for curing atrial fibrillation? Circulation. 2003;108:657–660. doi: 10.1161/01.CIR.0000086980.42626.34. [DOI] [PubMed] [Google Scholar]
- 19.Katritsis D, Ellenbogen KA, Camm AJ. Recurrence of left atrium-pulmonary vein conduction following successful disconnection in asymptomatic patients. Europace. 2004;6:425–432. doi: 10.1016/j.eupc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Lemola K, Hall B, Cheung P, et al. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004;1:197–202. doi: 10.1016/j.hrthm.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 21.Lemola K, Oral H, Chugh A, et al. Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. J Am Coll Cardiol. 2005;46:1060–1066. doi: 10.1016/j.jacc.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 22.Sawhney NAR, Jones L, Tate C, Panutich M, Feld GK. Segmental Pulmonary Vein Isolation is an Effective Initial Strategy in Patients with Symptomatic Paroxysmal Atrial Fibrillation. Heart Rhythm. 2008;5:S269. [Google Scholar]