Abstract
Objectives
To assess whether additional ablation in the right atrium(RA) improves termination rate in long-lasting persistent atrial fibrillation(PsAF).
Background
Prolongation of atrial fibrillation(AF) cycle length(CL) measured from the left atrial appendage predicts favorable outcome during catheter ablation of PsAF. However, in some patients despite prolongation of AFCL in the left atrium(LA) with ablation, AF persists. We hypothesized that this is due to RA drivers and these patients may benefit from RA ablation.
Methods
148 consecutive patients undergoing catheter ablation of PsAF(duration 25±32 months) were studied. AFCL was monitored in both atria during stepwise ablation commencing in the LA. Ablation was performed in the RA when all LA sources in AF had been ablated and a RA-LA gradient existed. The procedural endpoint was AF termination.
Results
Two distinct patterns of AFCL change emerged during LA ablation. In 104patients(70%), there was parallel increase of AFCL in LA and RA culminating in AF termination (baseline: LA 153ms[140,170], RA 155ms[143,171]; after ablation: LA 181ms[170,200], RA 186ms[175,202]). In 24 patients(19%), RA AFCL did not prolong, creating a right-to-left frequency gradient, (baseline: LA 142ms[143,153], RA 145 ms[139,162]; after ablation: LA 177 ms[165–185], RA 152ms[147,175]). These patients had a longer AF history (23versus12 months, p=0.001), and larger RA diameter (42versus39mm, p=0.005) and RA ablation terminated AF in 55%. In the remaining 20 patients, biatrial ablation failed to terminate AF.
Conclusions
A divergent pattern of AFCL prolongation after LA ablation resulting in a right-to-left gradient demonstrating that the right atrium is driving AF in about 20 % of PsAF.
Keywords: arrhythmias, atrium, fibrillation, ablation
INTRODUCTION
Left atrial (LA) tissue is often necessary to ablate in addition to pulmonary vein isolation (PVI) to achieve optimal results in patients with long-lasting persistent atrial fibrillation (PsAF) (1–12). During ablation, prolongation of AF cycle length (AFCL) occurs in the left atrium with its magnitude predicting procedural termination of AF. However, the need for right atrial (RA) ablation has not yet been clearly established in studies using surgery or catheter ablation (13) with some studies showing no utility of RA ablation while other studies have shown benefit. (14) As a result, it is unclear whether it is possible to identify a subset of patients who may benefit from additional RA ablation for long-lasting PsAF.
We hypothesized that failure to terminate AF after LA ablation is due to RA drivers and these patients may benefit from additional RA ablation. Furthermore, we evaluated the clinical and procedural factors predictive of the need for RA ablation, the extent of ablation required, its impact on AF termination and long term clinical outcome.
METHODS
Study Population
This study population consisted of 148 consecutive patients (119 males, age 58±10 years) with symptomatic long-lasting PsAF referred for catheter ablation. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics
| LA Termination (n=104) |
RA Termination (n=24) |
Non Termination (n=20) |
P | |
|---|---|---|---|---|
| Age | 58±10 | 60±11 | 58±11 | 0.72 |
| Males | 79% | 83% | 80% | 0.89 |
| AF duration | 12 [6,20]*† | 23[12,48]* | 35[12,72]† | *†<0.001 |
| HTN | 27% | 23% | 28% | 0.92 |
| SHD | 41% | 46% | 50% | 0.74 |
| Emboli | 5% | 5% | 12% | 0.56 |
| HF | 19% | 8% | 26% | 0.76 |
| No AAD | 2.5 [2,3] | 3[1,3] | 3[2,3] | 0.75 |
| LVEDD | 53[48,57] | 52[48,61] | 54[50,60] | 0.33 |
| LVESD | 36 [30,41] | 35[28,44] | 39[34,44] | 0.45 |
| LVEF | 56±13 | 59±12 | 55±14 | 0.49 |
| LA Para | 48±8* | 48±7*† | 57±9*† | *†<0.001 |
| LA LONG | 61±7* | 61±7*† | 67±9† | *†0.01 |
| LA TRANS | 45[42,50]* | 43[40,47]*† | 51[45,57] † | *†0.009 |
| RA LONG | 54[49,58]* | 56[52,61] | 58[53,66]* | *0.02 |
| RA TRANS | 39±7* | 42±7 | 45±8* | *0.005 |
HTN: hypertension, SHD: structural heart disease; HF: heart failure: AAD: antiarrhythmic drug; LVED left ventricular diastolic and systolic diameter, LA: left atrium; RA: right atrium, Para: parasternal, Long: longitudinal, Trans:transversal
Statistically significant difference between marked variables
Data compared using ANOVA for means, ANOVA on ranks for medians and Chi square for categorical data.
Electrophysiologic Study
All patients provided written informed consent. Antiarrhythmics were discontinued ≥5 half-lives prior to ablation with the exception of amiodarone. Prior to the procedure, patients were on oral anticoagulants targeting an INR of 2–3 for at least 1 month, and transesophageal echocardiography was performed within 5 days to exclude atrial thrombus.
Surface electrocardiogram and bipolar endocardial electrograms were monitored continuously and stored on a computer-based digital amplifier/recorder system (Labsystem Pro; Bard Electrophysiology). Intracardiac electrograms were filtered from 30–500Hz.
The following catheters were introduced via the right femoral vein for the electrophysiological study: (i) a steerable quadripolar or decapolar catheter (5mm electrode spacing, Xtrem, ELA Medical) was positioned within either the coronary sinus (CS) with the proximal electrode positioned at 4–5 o’clock along the mitral annulus or the LAA or the lateral RA; (ii) a 10-pole circumferential catheter was used for mapping the pulmonary veins (Lasso; Biosense-Webster), and was introduced following transeptal access through a long sheath (SLO, St Jude Medical, Sylmar, California) perfused continuously with heparinized D5W; Following PVI, this catheter was placed in either the LAA or RAA; (iii) an irrigated-tip radiofrequency (RF) ablation catheter with a distal 3.5mm tip (Thermocool, Biosense-Webster). For RA ablation the ablation catheter was introduced via the long sheath. Following transeptal puncture a single bolus of 50 IU/kg of heparin was administered.
Study Protocol
The procedural endpoint was termination of AF to either an intermediate atrial tachycardia (AT) or directly to sinus rhythm (SR) by RF application during the index procedure. Following restoration of SR, PV isolation was rechecked as well as conduction across any of the linear lesions performed.(15) (16) Additional RF applications were performed to achieve PVI and linear block. No attempt at reinduction was made. RF duration in the RA was limited to 25 minutes as LA ablation of fractionated activity is normally completed within this time. If AF persisted, SR was restored by electrical cardioversion.
Left Atrial Ablation
Ostial Pulmonary vein isolation was performed with the endpoint of the abolition or dissociation of electrical activity in all PVs. After PVI, the Lasso catheter was withdrawn from the long sheath and advanced via an introducer to the right atrial appendage (RAA) for monitoring RA AFCLwhile the ablation catheter or the decapolar catheter was advanced in the LAA for monitoring AFCL.
Electrogram-guided ablation was performed at LA sites displaying complex electrogram features: continuous electrical activity, complex rapid and fractionated electrograms, a gradient of activation (a temporal gradient of at least 70ms between the distal and proximal bipoles on the roving distal ablation electrode) that may represent local circuits.(8) Areas commonly targeted in ablation of fractionated electrograms included, the inferior LA/CS, base of the left atrial appendage (LAA), the LA roof and the LA septum, although any areas harboring fractionated electrograms were targeted. When ablation of the inferior LA did not result in organization of the CS, additional ablation within the CS was performed. The endpoint of electrogram-guided ablation was the transformation of complex fractionated electrograms into discrete electrograms and slowing of local CL compared with LAA AFCL or the elimination of electrograms.
Linear ablation was performed if AF persisted following the previous steps, and targeted the LA roof then the mitral isthmus with the endpoint of significant reduction (>80%) or abolition of local electrograms.
Right Atrial Ablation
Ablation was carried out in the RA if a frequency gradient from RA to LA (RAA AFCL <LAA AFCL) was observed after elimination of all LA sources in AF. Complex fractionated atrial electrograms were targeted using the same criteria as in the LA.
Right Atrial Substrate Ablation. Ablation was performed at the anterior, lateral and posterior RA;
Ablation in the RAA was guided by the Lasso catheter which was inserted into the body of the RAA, with ablation proximal to the catheter. The endpoint of ablation was organization and slowing of RAA electrograms and not RAA isolation.
Cavotricuspid Isthmus (CTI) Ablation was performed in all patients to achieve bidirectional conduction block(17).
Superior Vena Cava (SVC) Isolation was performed (using the same technique and endpoint as used for the PVs) only if it was identified as an AF source based on descending RA activation using either the decapolar or RF catheter, with distal to proximal activation within the SVC (18).
Atrial fibrillation cycle length
The effect of ablation was monitored as previously described(19) by evaluation of the AFCL. AFCL in the RAA was measured with the RF or decapolar catheter prior to ablation and continuously monitored using the lasso catheter following PVI. In the LAA, AFCL was measured prior to ablation, after PVI, after ablation of left atrial tissue and after linear lesion using the RF catheter. The AFCL was averaged over 30 seconds of consecutive cycles using automated CL monitoring software (Labsystem Pro; Bard Electrophysiology) (Figure1). The electrograms within the body of the appendages are usually unfractionated and of high amplitude (0.5–2.0 mV) thereby facilitating unambiguous automatic annotation. Each automated annotation was verified manually and corrected using online callipers at a paper speed of 100mm/s. In addition, the sites of AF termination were noted. A significant impact of ablation was defined by termination of AF or prolongation of the AFCL by 5ms or more.
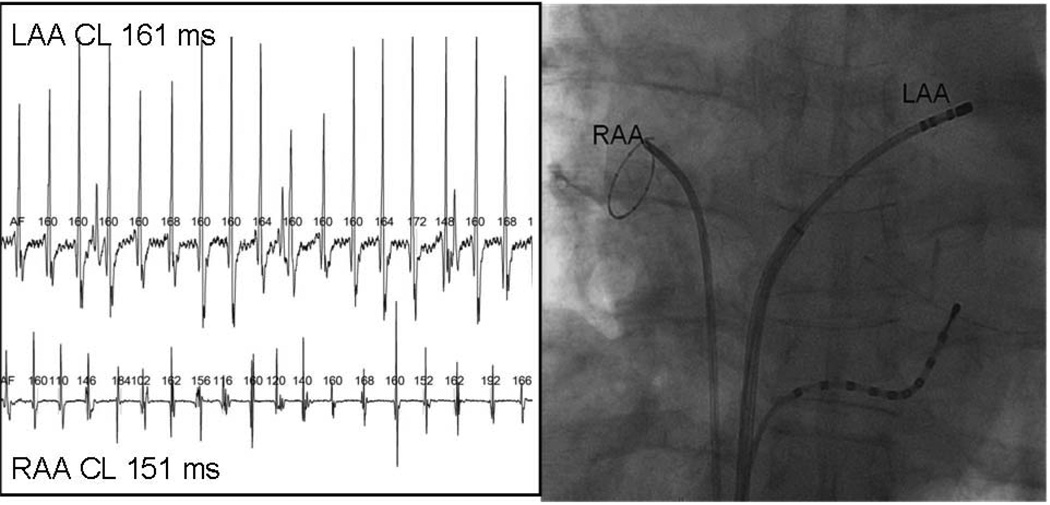
Figure 1.
Measurement of Atrial Fibrillation Cycle Length. A circular mapping catheter and the ablation catheter are placed in the right and left atrial appendages respectively. Using automated software with manual correction where necessary the AFCL is measured synchronosly using unambiguous recordings. The mean AFCL is determined from 30 seconds of continuous recording and in this example the cycle length of the right atrium is 151ms and in the left is 161ms.
Termination of AF was defined as a transition from AF to SR directly or via one or more intermediate atrial tachycardias (AT) without antiarrhythmics. AF was defined by beat-to-beat variability in cycle length and f wave morphology, while AT was defined as an organized atrial rhythm with a stable cycle length, a consistent endocardial activation sequence in both atria and a monomorphic P wave. Organized AF (type 1 AF) was defined as irregular atrial cycle length with beat to beat variation of ≥ 20 ms, and a consistent activation pattern for 75% of the time (20, 21). A frequency gradient between the AFCL in the LA and RA was considered present if a difference of ≥5 ms was observed.
Ablation parameters
RF energy was delivered to atrial tissue with a power of 25–35 watts using irrigation rates of 5–60ml/min (0.9% saline via a Cool Flow pump; Biosense-Webster) to achieve the desired power delivery. Within the CS, power was reduced to 15–20Watts distally and 30Watts at the CS ostium. Power was limited to 25 Watts in either appendage. Temperature was limited to 50°C. The duration of RF delivery was measured at each point.
Follow-up
All patients were monitored in hospital for at least 5-days following their procedure for extensive ambulatory monitoring. Following ablation, all antiarrhythmic drugs were not initiated unless otherwise indicated. Patients were re-evaluated at 1, 3, 6, and 12 months, and in the absence of AF or symptoms, followed up with their referring physician. At each visit, exercise testing and ambulatory 48 hour monitoring were performed to detect asymptomatic arrhythmias. In the event of symptomatic or asymptomatic arrhythmia recurrence, patients were offered additional ablation at least 3 months after the index procedure and after a trial of drug therapy (Class IC drug with the addition of Beta Blockers or in case of concomitant structural heart disease amiodarone was administrated). Cessation of anticoagulant therapy was considered 6 to 9 months after the last procedure in the absence of recurrence or concomitant indications.
Statistical Analysis
Data are expressed as mean ± standard deviation for normally distributed continuous variables and as the median [25th, 75th percentile] for non-normally distributed variables. Data are expressed as absolute numbers and percentages for categorical variables. Continuous variables were compared between groups using Student’s t-test, or Mann-Whitney rank test, and within each group using a paired-t-test or Wilcoxon signed rank test. Categorical variables were compared with chi-square or Fisher exact test. Statistical significance was established at p<0.05.
Multivariate analyses were performed using a multiple linear regression test and included all parameters with a significance <0.1 from the univariate analysis. Kaplan-Meier analyses for AF free-survival were performed for all 3 groups and were compared using a log-rank analysis.
RESULTS
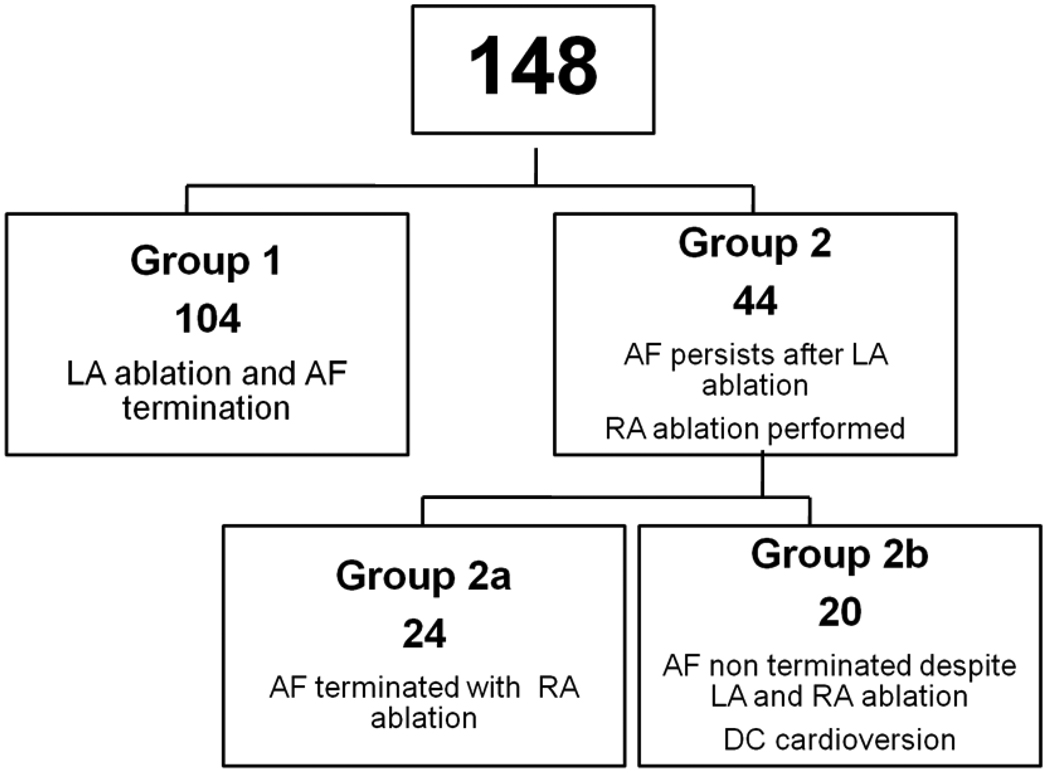
SR was restored by ablation without pharmacological or DC cardioversion in 128 patients (86%)(figure 2). The total procedure and fluoroscopic times were 252±60min and 79±19min (3.75 frames/s). The total RF ablation duration delivered in both atria was 85±25min. PVI was achieved in all patients. During LA ablation two distinctive patterns of AFCL evolution were observed in patients in whom procedural termination of AF was achieved. AFCL in each group and at every step is depicted in Table 2
Figure 2.
Patient Flow Chart
Table 2.
Procedural characteristics and clinical outcome
| LA Termination (n=104) |
RA Termination (n=24) |
RA non Termination (n=20) |
P | |
|---|---|---|---|---|
| Baseline RAA CL (ms) | 155 [143,171]* | 145 [139,162] | 144 [130,155]* | *0.04 |
| Baseline LAA CL | 153 [140,170]* | 142 [143,153] | 140 [133,158]* | *0.03 |
| RAA CL after PVI | 170 [152,184]* | 151 [142,161]* | 153[129,173] | *0.02 |
| LAA CL after PVI | 164 [152,180] | 160 [153,170] | 155 [137,165] | 0.49 |
|
RAACL after LA ablation |
186 [175,202]*† | 152[147,173]* | 160 [143,175] † | *†<0.001 |
|
LAA CL after LA ablation |
181 [170,200] | 177 [165,185] | 165 [156,195] | 0.09 |
| Total RF duration (min) | 81±24* | 89±23 | 104±19* | *<0.001 |
| Procedure duration | 240 [200,286] | 270 [231,330] | 270 [215,290] | 0.08 |
| Fluoroscopic duration | 82 [62,97] | 85 [76,101] | 94 [79,102] | 0.12 |
|
Long term clinical success n (%) |
97 (93%)* | 21 (88%)† | 11 (55%)*† | *†<0.001 |
RAA: Right atrial appendage; CL: Cycle length, LAA: Left atrial appendage; PVI: Pulmonary vein isolation, RF: Radiofrequency.
Statistically significant difference between marked variables
Data compared using ANOVA for means, ANOVA on ranks for medians and Chi square for categorical data.
Group 1: Parallel increase in AFCL during LA ablation
In 104 patients (70%), there was a parallel increase of AFCL in the LA and RA culminating in AF termination. At baseline, AFCL was 153[140,170]ms in LA and 155[143,171]ms in RA; prolonging after PV isolation to 164[152,180]ms and 170[152,184]ms (p<0.001 LA versus RA), and during LA ablation prior to termination to 181[170,200]ms and 186[175,202]ms (p=0.009 LA versus RA) respectively (figure3a). In addition to the parallel pattern of evolution, AFCL remained significantly shorter in LA than RA prior to AF termination. AF terminated directly to SR in 19 (18%) or via an intermediate AT in 85 (82%).
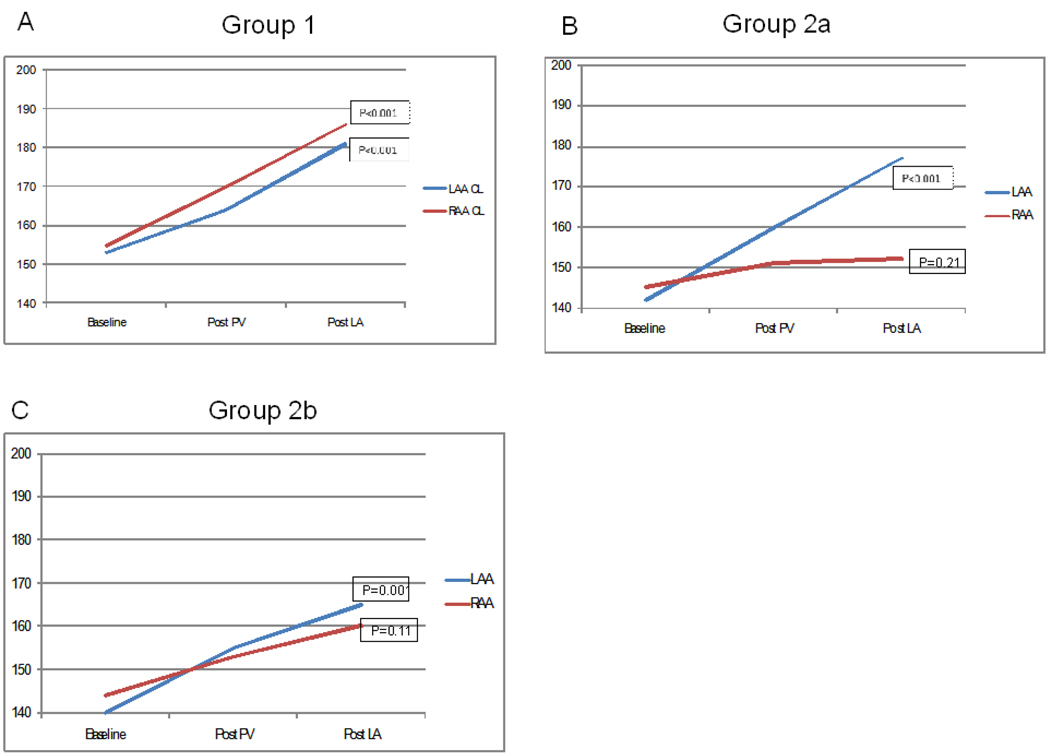
Figure 3.
Evolution of Atrial Fibrillation Cycle Length During Ablation. In group 1, AFCL measured from both the right and left atrial appendage prolongs in parallel, with termination of AF occuring in the left atrium. In Group 2a, where termination occurs during the right atrial ablation, the AFCL prolongs in the left atrium but not in the right atrium, with left atrial ablation. In Group 2b, the AFCL measured in the right atrium fails to prolong following PV isolation.
Data compared using ANOVA on ranks to compare AFCL at baseline and after each step of the ablation inside each atrium, the p value representing the difference in AFCL occurring during ablation inside each atrium.
A total of 171 different ATs were mapped in 85 patients (1.9 ± 1.1 per patient), and were macro-reentrant in 90 (53%) and focal in 81 (47%). The mechanisms of 6 ATs could not be defined due to inadvertent termination or other factors. Macro-reentrant tachycardias were terminated by linear ablation at the mitral isthmus (45), LA roof (22) or CTI (23). Eighty-one centrifugal AT were ablated, 47% consistent with focal mechanisms and 53% with localized reentry.
RF delivery in the LA lasted 34±14 min for PVI, 19±9min for electrogram-based ablation and 20±15 min for the roof and mitral isthmus lines.
Group 2: Divergent progression of AFCL during LA ablation
In 44 patients (30%), AFCL remained shorter in the RA than the LA, and AF did not terminate after LA ablation. In these patients, AFCL at baseline was 140[133,156]ms in LA and 145[135,160]ms in RA; changing after PVI to 160[152,170]ms in LA and 151[142,162]ms in RA (p<0.05 LA versus RA), and during LA ablation prior to termination to 177[164,194]ms in LA, and 156[146,175]ms in RA (p<0.001 LA versus RA)(Figure3b,c).
RA ablation terminated AF in 24 of these patients (55 %, Group 2a) directly to SR in 3 and via intermediate AT in 21 (87%). The remaining 20 patients underwent electrical cardioversion (45%, Group2b).
Group 2a. AF Termination after RA ablation
Termination of AF by ablation in the RA was preceded by an increase in RA AFCL from 145[139,162]ms to 181[171,209]ms (p=0.002). RF applications for 13±7 min at these sites increased right and left AFCL by 14±9ms and 6.2±5 ms, respectively. Sites where ablation prolonged RA AFCL were widely distributed but preferentially clustered on the anterior RA, RAA, and lateral RA. Ablation at the anterior RA and the RAA significantly prolonged AFCL (≥5 ms) in 14 patients (58%) while RF energy delivered to other sites such as posterior RA (3.4±2.6 min), SVC (2.7±1.6 min), right septum (1.5±2.2 min) and CTI (7.1±3.7 min) produced a smaller AFCL prolongation (mean of 4.6±5 ms, 3±5 ms, 4±7.4 ms, 4±7.4 ms and 4.3±7.3 ms respectively).
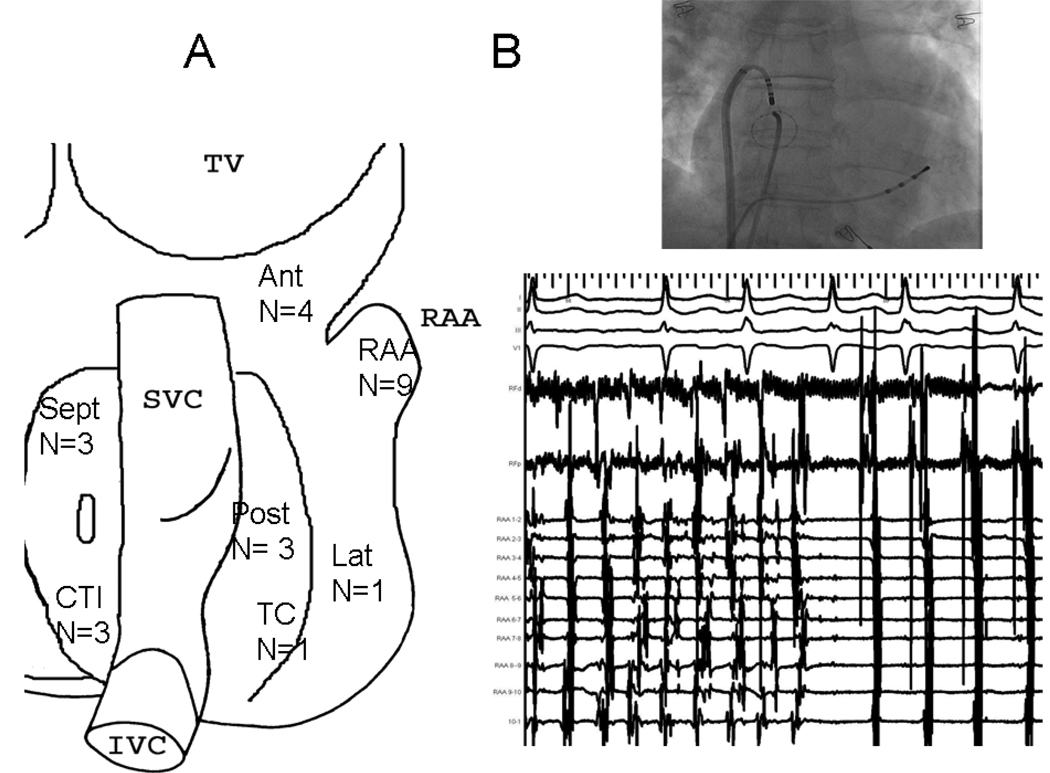
Sites where ablation terminated AF were the RAA and anterior RA in 13, posterior RA in 3, CTI in 3, right septum in 3, lateral RA in one and terminal crest in one (figure 4). Electrograms at these sites demonstrated very rapid activity with an average local CL of 136±66 ms.
Figure 4.
Sites of AF Termination Within The Right Atrium. Ant: Anterior Right Atrium, RAA: Right atrial appendage, SVC, Superior Vena Cava, Sept: Right Atrial Septum, Lat, Lateral Right Atrium, TC, Terminal Crest, Post: Posterior Right Atrium, CTI: Cavotricuspid Isthmus, IVC: Inferior Vena Cava.
A total of 36 AT were mapped and ablated in 21 patients after RA ablation (1.6 ±0.9 per patient). Underlying mechanisms were macrorentry in 17 (9 perimitral flutter, 5 rotating around the right PVs and 3 CTI dependent flutters), and centrifugal AT in 13 (10 focal and 3 localized reentry): 3 from the PVs, 3 from anterior LA, 2 around fossa ovalis, 2 from RA sites and 3 from other sites and ablation restored SR.
In these patients, RF delivery was 33±13 min for PVI, 23±9min for LA electrogram based ablation, and 16±7 min for roof and mitral isthmus lines. In the RA, total RF delivery was 9±5.8 min with an additional 3.2±2.6 min for the CTI line
Group 2b: Failure to terminate AF
In 20 patients AF did not terminate despite bi-atrial ablation, and DC cardioversion was needed to restore SR. In this subgroup, baseline AFCL was 140[133,158]ms and 144[130,155]ms in LA and RA respectively, (p=ns compare to group2a). LA ablation of fractionated electrograms and linear lesion at the LA roof and at the mitral isthmus prolonged AFCL in both atria but to a lesser extent (LA 165[156,195]ms and RA 160[143,175]ms, p<0.001).
Impact of Ablation
The gradient after LA ablation between right and left AFCL was significantly smaller in group 1 (3 [2–5] ms) versus group 2 (2a: 17 [10–30] ms and 2b: 18 [7–21]) ms, p<0.001.
Compared to Group 2a, Ablation of electrograms at the anterior RA and RAA in Group 2b resulted in a smaller increase in global AFCL (14±9ms versus 6±5ms, p=0.1). Ablation at the lateral RA increased AFCL by 7±4 ms versus 6±5ms, p=ns, while targeting other sites such as posterior RA, right septum and CTI resulted in smaller increases in global AFCL (3.1±1.6 ms, 4.5±0.7 ms and 3±2.6 ms respectively, p=ns). At all RA sites, RF delivery was significantly longer for group 2b, than in group 2a in whom AF terminated (total RA ablation: 22±8 min versus 9±6 min, p<0.001). LA electrogram based ablation was also longer for group 2b than 2a (31±15 min versus 23±10min, p<0.05).
Factors predictive of Right Atrial ablation
The pre-procedural factors that were predictive of the need for RA ablation in univariate analysis were a longer duration of continuous AF (p<0.001) and a larger LA parasternal dimension (p=0.03), RA size (p<0.01)(Table 3). In a multivariate model including duration of AF, LA and RA size, and baseline AFCL both duration of continuous AF (p<0.01) and RA size (p<0.05) were predictive of the need for RA ablation. The sensitivity and specificity of a duration of continuous AF greater than 20 months 79% and 62% respectively, with an area under the curve of 0.75, p=0.0001. The sensitivity and specificity of a RA diameter of >38mm were 49% and 76% respectively, with an area under the curve of 0.64, p=0.01.
The procedural factors that were predictive of the need for RA ablation in univariate analysis were baseline LA AFCL (p=0.01), baseline RA AFCL (p=0.02), RA AFCL following PVI (p<0.01), RA AFCL following LA ablation (p<0.001) and LA AFCL following LA ablation (p<0.05)(Table 4). In a multivariate model including baseline LA AFCL, baseline RA AFCL, RA AFCL following PVI, RA AFCL following LA ablation and LA AFCL following LA ablation only RA AFCL following LA ablation (p<0.001) and LA AFCL following LA ablation (p<0.001) were predictive of the need for RA ablation. The sensitivity and specificity of a LA AFCL of ≤165ms following LA ablation was 42% and 88% respectively, with an area under the curve of 0.64, p=0.01. The sensitivity and specificity of a RA AFCL of ≤160ms following LA ablation was 62% and 97% respectively, with an area under the curve of 0.84, p=0.0001.
Table 3.
Pre-Procedural Characteristics Used in Logistic Regression Model
| LA termination (n=104) |
RA ablation (n=44) |
P | |
|---|---|---|---|
| AF duration | 12[6, 20] | 24[12, 66] | <0.001 |
| LVEDD | 53[48, 57] | 54[48, 60] | 0.29 |
| LVESD | 36 [30, 41] | 38[31, 44] | 0.32 |
| LVEF | 56±13 | 58±13 | 0.53 |
| LA Para | 48±8 | 52±9 | 0.03 |
| LA LONG | 61±7 | 63±8 | 0.13 |
| LA TRANS | 45[42, 50] | 46[43, 53] | 0.11 |
| RA LONG | 54[49, 58] | 56[52, 65] | 0.01 |
| RA TRANS | 40[34, 44] | 42[39, 50] | 0.01 |
LVED left ventricular diastolic and systolic diameter, LA: left atrium; RA: right atrium, Para: parasternal, Long: longitudinal, Trans:transversal
Data compared using Student t-test for means and Mann-Whitney rank test for medians. Univariate data p≤0.25 used in multiple Logistic Regression Model
Table 4.
Procedural Characteristics used in Logistic Regression Model
| LA Termination (n=104) |
RA ablation (n=44) |
P | |
|---|---|---|---|
|
Baseline RAA CL (ms) |
155[143, 171] | 145[135, 160] | 0.02 |
| Baseline LAA CL | 153[140, 170] | 140[133, 156] | 0.01 |
| RAA CL after PVI | 170[152, 184] | 151[142, 162] | 0.005 |
| LAA CL after PVI | 164[152, 180] | 160[152, 170] | 0.49 |
|
RAACL after LA ablation |
186 [175,202] | 156[146, 175] | <0.001 |
|
LAA CL after LA ablation |
181 [170,200] | 177[164, 194] | 0.04 |
RAA: Right atrial appendage; CL: Cycle length, LAA: Left atrial appendage; PVI: Pulmonary vein isolation.
Data compared using Mann-Whitney rank test. Univariate data p≤0.25 used in multiple Logistic Regression Model
Factors predictive of AF termination after RA ablation
In a multivariate model including factors identified by univariate analysis as potential predictors (duration of continuous AF, LA and RA size) only LA size ≤ 51mm (OR 0.87, IC 0.79–0.96, p=0.003) was independently predictive of AF termination after RA ablation.
Follow-up and subsequent clinical outcome
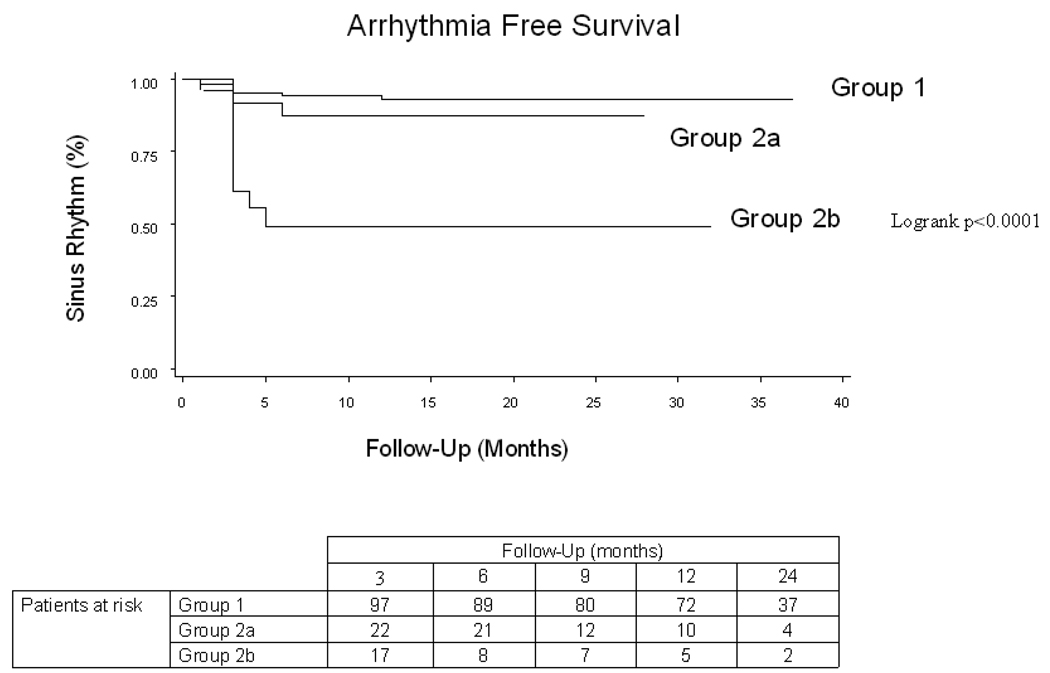
With a follow up of 22±9 months and a mean of 1.5 procedures per patient 129 patients (87%) were in SR. There was a disparate clinical outcome between the 3 groups (figure 5). The success rate after a single procedure was 46% in patients in whom AF terminated and 10% in whom AF did not terminate (p=0.005). After the last procedure, stable SR was maintained in 97 (93%) of patients in group 1 (53 patients with a repeat procedure and 86 patients off AADs), 21 (88%) in group 2a (9 patients with a repeat procedure and 17 patients off AADs), and only 11 (55 %) of patients in group 2b (16 patients with a repeat procedure and 9 patients off AADs).
Figure 5.
Arrhythmia Free Survival By Site of Arrhythmia Termination. Kaplen Meier analysis of the recurrence of any atrial arrhythmia (Atrial fibrillation or Atrial Tachycardia). Group 1 Left atrial termination, Group 2a Right Atrial Termination, Group 2b Non-termination.
ADVERSE AFFECTS
Two patients developed pericardial tamponade (during left atrial ablation) that required pericardiocentesis. In another patient, a transient ischemic attack occurred on the day following the procedure.
DISCUSSION
This study presents new information on the relative role of the left and right atria in the maintenance of long-lasting PsAF. We found that i) Biatrial monitoring of AFCL revealed 2 patterns of AFCL evolution: parallel RA and LA CL prolongation in the majority of patients and divergent evolution of AFCL in 20% of patients, with a right to left frequency gradient ii) In patients in whom LA and RA AFCL prolonged in parallel, LA ablation alone resulted in AF termination; iii) In patients with a RA to LA frequency gradient, ablation targeted to the RA terminated AF in 50% of patients; iv) In these patients, the anterior RA and RAA appeared to be critical structures to maintain AF and ablation at these sites resulted in termination of AF in 55% of all patients where termination occurred in the RA; v) Pre-procedural and procedural independent predictors of the need for RA ablation were duration of continuous AF, RA size, and right and left AFCL following LA ablation.
Previous studies
Prior studies have indicated that the RA may drive AF in some cases and dominant frequency (DF) mapping in persistent AF uncovered in RA DF max sites in 16% of patients(33). There are clear reports of AF termination during exclusive or dominant ablation in the RA, although these cases are uncommon (22, 23).
In the present study in the majority of patients with long-lasting PsAF, LA ablation prolonged AFCL in both atria culminating in AF termination, clearly indicating that the LA was driving the RA, and thus the fibrillatory process. It follows that RA ablation is not appropriate for all patients, which may explain why some studies do not show a benefit of indiscriminate RA ablation(24)·(13).
However, our results are similar to other studies in which both the LA and RA have been targeted. In a recent study using biatrial ablation, AF termination occurred in the RA in 26% of patients.(14) Interestingly, as in our study there was a frequency gradient from the RA to the LA in these patients. In an earlier study based on electrogram targeted ablation, 60% of PsAF could be terminated of whom 15% occurred in the RA.(1) A subsequent study compared LA ablation to biatrial ablation in 80 patients with PsAF; procedural termination of AF was more frequent with biatrial than LA ablation (85% vs 24%) (23). However, AF-free survival without anti-arrhythmic medications in that study was limited to 35–47%. A series of small biatrial mapping studies in chronic AF have occasionally identified rapid repetitive activations in the lateral RA.(25)
Role of the right atrium
In 30% of our total population, AFCL in the RA did not prolong in parallel with AFCL in the LA, resulting in a right to left frequency gradient, suggestive that the substrate resides in the RA. This hypothesis was confirmed by AF termination with RA ablation in more than half of these patients. Interestingly the RA AFCL was shorter in these patients following PVI, and it may be that biatrial monitoring of AFCL with ablation targeted to the chamber with the shortest AFCL following PVI will avoid unnecessary ablation.
While sites associated with AF termination in the RA were widely distributed, the two predominant areas were the anterior RA and RA appendage. Although RA focal or reentrant tachycardias have been mainly described in superior vena cava, crista terminalis or atrial septum, the overlapping heterogeneity of trabeculated fibers between RAA and RA body(26) may constitute a critical milieu for perpetuation of AF in some patients as with the LAA.
In patients requiring RA ablation to terminate AF, 88% converted to SR through an intermediate AT. It is notable that these ATs were mainly left atrial (73%). These data support the multiple concurrent driver hypothesis of AF.(27) Somewhat speculatively, it is possible that the elimination of LA sources for AF by ablation enabled the emergence of slower RA sources. Successful ablation of these sources, in turn, terminated AF. Further studies are needed to determine if the LA AT to which AF subsequently terminated were due to preceding LA ablation, or reflected organization from still slower residual LA sources for AF. Multivariate analysis demonstrated that duration of long-lasting PsAF, RA size and right and left AFCL following LA ablation were independent predictors of the need of RA ablation. These variables can help identify patients who will require RA ablation. This suggests that as the AF process evolves initial remodelling occurs in the LA but then later the RA becomes recruited into the pathological process.
The present study does not provide information on whether some patients may have benefited from RA ablation alone. An earlier study showed that AF may originate solely from the RA could effectively be ablated by targeting the crista terminalis (28), although such patients only constituted 3% of the screened population.
Limitations
The main limitation of this study is that ablation was targeted to the RA only after ablation in the LA was completed. However, a right to left frequency gradient was often apparent following PVI, suggesting that the RA could have been targeted earlier. This study may therefore underestimate the contribution of the RA in patients with long-lasting PsAF. Secondly, electrogram analysis based on visual inspection is somewhat subjective(29), however, this is true for prior studies, and attempts to quantify successful ablation sites using frequency analysis in long-lasting PsAF have had conflicting results(30, 31). Although considerable atrial damage may result from ablation to terminate long-lasting PsAF(8), echocardiography showed that atrial systolic function was retained in all patients in SR.(32) Thirdly we measured the impact of ablation via changes in AFCL, yet it is possible that the fibrillatory process was also modulated by ablation that did not alter AFCL. Finally it is also possible that critical drivers may be present in the RA and conduct to the LA without a frequency gradient.
CONCLUSIONS
Ablation of RA substrate is required to terminate long-lasting PsAF and achieve durable long-term results in 20 % of patients. These patients tend to have a longer duration of AF, larger RA size and shorter right and left AFCL after LA ablation resulting in a right to left frequency gradient. Although these patients have lower overall success than patients in whom LA ablation alone is successful, additional ablation within the RA terminated AF in over half with a similar long-term outcome to those who had procedural termination in the LA. AF termination whether occurring in the right or left atrium is associated with an excellent long-term outcome. Further studies are needed to elucidate the complex interplay between left and right atrial sources for AF
ABBREVIATIONS
- AF
Atrial fibrillation
- AT
Atrial tachycardia
- CL
Cycle length
- CS
Coronary sinus
- CTI
Cavotricuspid isthmus
- DC
Direct current
- DF
Dominant frequency
- INR
International normalised ratio
- LA
Left atrium
- LAA
Left atrial appendage
- PV
Pulmonary vein
- PVI
Pulmonary vein isolation
- Ps
Persistent
- RA
Right atrium
- RAA
Right atrial appendage
- RF
Radiofrequency
- SR
Sinus rhyth
- SVC
Superior vena cava
References
- 1.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 4.Rostock T, Rotter M, Sanders P, et al. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006;3:27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt C, Estner H, Hecher B, et al. Radiofrequency ablation of complex fractionated atrial electrograms (CFAE): preferential sites of acute termination and regularization in paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1039–1046. doi: 10.1111/j.1540-8167.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 7.Knecht S, Hocini M, Wright M, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, O'Neill MD, Hocini M, et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008;51:1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 Suppl 1:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 11.Elayi CS, Verma A, Di Biase L, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658–1664. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Arentz T, Weber R, Burkle G, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–3063. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Chugh A, Good E, et al. A randomized evaluation of right atrial ablation after left atrial ablation of complex fractionated electrograms for long lasting persistent atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2008;1:6–13. doi: 10.1161/CIRCEP.107.748780. [DOI] [PubMed] [Google Scholar]
- 14.Rostock T, Steven D, Hoffmann B, et al. Chronic Atrial Fibrillation Is a Biatrial Arrhythmia: Data from Catheter Ablation of Chronic Atrial Fibrillation Aiming Arrhythmia Termination Using a Sequential Ablation Approach. Circ Arrhythmia Electrophysiol. 2008;1:344–353. doi: 10.1161/CIRCEP.108.772392. [DOI] [PubMed] [Google Scholar]
- 15.Hocini M, Jais P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- 16.Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 17.Cauchemez B, Haissaguerre M, Fischer B, Thomas O, Clementy J, Coumel P. Electrophysiological effects of catheter ablation of inferior vena cava-tricuspid annulus isthmus in common atrial flutter. Circulation. 1996;93:284–294. doi: 10.1161/01.cir.93.2.284. [DOI] [PubMed] [Google Scholar]
- 18.Shah D, Haissaguerre M, Jais P, Hocini M. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003;26:1631–1635. doi: 10.1046/j.1460-9592.2003.t01-1-00243.x. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 20.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 21.Haissaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–625. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 22.Calo L, Lamberti F, Loricchio ML, et al. Long-term follow-up of right atrial ablation in patients with atrial fibrillation: J Cardiovasc Electrophysiol. 2004;15:37–43. doi: 10.1046/j.1540-8167.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- 23.Calo L, Lamberti F, Loricchio ML, et al. Left atrial ablation versus biatrial ablation for persistent and permanent atrial fibrillation: a prospective and randomized study. J Am Coll Cardiol. 2006;47:2504–2512. doi: 10.1016/j.jacc.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Guden M, Akpinar B, Caynak B, et al. Left versus bi-atrial intraoperative saline-irrigated radiofrequency modified maze procedure for atrial fibrillation. Card Electrophysiol Rev. 2003;7:252–258. doi: 10.1023/B:CEPR.0000012393.09666.26. [DOI] [PubMed] [Google Scholar]
- 25.Wu TJ, Doshi RN, Huang HL, et al. Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Cardiovasc Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 26.Ho SY, Anderson RH, Sanchez-Quintana D. Gross structure of the atriums: more than an anatomic curiosity? Pacing Clin Electrophysiol. 2002;25:342–350. doi: 10.1046/j.1460-9592.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- 27.Rotter M, Dang L, Jacquemet V, Virag N, Kappenberger L, Haissaguerre M. Impact of varying ablation patterns in a simulation model of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:314–321. doi: 10.1111/j.1540-8159.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin YJ, Tai CT, Kao T, et al. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Estner H, Luik A, et al. Automatic 3D Mapping of Complex Fractionated Atrial Electrograms (CFAE) in Patients with Paroxysmal and Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2008 doi: 10.1111/j.1540-8167.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 31.Lazar S, Dixit S, Callans DJ, Lin D, Marchlinski FE, Gerstenfeld EP. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006;3:889–895. doi: 10.1016/j.hrthm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi Y, O'Neill MD, Hocini M, et al. Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J Am Coll Cardiol. 2007;49:1306–1314. doi: 10.1016/j.jacc.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 33.Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]