Abstract
Objectives
We examined the relationship between chronic caregiving stress and endothelial function.
Background
Evidence suggests that caregiving stress is associated with pathophysiologic processes related to atherosclerosis. Endothelial dysfunction is a possible underlying mechanism explaining the relationship between caregiving stress and cardiovascular morbidity. We investigated the relationship between chronic caregiving stress and endothelial dysfunction assessed by reactive hyperemia induced flow-mediated dilation (FMD).
Methods
Seventy eight elderly individuals participated in the study. Fifty-five were providing in-home care to a spouse with Alzheimer’s disease (AD) and 23 were married and living with a healthy, non-demented spouse. ANCOVA was used to examine relations between advancing dementia severity (Clinical Dementia Rating scores) and FMD and nitroglycerine-induced vasodilation of the brachial artery. Multiple linear regression was used to examine the relationship between years of caregiving and FMD.
Results
Clinical Dementia Rating (CDR) scores were significantly related to FMD (p = 0.033) with participants caring for a spouse with moderate-to-severe dementia showing significantly worse FMD than those caring for a spouse with mild dementia (p = 0.028) and non-caregivers (p = 0.032). Within the caregiver sample, years of caregiving was significantly related to FMD (r = −.465, p < .001).
Conclusions
These results suggest that the chronic stress of caregiving is associated with impaired endothelial function, which may be a potential mechanistic link between the observed increased risk for cardiovascular disease in elderly caregivers.
Keywords: Flow-Mediated Dilation, Cardiovascular Disease, Stress, Caregiving, Alzheimer’s Disease
INTRODUCTION
The atherosclerotic disease process is mediated by a number of complex pathophysiologic processes resulting in thickening of the arterial walls nearest to the lumen. Dysfunction of the endothelial lining plays a key role in the development and progression of atherosclerosis(1,2). Among its numerous functions, the endothelium is involved in inhibiting platelet aggregation, contributing to formation and secretion of growth-regulatory molecules and cytokines, and release of a number of chemical mediators such as nitric oxide (NO), a potent vasodilator released in response to shear stress(1,3). Bioavailability of NO is indicative of cardiovascular health(4); however dysfunction of the endothelium may disrupt its ability to balance vasoconstriction and vasodilation, resulting in compensatory responses that interfere with the maintenance of vascular homeostasis(5). Endothelial injury results in a number of changes to its function that may promote procoagulant properties, inflammatory responses, and vasoconstriction that contributes to arterial thickening(5,6), atherosclerotic lesion formation(2), and ultimately to the development of atherosclerosis(1,3).
Brachial artery flow-mediated dilation (FMD) is a non-invasive method designed to assess endothelial function of the peripheral conduit artery in humans(7). FMD highly correlates with invasive quantification of the vasomotor responses of epicardial arteries to Acetylcholine (Ach), which is adopted as the gold standard(8,9). The FMD technique uses upper arm occlusion to induce distal hypoxia followed by the reactive hyperemia and local vasodilation after cuff deflation. The distal vasodilation, in turn, will induce large increase in the shear stress upstream, in the brachial artery. In response to the increased shear, the brachial artery endothelial cells increase the production of NO causing the vascular smooth muscle to relax and the artery to dilate(3).
Impaired flow-mediated dilation (FMD) has been prospectively associated with increased risk for cardiovascular events(10,11), such as postoperative events in patients undergoing vascular surgery. Furthermore, Shimbo and colleagues(12) found that impaired brachial FMD was predictive of incident cardiovascular events in asymptomatic, lower risk individuals in a population-based study. However, the predictive value of FMD in this study was not independent of cardiovascular risk factors. Factors associated with worse FMD include increasing age (with declining function occurring earlier for men than women)(13,14), increased systolic blood pressure (SBP)(13), smoking(13), and total to high density lipoprotein cholesterol ratio(15). In contrast, use of cholesterol lowering medication has been associated with improved FMD(16,17).
Importantly, it has been hypothesized that atherosclerosis may be exacerbated by repeated and sustained sympathetic nervous system activation resulting from exposure to environmental and psychological stressors(18,19). Indeed, the chronic stress of caring for a disabled loved-one (e.g., with Alzheimer’s disease), has been associated with increased cardiovascular risk(20–22). Chronic caregiving stress and factors associated with the stress process have also been associated with a number of physiological processes related to mechanisms associated with atherosclerosis including sympathetic reactivity(23,24), coagulation(25,26), and platelet activation(24). However, the association between chronic caregiving stress and endothelial dysfunction has yet to be demonstrated.
Given the evidence that caregiving stress is associated with pathophysiologic processes related to atherosclerosis, this study aimed to investigate the potential relationship between chronic caregiving stress and endothelial dysfunction (i.e., impaired FMD) in a population of 55 elderly AD caregivers and a control group of 23 age and gender-equivalent non-caregivers. Endothelial dysfunction was conceptualized as a possible underlying mechanism explaining the relationship between caregiving stress and cardiovascular morbidity(27). While caregiving stress has been conceptualized in a number of ways (e.g., number of care recipient problem behaviors; role overload; burden), these measures typically reflect snapshots of caregiver stress at specific moments in time rather than the accumulated wear-and-tear that caregivers may experience over time. Therefore, we assessed chronic stress with two measures: a) the clinical dementia rating (CDR) of participants’ spouses, and b) the number of years participants were providing in-home care for their spouses. We hypothesized that higher CDR and more years of caregiving would be associated with impaired endothelial functioning, as indexed by reduced FMD.
METHODS
Participants
Seventy-eight elderly individuals participated in this study. Of these, 55 were providing care to a spouse with Alzheimer’s disease (AD) and 23 were married to a healthy, non-demented spouse (i.e., “healthy controls”). All participants were enrolled in the “Alzheimer’s Caregiver Study” at the University of California San Diego (UCSD), which was designed to examine physiological and psychological mechanisms of increased health risk in spousal caregivers. Participants were required to be free from major illnesses (e.g., cancer), at least 55 years of age, married, and living with their spouses at the time of enrollment. Participants were excluded if they suffered from extreme hypertension (>200/120 mmHg). A total of 127 participants were screened for the study. Of these, 88 (69.3%) were eligible to participate and 29 (22.8%) were ineligible. The remaining 10 (7.9%) participants were screened eligible but chose not to participate in the study. Of the 39 participants who were ineligible, the most common reasons for being excluded were a) the individual was previously, but not currently a caregiver (e.g., spouse passed away; n = 10), b) non-spouse caregiver (e.g., caring for a parent with AD; n = 7), c) current or recent serious medical condition (e.g., cancer requiring chemotherapy; n = 4), and d) not currently living in San Diego or surrounding community (n = 2). Participants were recruited through referrals from the UCSD Alzheimer’s Disease Research Center (ADRC), community agencies serving caregivers, local caregiver support groups, community health fairs, and referrals from other participants.
Measures
Participants were interviewed on several demographic and health variables. Because of their potential relationship to FMD, participants provided information regarding their age, smoking history, medication usage over the past 30 days, and years of caregiving (years since spouse was diagnosed with AD). For non-caregivers, a value of ‘0’ was assigned for years of caregiving.
Brachial Artery Flow-Mediated Dilation (FMD) and Nitroglycerine Mediated Dilation (NMD)
Tests of endothelium-dependent (FMD) and endothelium-independent (NMD) response of the right brachial artery to the increased blood flow and NO, respectively, were performed by a single technician with the modified method first described by Celermayer et al.(7). All measurements were done after 15 minutes of relaxation in the supine position, between 11:00 a.m. and 1:00 p.m., after fasting and without vasoactive medications. The occlusion cuff was placed on the right upper arm and the brachial artery was scanned, in longitudinal section 4–10 cm proximal to the antecubital fossa, using an Acuson Cypress portable ultrasound system with 5.4 – 6.6 MHz linear array transducer (Model 7L3; Siemens Medical Solutions USA, Mountain View, CA).
After the brightest views of the anterior and posterior artery walls had been obtained, three baseline images were saved. Then, the occlusion cuff was inflated to 50 mmHg above SBP thereby producing distal hypoxia for five minutes. After the cuff was deflated, arterial images were saved every 15 seconds during the first minute post-occlusion and then once every 30 seconds for an additional 8 minutes. A single technician, blind to the caregiver status of the participant, measured artery diameters manually from the saved digital ultrasound images with the Acuson Cypress built-in vascular measurements software module (Siemens Medical Solutions USA, Mountain View, CA). All measurements were done by placing electronic calipers on the anterior and posterior intima line (i-i line). FMD was calculated as the maximum percentage change in the brachial artery diameter, FMD%(max), from the average baseline diameter value, DFMD(b), to the maximum diameter value after the cuff deflation, DFMD(max) :
Fifteen minutes after the FMD test, baseline brachial artery diameter was determined again by averaging three diameter measurements taken immediately prior to applying nitroglycerin. Then, 400µg nitroglycerin (GTN) tablet (NitroQuick®, ETHEX Corp., St. Louis, MO) was given sublingually to induce vasodilation. Ultrasound scans were acquired continuously and brachial artery diameter was measured once every minute during the 7 minutes. NMD was calculated as the maximum percentage change in the brachial artery diameter, NMD%NMD(max), from the baseline value DNMD(b) to the maximum value obtained with sublingual GTN, DNMD(max):
Dementia Rating of Spouses
Participants were interviewed using the Clinical Dementia Rating (CDR) scale(28), whereby participants indicated the extent to which their spouses exhibited symptoms of dementia in 6 domains: a) memory, b) orientation, c) judgment & problem-solving, d) community affairs, e) home and hobbies, and f) personal care. Based on responses to these items, an overall dementia severity score is given, whereby a score of ‘0’ = ‘no dementia’, 1 = ‘mild’ dementia, 2 = ‘moderate dementia’, and 3 = ‘severe dementia’. By study design, non-caregivers were required to be married to non-demented spouses, and so all spouses of non-caregivers were scored ‘0’ (no dementia). In addition, all caregivers were required to have spouses with at least mild dementia, so spouses of caregivers had CDR scores of at least ‘1’.
Perceived Stress
Each participant was administered the Role Overload scale(29), which assesses overall stress experienced by the individual. This scale consists of 4 items (e.g., “You have more things to do than you can handle”) rated by the participant on a 4-point Likert scale ranging from 1 = Not at all to 4 = Completely.
Blood Pressure
Prior to FMD analysis, a total of 3 resting blood pressure measurements were collected by a research nurse over a 15 minute resting period. The mean of the three measurements was taken as the participant’s mean resting blood pressure.
Blood lipids
Total cholesterol (T-C) and high-density lipoprotein cholesterol (HDL-C) were determined by standard methodology at the clinical chemistry laboratories at the UCSD medical center. The T-C/HDL-C ratio was computed as an index of dyslipidemia.
Statistical Analyses
CDR and FMD
Analysis of covariance (ANCOVA) was used to assess the relationship between CDR group and FMD, in which FMD was our dependent variable and CDR score was our primary independent variable. Preliminary examination of CDR scores indicated that only 5 care-recipients were classified as ‘severely demented’ (i.e., CDR=3). Therefore, participants in this category were grouped with those with a CDR score of ‘2’, thereby resulting in three CDR groups for analysis. These three groups corresponded to the CDR score for the care-recipient (i.e., CDR0, CDR1, and CDR2). Because of their potential correlation with FMD, the following covariates were included in this analysis: a) age, b) gender, c) smoking history (yes/no), d) mean resting SBP, e) total-to-high density lipoprotein cholesterol ratio, f) current use of cholesterol-lowering medication (yes/no), and g) role overload. A significant omnibus test was followed by post-hoc ‘least significant difference’ (LSD) tests to determine differences between the three CDR groups.
Because administration of NTG produces dilation of the brachial artery independent of the endothelium, NMD should not be dependent on chronic stress. To demonstrate this effect, a second ANCOVA was conducted in which NMD was entered as our dependent variable and CDR group was our independent variable. Clinical covariates used in our first analysis were also entered in this analysis.
Years Caregiving and FMD
We conducted a second set of analyses to examine the relationship between ‘years of caregiving’ and FMD. A first analysis was conducted using an ANCOVA analysis, whereby participants were divided into three groups: a) non-caregivers (N=23), b) caregivers with “few” years caregiving (i.e., <4 years; N=26), and c) caregivers with “many” years caregiving (i.e., ≥4 years; N=28). Differentiation of “few” vs. “many” years caregiving was determined via a median split. Covariates were the same as in our previous analysis (see above).
In a second analysis, linear regression was used to examined the relationship between linear years of caregiving and FMD within our caregivers only. Covariates used in this analysis were the same as with our other analyses (described above).
RESULTS
Sample Characteristics
Demographic and clinical characteristics of caregivers and non-caregivers are presented in Table 1. Group comparisons of these characteristics, using t-tests and chi-square analyses for linear and bivariate variables, indicated that the two groups were statistically similar on all variables except smoking history, for which caregivers were more likely to report a history of smoking (p=.044).
Table 1.
Characteristics of the sample.
| Variable | Caregivers (N = 55) |
Non-caregivers (N = 23) |
t-value | χ2 | p-value |
|---|---|---|---|---|---|
| Age, M (SD) | 74.2 (7.6) | 74.3 (7.8) | −0.03 | 0.975 | |
| Female, n (%) | 38 (69.1) | 18 (78.3) | 0.67 | 0.412 | |
| Race, n (%) | |||||
| White, non-Hispanic | 50 (90.9) | 19 (82.6) | 3.54 | 0.316 | |
| Hispanic | 4 (7.3) | 3 (13.0) | |||
| Black | 1 (1.8) | 0 (0.0) | |||
| Asian | 0 (0.0) | 1 (4.3) | |||
| Education, n (%) | |||||
| < High School | 0 (0.0) | 2 (8.7) | 7.18 | 0.127 | |
| High School Graduate | 23 (41.8) | 6 (26.1) | |||
| Some College | 7 (12.7) | 2 (8.7) | |||
| College Graduate | 13 (23.6) | 5 (21.7) | |||
| Professional Degree | 12 (21.8) | 8 (34.8) | |||
| Taking Cholesterol Med(s), n (%) | 27 (49.1) | 8 (34.8) | 1.34 | 0.247 | |
| Systolic Blood Pressure (mm Hg), M (%) | 135.1 (16.1) | 131.8 (14.6) | 0.85 | 0.397 | |
| Ever Smoked, n (%) | 28 (50.9) | 6 (26.1) | 4.06 | 0.044 | |
| T-C / HDL-C ratio, M (SD) | 3.6 (1.0) | 3.4 (0.8) | 0.46 | 0.645 | |
Relationship between CDR score and FMD
Mean baseline brachial artery diameter (M±SD) for CDR0 was 0.35±0.07. For CDR1 and CDR2 the mean±SD was 0.33±0.04 and 0.37±0.07, respectively. ANOVA analysis indicated no significant differences by group (F=2.41, df=2,75, p=0.097).
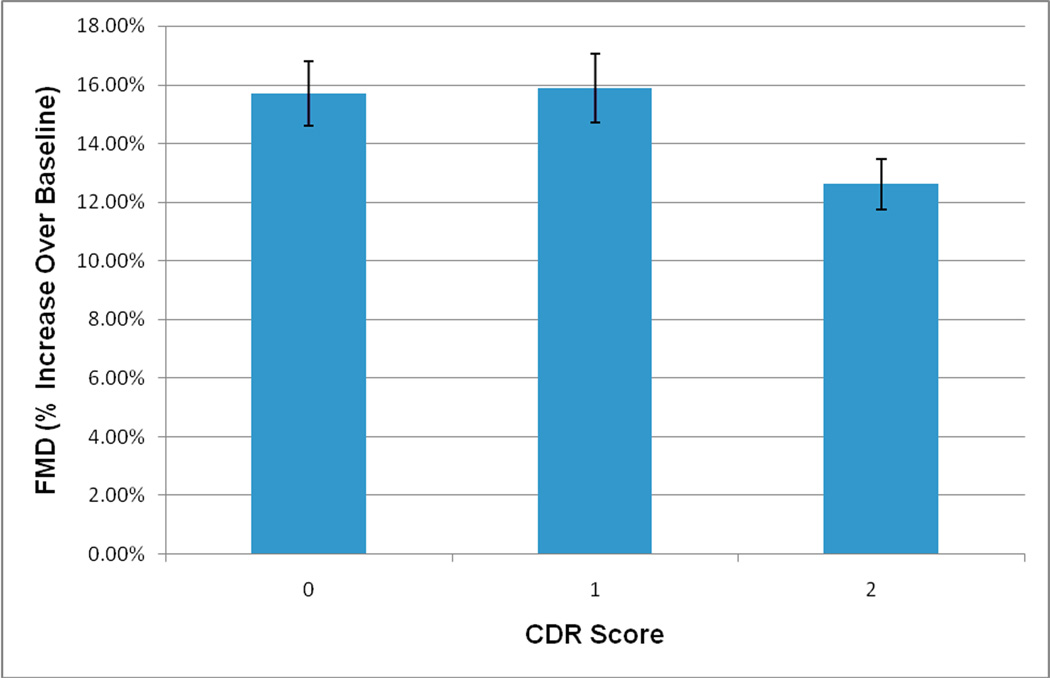
Results of our ANCOVA analysis indicated that CDR score was significantly related to FMD (F=3.60, df=2,69, p=0.033). Post-hoc analyses indicated that participants in the CDR2 group (M=12.52±5.01) had significantly worse FMD compared to those in the CDR1 group (M=16.01±5.20; p=0.028) and CDR0 group (M=15.74±6.09; p=0.032). CDR groups 0 and 1 did not significantly differ in FMD (p=0.918). Means and SE for covariate-adjusted FMD are presented in Figure 1.
Figure 1. FMD by CDR rating. Bars represent means +/− standard error of the mean.
Covariates include age, gender, taking any cholesterol-lowering medication (yes vs. no), systolic blood pressure, ever-smoked (yes vs. no), and T-C/HDL-C ratio. Subjects per CDR score were as follows: CDR = 0 (n = 23), CDR = 1 (n = 20), CDR = 2 or 3 (n = 35). Participants with CDR2 or 3 had significantly worse FMD than those with CDR1 (p = 0.028) and CDR0 (p = 0.032)
Relationship between CDR score and NTG-G
As mentioned above, we repeated our initial analysis using NMD as our dependent variable and CDR score as our primary independent variable. For this analysis, data were missing from 8 participants. The number of participants with missing data for CDR groups was as follows: CDR0=3, CDR1=2, and CDR2=3. The most common reasons for missing data were: a) refusal of NTG (n=6), history of negative reaction to NTG (n=1), and a low resting pulse (n=1).
Results of the NMD analysis indicated a non-significant effect of CDR group (F=0.86, df=2,61; p=0.427). Also, age was significantly related to NMD, with older participants having significantly worse NMD (p=0.029). None of the other covariates was significant (all p-values >0.05). Post-hoc exploratory analyses for CDR group indicated no differences between CDR0 and CDR1 p=0.279), CDR0 and CDR2 (p=0.980), or CDR1 and CDR2 (p=0.223). The mean±SD for CDR0, CDR1, and CDR 2 were 25.87±9.05, 29.06±9.28, and 25.92±9.55, respectively.
Relationship between years of caregiving and FMD
One caregiver had missing data for years of caregiving and was excluded from our analyses. Results of our ANCOVA analysis indicated a significant omnibus test for group differences (F=5.94, df=2,67; p=0.004). The covariate-adjusted FMD means (±SE) for non-caregivers was 15.5±0.01. The mean (±SE) FMD for caregivers with <4 years caregiving was 16.2±0.01, whereas FMD for those with ≥4 years caregiving was 11.7±0.01. Post-hoc LSD comparisons indicated that caregivers with <4 years caregiving were not significantly different from non-caregivers (p=0.690), whereas caregivers with ≥4 years caregiving had significantly worse FMD than non-caregivers (p=0.025).
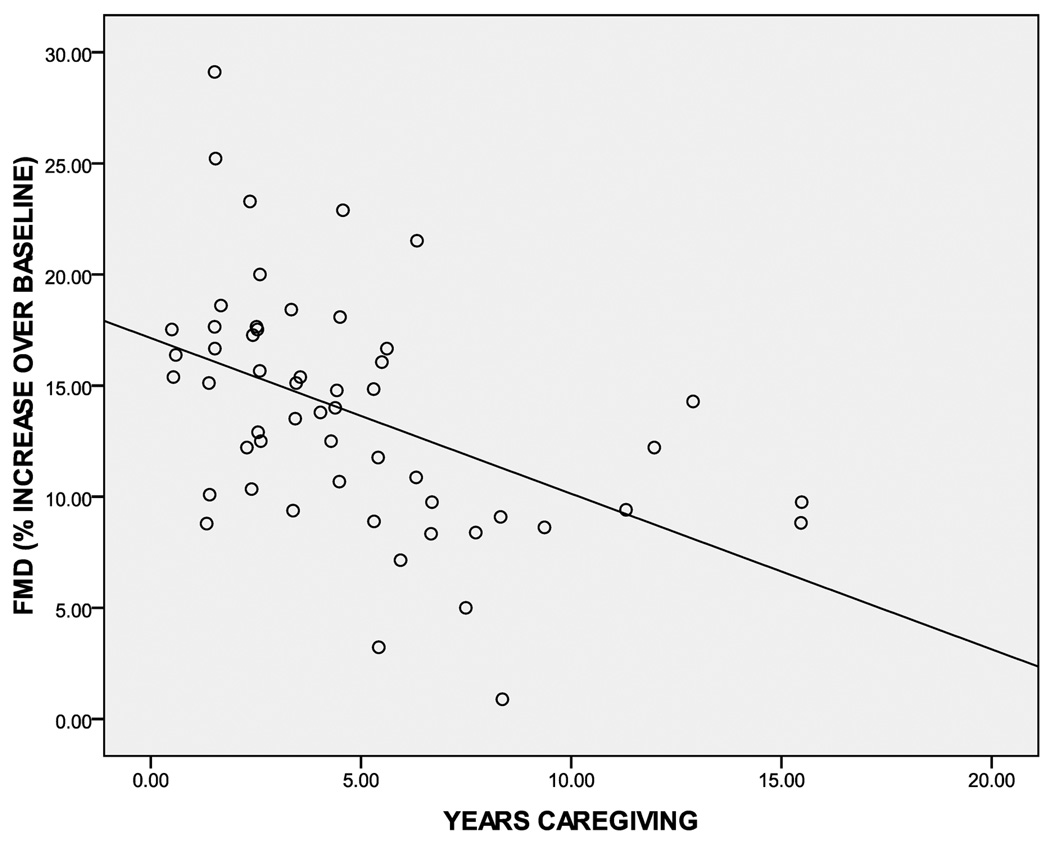
Results of our multiple regression model (with covariates) are presented in Table 2. Overall, our model explained 36.7% of the variance in FMD (adjusted R2=25.5%). Significant predictors in the model included taking cholesterol medication and years caregiving. That is, caregivers who were taking cholesterol-lowering medication had improved FMD, whereas a greater number of years caregiving was associated with worsened FMD (see Table 2). The bivariate correlation between years caregiving and FMD was −.465 (p<0.001), and is presented in Figure 2).
Table 2.
Multiple regression predicting FMD within caregivers.
| Variable | B | SE | β | t-value | p-value |
|---|---|---|---|---|---|
| Age | −0.12 | 0.09 | −0.17 | 1.31 | .197 |
| Female | 0.25 | 1.43 | 0.02 | 0.17 | .863 |
| Taking Cholesterol Medication | 3.24 | 1.38 | 0.31 | 2.35 | .023 |
| Systolic Blood Pressure | 0.01 | 0.04 | 0.02 | 0.16 | .871 |
| Ever Smoked | −2.13 | 1.33 | −0.20 | −1.61 | .115 |
| T-C / HDL-C ratio | 0.32 | 0.63 | 0.06 | 0.51 | .612 |
| Role Overload | −0.10 | 0.23 | −0.06 | −0.44 | .664 |
| Years Caregiving | −0.74 | 0.18 | −0.49 | −4.04 | <.001 |
| R2 | 36.7.5% | ||||
| Adj R2 | 25.5% | ||||
Note. For the overall model, F = 4.98 and degrees of freedom = 7, 69.
Figure 2. Scatterplot showing the unadjusted correlation between years of caregiving and FMD within all caregivers (n = 54).
Pearson correlation was r = −.465 (p <0.001).
Independent Predictability of CDR and FMD
A final ANCOVA analysis (within caregivers) examined the effect of CDR group and years caregiving in the same model. In this model, both CDR group (F=4.35, df=1,44; p=0.043) and years caregiving (F=13.67, df=1,44; p=0.001) were significant predictors of FMD. Specifically, caregivers of moderate/severely demented patients had worse FMD than those caring for mildly demented patients, and the longer caregivers had provided care the worse their FMD. These results suggest that CDR and years caregiving are independently predictive of FMD. Overall, this model explained 42.4% of the variance in FMD (adjusted R2=30.6%).
DISCUSSION
This study of 78 elderly participants suggests that the chronic stresses of caring for a spouse with AD may be associated with worse endothelial functioning, as measured by brachial artery FMD. Specifically, advancing dementia (i.e., CDR) and years of caregiving were associated with impaired endothelial functioning, independent of endothelial risk factors including age, gender, cholesterol-lowering medication use, SBP, smoking history, and T-C/HDL-C ratio. These results are consistent with and expand upon previous research showing increased cardiovascular risk in stressed caregivers(21,30), suggesting that cardiovascular risk in caregivers encompasses hemodynamic, inflammatory, and endothelial mechanisms. Non-invasive brachial flow-mediated dilation (FMD), measured by ultrasonograpy, is often used to assess endothelial dysfunction in peripheral conduit arteries and has been correlated with invasive measures of coronary artery endothelial dysfunction(8,9). The rapid flow of blood generates high shear stress at the endothelium causing the release of NO, which relaxes smooth muscle and dilates the blood vessel. We find no evidence for differences in the ability of the brachial artery smooth muscle to relax in long duration caregivers, as the NO donor nitroglycerine gave equivalent vasodilatation among groups. However, the diminished vasodilatation in response to increase in flow indicates impaired endothelial NO release among long duration caregivers. Impaired FMD is a first step in the development of atherosclerosis, but is partially reversible with angiotensin receptor blockers, statins and exercise(31).
While caregivers of patients in the early stages of dementia may be required to adapt to caregiving-related stresses, we conceptualized that these caregivers had not experienced the chronic buildup, or wear-and-tear, that mid-to-late stage caregivers had experienced. This wear- and- tear likely includes repeated hyperemia-induced shear stress on the endothelium, with this “buildup” of stress likely reaching threshold during the mid-to-late stages of dementia. The end result is believed to be early signs of endothelial dysfunction. This hypothesis was supported by both our analyses. Caregivers providing care to a spouse with mild dementia (CDR=1) did not show significant impairment in FMD relative to non-caregivers (i.e., “non-stressed”). However, those providing care to a spouse with moderate-to-severe dementia demonstrated significantly impaired FMD relative to non-caregivers. The FMD difference between caregivers of moderate/severe dementia (CDR2) and non-caregivers (CDR0) was approximately 3.2%. Based on a large-scale FMD study of the elderly(32), this difference suggests the odds of a participant in the CDR2 group having an earlier adverse cardiovascular event over a 5-year period are approximately 1.31 times greater than non-caregivers. Regarding our years of caregiving analysis, the magnitude of effect (i.e., effect size) (r=−.465) was in the medium-to-large range when using Cohen’s(33) definition of small, medium, and large effect sizes (i.e., r=0.1, 0.3, and 0.5, respectively). These effect sizes suggest clinically meaningful relationships between chronic stress and endothelium functioning.
Although these results are promising, causal interpretation of these data is premature. However, if confirmed, these results suggest that the chronic caregiving stress may lead to early signs of atherosclerosis. As with any patient, clinicians are encouraged to monitor and curb clinical risk factors of atherosclerosis, including hyperlipidemia and blood pressure, particularly given that reversal of these factors has been shown to improve endothelial functioning(2). This is particularly relevant for caregiving populations given their increased risk for CVD. Accordingly, clinicians would be encouraged to monitor caregiver-related stress and make appropriate recommendations for stress-reduction such as referring caregivers to interventions that are efficacious for reducing distress in caregivers. In this regard, a number of interventions for caregivers have been found efficacious, including cognitive-behavioral, psychoeducational, and multicomponent interventions(34), all of which teach caregivers specific, behavioral strategies for managing stress and improving well-being. In addition, given that disruptive patient behaviors are often the most stressful aspect of caregiving(35), clinicians might consider recommending efficacious interventions for reducing disruptive patient behaviors(36).
In addition to the cross-sectional nature of this study, other limitations should be noted. First, our sample was relatively small, which limited the number of covariates we could include in our model. A related limitation is that our sample included only 5 participants caring for a spouse with “severe” dementia. This led us to group these participants with those in the “moderate” dementia group. It should be noted that FMD for participants caring for “severely” demented patients (12.42±2.44) was similar to those in the moderate group (12.64±0.95), and that our correlation analysis showed participants who had provided the longest care showed the worst FMD functioning. These analyses suggest that chronic wear-and-tear may indeed be associated with endothelial dysfunction. However, these results should be replicated in a larger sample that also includes more late-stage dementia caregivers.
We utilized LSD post-hoc analyses to examine between-group differences in FMD. However, these analyses do not control for multiple comparisons. However, our analyses can inform future researchers on developing planned comparisons which might include comparisons of caregivers of mild, moderate, and severe dementia to non-caregivers. We strongly encourage this line of research.
A final limitation was that we included comparatively healthy participants. That is, our inclusion/criteria limited our sample to healthy individuals without history of major illnesses (e.g., severe hypertension, cancer, heart disease, etc.). Thus, although highly distressed caregivers may be at greater risk of a CVD diagnosis(21) and mortality(37), participants in our study were likely not to have been diagnosed with CVD given our inclusion criteria. Nonetheless, the results provide preliminary data suggesting that caring for a spouse with AD may introduce circumstances that place caregivers at risk for future CVD.
In sum, we found that chronically stressed AD caregivers demonstrated impaired endothelial functioning, as assessed by reactive hyperemia-induced FMD. Because caregiving(21,22) and worse endothelial function(10,11) and have been associated with increased risk for cardiovascular events, these results suggest a potential mechanism by which the chronic stresses of caregiving lead to negative health outcomes. Future studies should examine FMD, cardiovascular events, and stress among caregivers over time to determine the associations among these variables. If chronic stress is indeed associated with endothelial dysfunction, clinicians are encouraged to monitor chronic stress and make appropriate referrals to efficacious stress-reduction programs, including psychosocial treatments(34,36).
Acknowledgments
Support: This study was supported by the National Institutes of Health/National Institute on Aging (NIH/NIA) through award AG 15301 (to Igor Grant, MD). Additional support was provided through award AG 03090 (to Brent T Mausbach, PhD).
ABBREVIATIONS LIST
- FMD
Flow-mediated Dilation
- AD
Alzheimer’s Disease
- CDR
Clinical Dementia Rating
- NO
Nitric Oxide
- SBP
Systolic Blood Pressure
- NMD
Nitroglycerine Mediated Dilation
- GTN
Nitroglycerine
- T-C
Total Cholesterol
- HDL-C
High Density Lipoprotein Cholesterol
- CVD
Cardiovascular Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr Dimsdale receives research grant support from Sepracor Pharmaceuticals for work unrelated to that discussed in this manuscript. Dr Ancoli-Israel serves as a consultant and is on the Scientific Advisory Board for Ferring Pharmaceuticals Inc., GlaxoSmithKline, Orphagen Pharmaceuticals, Pfizer, Respironics, sanofi-aventis, Sepracor, Inc., and Schering-Plough for work unrelated to this manuscript. She also has received Grants/Contracts from Sepracor, Inc. and Litebook, Inc. for work unrelated to this manuscript. All other authors report no disclosures.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? Journal of the American College of Cardiology. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 3.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 4.Allen JD, Cobb FR, Kraus WE, Gow AJ. Total nitric oxide following testing reflects endothelial function and discriminates health status. Free radical biology & medicine. 2006;41:740–747. doi: 10.1016/j.freeradbiomed.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: A diagnostic intsrument or and experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 8.Takase B, Uehata A, Akima T, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. The American Journal of Cardiology. 1998;82 doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Uehata A, Gerhard MD. Close relationship of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 10.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 11.Gokce N, Keaney JF, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 12.Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Barley J, Jeffrey S, Carter N, Deanfield J. Angiotensin-converting enzyme genotype is not associated with endothelial dysfunction in subjects without other coronary risk factors. Atherosclerosis. 1994;111:121–126. doi: 10.1016/0021-9150(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 15.Schnell GB, Robertson A, Houston D, Malley L, Anderson TJ. Impaired brachial artery endothelial function is not predicted by elevated triglycerides. Journal of the American College of Cardiology. 1999;33:2038–2043. doi: 10.1016/s0735-1097(99)00104-7. [DOI] [PubMed] [Google Scholar]
- 16.Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. European heart journal. 2008;29:1753–1760. doi: 10.1093/eurheartj/ehn166. [DOI] [PubMed] [Google Scholar]
- 17.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 18.von Känel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosomatic Medicine. 2001;63:531–544. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Widmaier EP, Raff H, Strang KT. Vander's human physiology: The mechanisms of body function. 10th ed. McGraw-Hill; 2006. [Google Scholar]
- 20.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mausbach BT, Patterson TL, Rabinowitz YG, Grant I, Schulz R. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychology. 2007;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: A prospective study. American Journal of Preventive Medicine. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 23.Roepke SK, Mausbach BT, Aschbacher K, et al. Personal mastery is associated with reduced sympathetic arousal in stressed Alzheimer caregivers. American Journal of Geriatric Psychiatry. 2008;16:310–317. doi: 10.1097/JGP.0b013e3181662a80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain, Behavior, and Immunity. 2008;22:493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschbacher K, von Känel R, Dimsdale JE, et al. Dementia severity of the care receiver predicts procoagulant response in Alzheimer caregivers. American Journal of Geriatric Psychiatry. 2006;14:694–703. doi: 10.1097/01.JGP.0000227969.36850.eb. [DOI] [PubMed] [Google Scholar]
- 26.Mausbach BT, von Känel R, Aschbacher K, et al. Spousal caregivers of patients with Alzheimer's disease show longitudinal increases in plasma level of tissue-type plasminogen activator antigen. Psychosomatic Medicine. 2007;69:816–822. doi: 10.1097/PSY.0b013e318157d461. [DOI] [PubMed] [Google Scholar]
- 27.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 29.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 30.von Känel R, Dimasdale JE, Mills PJ, et al. Effect of Alzheimer caregiving stress and age on frailty markers Interleukin-6, C-reactive protein, and d-Dimer. Journals of Gerontology, Series A: Biological and Medical Sciences. 2006;61A:963–969. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 31.Korkmaz H, Onalan O. Evaluation of endothelial dysfunction: flow-mediated dilation. Endothelium. 2008;15:157–163. doi: 10.1080/10623320802228872. [DOI] [PubMed] [Google Scholar]
- 32.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 34.Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychology and Aging. 2007;22:37–51. doi: 10.1037/0882-7974.22.1.37. [DOI] [PubMed] [Google Scholar]
- 35.Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. The Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 36.Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychology and aging. 2007;22:28–36. doi: 10.1037/0882-7974.22.1.28. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]