Abstract
Animal coloration has provided many classical examples of both natural and sexual selection. Methods to study color signals range from human assessment to models of receiver vision, with objective measurements commonly involving spectrometry or digital photography. However, signal assessment by a receiver is not objective but linked to receiver perception. Here, we use standardized digital photographs of female rhesus macaque (Macaca mulatta) face and hindquarter regions, combined with estimates of the timing of the female fertile phase, to assess how color varies with respect to this timing. We compare objective color measures (camera sensor responses) with models of rhesus vision (retinal receptor stimulation and visual discriminability). Due to differences in spectral separation between camera sensors and rhesus receptors, camera measures overestimated color variation and underestimated luminance variation compared with rhesus macaques. Consequently, objective digital camera measurements can produce statistically significant relationships that are probably undetectable to rhesus macaques, and hence biologically irrelevant, while missing variation in the measure that may be relevant. Discrimination modeling provided results that were most meaningful (as they were directly related to receiver perception) and were easiest to relate to underlying physiology. Further, this gave new insight into the function of such signals, revealing perceptually salient signal luminance changes outside of the fertile phase that could potentially enhance paternity confusion. Our study demonstrates how, even for species with similar visual systems to humans, models of vision may provide more accurate and meaningful information on the form and function of visual signals than objective color measures do.
Keywords: color signaling, communication, receiver perception, visual discrimination threshold modeling
The range of animal colors and patterns displayed across many taxa includes signals representing classical examples of both natural selection, such as antipredator coloration (Stevens and Cuthill 2006) and cuckoo host egg mimicry (Davies et al. 1996), and sexual selection, from the colorful spots on the peacock's tail (Loyau et al. 2005) to the red and blue colors of the mandrill's nose (Darwin 1876; Setchell 2005). Present-day studies of such signals often achieve objective color measurement, using methods such as spectrometry and digital photography (Andersson and Prager 2006; Stevens et al. 2007, 2009). However, color assessment and interpretation are not objective but directly linked to the receiver's visual system and subsequent cognitive processing (Endler 1990). Though many studies of animal coloration have presented objective color analysis, studies presenting biologically relevant measures are still rare. Even in birds, one of the most widely studied groups with respect to coloration, exhibiting an extraordinary array of visual signals, color is still often represented by measures of reflectance spectra shape (Andersson and Prager 2006) that do not relate to perception. Only more rarely have measures been presented that relate directly to the visual processing of the likely receiver(s) (e.g., Siddiqi et al. 2004; Håstad et al. 2005; Lovell et al. 2005; Stevens and Cuthill 2006; Cassey et al. 2009; Langmore et al. 2009).
Color signals are less well studied in mammals than in many other animal taxa, partly because they tend not to exhibit the variety of colors seen in groups such as birds, insects, reptiles, or fish. However, this does not mean that visual cues are not important in mammals (e.g., for camouflage). One exceptional mammalian group is the primates, which exhibit a remarkable diversity of skin and pelage colors and markings (Bradley and Mundy 2008; Higham 2009). Until recently, there had been few studies of the adaptive function of primate color displays, but recent increased interest has seen many more studies exploring the underlying genetic correlates (e.g., Setchell et al. 2009) and hormonal mechanisms (e.g., Higham et al. 2008; Dubuc et al. 2009) of color variation, as well as the adaptive functions of the colors expressed (e.g., Setchell 2005; Bergman and Beehner 2008; Higham et al. 2008; Dubuc et al. 2009; Marty et al. 2009). These studies have typically used digital photography to obtain standardized color images and gray-scale values of red, green, and blue (RGB) channels, representing color using measures such as the ratio of red to green (R:G) (e.g., Bergman and Beehner 2008; Dubuc et al. 2009), or using principal components analysis to collapse RGB values into one “color” score (e.g., Higham et al. 2008; Marty et al. 2009).
In one recent study, Dubuc et al. (2009) investigated whether variation in the red coloration exhibited in the face and hindquarter regions of female rhesus macaques (Macaca mulatta) contained information about the timing of the fertile phase. The rhesus macaque is one of a number of catarrhines that exhibit variation in female sexual skin during the ovarian cycle, with either a change in morphology (“sexual swelling”) and/or color (Dixson 1998). Adult female rhesus macaques do not exhibit sexual swellings, but do express changes in the skin color of the face and hindquarters that are pronounced and visible to human observers (e.g., Zuckerman et al. 1938). These changes occur in response to increased estrogen levels and resultant increased vascularization and blood flow to the skin (Dixson 1998). Dubuc et al. (2009) collected objective measurements of rhesus face and hindquarter regions using digital photography. They combined this with fecal samples to determine the timing of the female fertile phase, to assess whether color variation covaried with respect to this timing, and hence could convey information about this to others. They took the R:G ratio as their representation of color (following Bergman and Beehner 2008) and found that facial, but not hindquarter, color varied specifically with respect to the timing of the fertile phase.
Though objective, an analysis of standardized image values does not reveal whether differences in the R:G ratio are perceptually salient to rhesus macaques. However, utilizing information about the rhesus visual system, such as receptor spectral sensitivities and abundances, we can model changes in coloration in terms of rhesus vision, including the ability of the receiver to discriminate between color signals (Vorobyev and Osorio 1998; Stevens et al. 2009). Some studies of nonmammals have used such modeling techniques to determine, for example, songbird visibility to conspecifics and avian predators (Håstad et al. 2005), and whether cuckoo eggs are camouflaged (Langmore et al. 2009). However, as far as we are aware, no study of a mammalian color signal has used such methods to analyze and interpret signal variation (though Osorio et al. 2004 modeled fruit visual discriminability to primates). Further, though it is apparent that such modeling has a lot to offer in species with very different visual systems to humans (and to RGB cameras), the utility of such modeling in signal analysis of species that are also RGB trichromats remains unclear. In addition, most color studies of any taxa base their modeling on reflectance spectra, whereas the majority of studies of free-ranging animals utilize digital photography, partly due to the difficulty of obtaining spectra from live individuals (Stevens et al. 2009).
In the present study, we compare the analyses of Dubuc et al. (2009) using camera R:G sensor ratios, with those of modeled quantal catch values of the rhesus receptors, and discrimination threshold modeling determining how perceptually different signal variation is likely to appear to rhesus macaques. We do this for both color and luminance—2 crucial aspects of perception for discriminating visual signals (Osorio and Vorobyev 2005), aiming to: 1) demonstrate how visual signals can be analyzed with respect to a specific visual system, especially using field data collected by digital photography rather than with a spectrometer; 2) reassess the original analysis of Dubuc et al. (2009) using the R:G ratio of digital camera sensors, compared with the analogous ratio of rhesus macaque color receptors; 3) undertake visual discrimination modeling, to determine how color variation is likely to be perceived by the relevant receivers, rhesus macaques; and 4) determine whether such analyses offer new insight into the function of female rhesus macaque color variation and signals of fertility.
METHODS
Details on the study site and population, field data collection and hormonal analyses, are contained in Dubuc et al. (2009). Here, we give brief descriptions of these methods and then present our new modeling and analyses.
Study population and endocrine methods
Dubuc et al. (2009) studied free-ranging rhesus macaques on Cayo Santiago, Puerto Rico, and all data were collected between 22nd April and 12th July 2007. The study group (Group V) comprised 22 adult females (≥7 years old), 9 nulliparous females (3–5 years old), and 15–20 sexually active males (≥4 years old). Data presented in this paper are from 10 ovarian cycles (face images) and 8 ovarian cycles (hindquarter area images) from 8 parous adult females (average age: 9.3 years, range: 6–17). Fecal samples were collected from focal females directly after defecation and homogenized, with 0.5–2 g wet weight of feces collected on ice, and stored at −20 °C. Samples were analyzed at the German Primate Centre for progestogen metabolites using a validated (Shideler et al. 1990) pregnanediol glucuronide (PdG) microtiterplate enzymeimmunoassay. Sensitivity of the assay at 90% binding was 12.5 pg/well, and assay variation coefficients were: interassay variation, 10.6% (high, n = 37) and 14.9% (low, n = 37) and intra-assay variation, 7.2% (high, n = 16) and 9.4% (low, n = 16). Progestogen metabolite profiles were used to determine the most likely ovulation dates (the “ovulation window”). Ovulation was considered to have occurred when PdG concentrations rose above a threshold of the mean plus 2 standard deviations of 3–5 preceding baseline values, maintained for at least 3 consecutive samples (Jeffcoate 1983; Heistermann et al. 2001). Given a time lag of 24–56 h in hormone metabolite excretions in macaque feces (Wasser et al. 1994) and to account for oocyte life span (France 1981), Dubuc et al. (2009) defined the ovulation window as days −2 and −3 relative to the defined PdG rise (e.g., Heistermann et al. 2001) and set the last day of this window as day 0. The fertile phase was defined as a 5-day period including the 2-day ovulation window and the 3 days preceding it to account for sperm life span in the female tract (Wilcox et al. 1995). The 5 days preceding and the 5 days following the fertile phase were referred to as prefertile and postfertile phases.
Collection of digital color images and color R:G ratio
Dubuc et al. (2009) collected digital images of female faces and hindquarters, captured from 1–3 m away from subjects using a Canon EOS Digital Rebel XTi camera with a 10.1 megapixel CMOS censor and an EF28-135mm f/3.5-5.6 IS USM lens. Photographs were taken in RAW format to avoid lossy compression (Stevens et al. 2007) and converted to uncompressed 16-bit TIFF files for analysis. In order to standardize images for ambient light and camera settings, Dubuc et al. (2009) employed the “sequential method” (Higham 2006; Bergman and Beehner 2008; Higham et al. 2008; Stevens et al. 2009), in which a second photograph is taken of a color rendition chart (GretagMacbeth ColorChecker) immediately after the animal image, in the same location as the subject, using identical camera settings. Dubuc et al. (2009) used the in Camera plug-in (Pictocolor Corporation, Burnsville, MN, v. 4.0.1) for Adobe Photoshop (CS2, 9.0.1) (Bergman and Beehner 2008) to linearize and standardize image RGB values (Stevens et al. 2007). They measured RGB values for numerous points on the face and hindquarter using Jasc Paint Shop Pro 7 and from these calculated mean values for each image. In the present study, we use these values to produce R:G for the camera (as in Dubuc et al. 2009) as well as values of camera luminance ([R + G]/2).
Modeling receptor quantal catch
In the present analysis, we modeled the quantal catch of the rhesus macaque retinal receptors in response to the colors seen. We used the program ColourWorker (http://www.chrometrics.com) to determine the reflectance spectrum of the signal from the photographs as if it had been directly measured with a spectrometer. This technique estimates reflectance spectra from the RGB image values based on evidence that natural reflectance spectra are restricted to a limited number of types (Chiao et al. 2000). We combined the image of the animal with the corresponding image of the color chart to provide a standard within the image. In addition to this, the program requires samples of spectrometer-derived reflectance spectra, which should encompass the natural range of the signal being estimated. For this, we used samples of red signals from: long-tailed macaque (Macaca fascicularis), red uakari (Cacajao rubicundus), and Mandrill (Mandrillus sphinx) taken from http://vision.psychol.cam.ac.uk/spectra/. ColourWorker also came with a sample of human skin spectra, which correspond to the lower range of the signal. We obtained mean spectra from 10 reflectance spectra of the rhesus red areas from each image. We calculated the quantal catch of the rhesus longwave (LW), mediumwave (MW), and shortwave (SW) cones in response to the colors, using the reflectance spectra and a standard daylight “D65” irradiance spectrum using the following equation (Endler and Mielke 2005; Stevens et al. 2009):
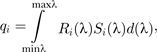 |
(1) |
where qi is the quantal catch of receptor type i, λ the wavelength, Si the spectral sensitivity of receptor i, and R(λ) is the spectrum of light entering the eye (a product of the reflectance and irradiance spectra or radiance directly measured). The code d(λ) indicates that values are integrated over the visible spectrum. For the rhesus LW and MW cones, we used microspectrophotometry data from Bowmaker et al. (1978). However, we were unable to obtain data on the spectral sensitivity of the rhesus SW cone, so we used data for the human SW cone (Dartnall et al. 1983). The error associated with this should be very minor because: 1) human and macaque peak receptor sensitivity is very similar, and the blue cone sensitivity changes little between trichromatic primates (e.g., Baylor et al. 1987; Schnapf et al. 1988; Osorio and Vorobyev 2008; though this does not necessarily mean that humans and macaques have similar color vision due to differences in the relative proportion of receptors; Knoblauch et al. 2006); 2) the LW and MW cones contribute to luminance perception in trichromatic primates (Osorio and Vorobyev 2005); 3) the SW cone is rare compared with the other 2 cone types, being low in number and absent from the fovea; and 4) the signal is red, not blue, and so does not reflect significantly at short wavelengths. It is worth noting, however, that such a substitution would be less appropriate in studies of color signals that are basically blue (e.g., the scrotal color of male vervet monkeys, Chlorocebus aethiops; Gerald 2001). Having calculated the receptor catches in response to each image, we used these data in a range of analyses (see below).
Threshold discrimination modeling
Using the quantal catch data of the rhesus receptors, we used a log form of the Vorobyev–Osorio receptor noise model (Vorobyev and Osorio 1998) to determine how different 2 colors are likely to appear to the rhesus visual system. The model uses data on the abundance of the different receptor types and estimates noise in the photoreceptors that limits the ability of the viewer to distinguish between 2 signals (Vorobyev and Osorio 1998). The model first calculates the difference in response for each receptor type for 2 different stimuli, as:
| (2) |
The model incorporates receptor noise, calculated for each cone type, as:
| (3) |
where wi is taken as a Weber fraction value and ni is the relative proportion of cone type i in the retinal integration area. Though there is interindividual variation in human cone ratios, on average, humans have a ratio of 1:16:32 for the SW, MW, and LW cones, respectively (Vorobyev and Osorio 1998). However, evidence indicates that macaques on average have a 1:1 ratio of LW to MW cones (e.g., Knoblauch et al. 2006; Jacobs and Deegan 1997), so we used cone abundance data in the proportion of 1:16:16. We used Weber fraction values of 0.08 for the SW cones and 0.02 for both the MW and the LW cones (Osorio and Vorobyev 1996; Osorio et al. 2004). For luminance modeling (see below), we used a Weber value of 0.08. We then used the following equation to determine the perceptual difference between 2 colors within the rhesus visual system (Vorobyev and Osorio 1998):
| (4) |
The units of the model (just noticeable differences [JNDs]) give values where numbers <1 mean that 2 signals cannot be discriminated, values approximately between 1–3 are discriminably different under good lighting conditions, and increasing values above 3 indicate that 2 signals are increasingly easy to tell apart, even as light levels deteriorate (Siddiqi et al. 2004). For the luminance (achromatic) signal, we used the following input: (LW + MW)/2, as luminance vision stems from a combination of the LW and MW cones in trichromatic primates (Osorio and Vorobyev 2005).
Data analysis
We used a total of 10 cycles for the face (2 cycles for 2 females, 1 cycle for 6 females) and 8 for the hindquarters (1 cycle per female) in analysis. A median of 12 images were available per 28-day ovarian cycle for facial skin (range: 10–15) and 11 for hindquarter skin (range: 7–14).
R:G ratios and luminance
Dubuc et al. (2009) performed general linear mixed models (GLMM) to determine whether R:G varied in such a way as to reveal information about the timing of the fertile phase, while controlling for multiple observations of the same females. The 5 days of the fertile phase were all numbered 0, the day directly preceding the fertile phase was labeled day −1, the day directly following it day 1, and so on (following Higham et al. 2008, 2009). To determine whether R:G reached highest values during the middle 5 days (the fertile phase), and as GLMMs test for linear relationships, this scale was squared (Higham et al. 2008, 2009). Here, we repeat these analyses using data from the period day −9 (5 days before the fertile period) through to day +5 (5 days after). We do this for the R:G values produced in Dubuc et al. 2009 (representing the camera sensor R:G values—CameraR:G), the R:G values produced in the present study (representing the rhesus retinal receptor LW:MW values—RhesusR:G), and for luminance values produced in the present study for the camera (CameraLuminance) and for rhesus (RhesusLuminance). Full models were: response—CameraR:G or RhesusR:G or CameraLuminance or RhesusLuminance, fixed effect (covariate)—day relative to the fertile period squared, and random effects—cycle number nested in female ID (face analyses) or female ID (hindquarter analyses). Composite profiles for Camera R:G, RhesusR:G, CameraLuminance, and RhesusLuminance were prepared, controlling for female baseline color by calculating each value for face and hindquarter color as percentages of that female’s maximum R:G or luminance score (occurring at any point during the study period). Mean values were then taken across all females for each day with respect to the date of ovulation (Dubuc et al. 2009).
Perceptual differences in color and luminance
The Vorobyev and Osorio (1998) model does not give absolute values that can be plotted and assessed independently but compares how different 2 stimuli are. As such, to obtain figures for color and luminance using this model, it is necessary to compare each image value with some other value. Our results based on quantal catch receptor values indicated that luminance was the important factor in determining the apparent changes in rhesus sex skin appearance (see below). Consequently, we compared each image's color and luminance values with the image containing that female's maximum luminance value, and measured all values with respect to this. This is very similar to controlling for female baseline values, as in the production of R:G and luminance plots above. As the model produces values that are by definition either perceptible (∼1 JND or more) or not (less than 1 JND) in some ways, it makes little sense to run statistics on these data. However, to present comparable analyses to those undertaken for R:G ratios and luminance, and to see whether JND values during the fertile period differed from the periods outside, we again undertook GLMMs. Full model structures were: response—JNDColor or JNDLuminance, fixed effect (covariate)—day relative to the fertile period squared, and random effects—cycle number nested in female ID (face analyses) or female ID (hindquarter analyses). To produce composite profiles across all females, we averaged across all female values for each day with respect to the day of ovulation.
We tested the distribution of all response variables tested in GLMMs. Kolmogorov–Smirnov analyses showed that none differed from a normal distribution (all tests P > 0.05) so that parametric tests were appropriate in all cases. All statistics were undertaken in SPSS 16.0.
RESULTS
R:G ratios and luminance
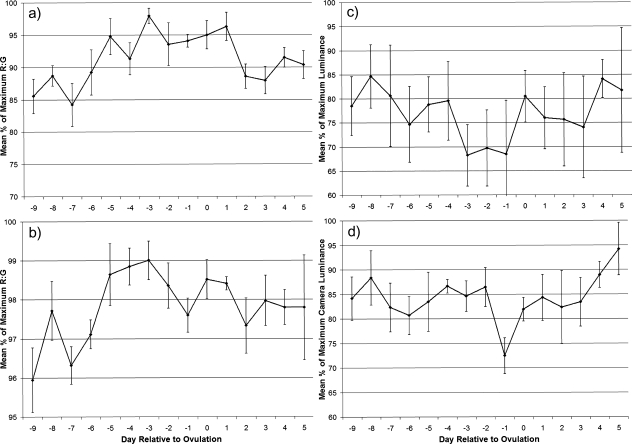
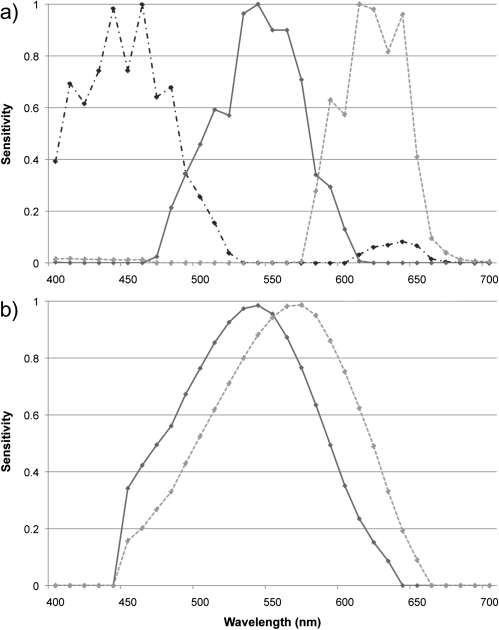
CameraR:G varied strongly with respect to the timing of the fertile phase for facial coloration (F1,64.4 = 13.82, P < 0.001; Figure 1a; Dubuc et al. 2009). RhesusR:G of facial color was less strongly related to the timing of the fertile phase but was nonetheless still significantly related to it (F1,65.8 = 7.41, P = 0.008; Figure 1b). RhesusLuminance was related to this timing (F1,64.7 = 5.68, P = 0.020; Figure 1c) but CameraLuminance was not (P > 0.1; Figure 1d). Supplementary Figure S1 shows an example average reflectance spectra for 1 set of female facial images, showing reflectance differences according to the female's fertile status. For the hindquarters, no measures were significantly related to the timing of the fertile phase (all P > 0.1, Supplementary Figure S2). Variation in CameraR:G is clearly much greater than that in RhesusR:G for both facial coloration (Figure 1a,b) and hindquarter coloration (Supplementary Figure S2a,b), with the low-level variation seen in RhesusR:G occurring over a very small range (note difference in y axis units for camera vs. rhesus plots for R:G). CameraLuminance showed the opposite relationship, being less variable than RhesusLuminance (Figure 1c,d). These differences are almost certainly due to the larger separation of spectral sensitivities of the LW and MW receptors of digital cameras compared with those of the rhesus macaque (Figure 2; see “DISCUSSION”).
Figure 1.
Composite facial skin color profiles throughout the ovarian cycle. Values represent the mean (±standard error of the mean) percentage of the maximum value reached for each cycle for: (a) CameraR:G (from Dubuc et al. 2009), (b) RhesusR:G, (c) RhesusLuminance, and (d) CameraLuminance.
Figure 2.
Spectral sensitivities of the MW and LW receptors for: (a) a Fuji digital camera (includes SW sensitivity) and (b) a rhesus macaque (data from Bowmaker et al. 1978). Note that the available data for rhesus macaques do not extend to wavelengths below 440 nm.
Perceptual differences in color and luminance
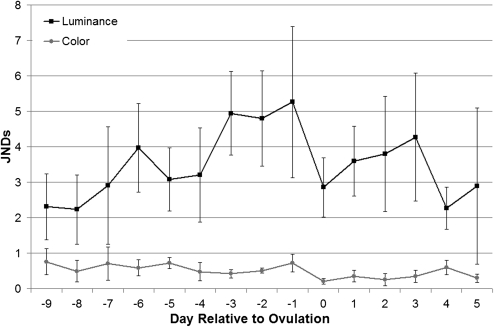
The chromatic differences in facial color during the fertile phase, which were highly significant in the above analyses, are probably imperceptible to rhesus macaques (JNDColor, F1,63.5 = 1.11, P = 0.295; Figure 3), being consistently less than 1 JND in variation from baseline. Despite camera measures showing no significant variation in luminance, there was significant variation in facial luminance as detectable by rhesus macaques that was linked specifically to the timing of the fertile phase (JNDLuminance, F1,67.4 = 4.43, P = 0.039; Figure 3). Facial luminance was on average 5 JNDs below baseline during the fertile phase (values are differences with respect to maximum luminance), which should be an easily perceived variation to rhesus macaques, even under less than optimal lighting conditions. This profile fluctuates through the 15-day period that includes the 5-day fertile phase and the 5 days on either side. For the 3 days before ovulation, female faces decrease in luminance by almost exactly 2 JNDs when compared with values either side (changing from a mean 3 to a mean 5 JNDs from maximum luminance). Further, once before the fertile phase (day −6) and once after fertile phase (day +3), there is a perceptible decrease in luminance when compared with values either side. These changes constituted either 1 JND (day +3) or 2 JNDs (day −6) decrease when compared with values 2 to 3 days earlier. There was little perceptible variation in hindquarter color (P > 0.1; Supplementary Figure S3), with total variation across all 15 days less than or around 1 JND from baseline. Though there was substantial variation in hindquarter luminance, this was unrelated to the timing of the fertile phase (P > 0.1; Supplementary Figure S3).
Figure 3.
Composite JND profiles. Values represent the mean (±standard error of the mean) difference per day relative to ovulation between the maximum luminance value reached for each cycle for face images.
DISCUSSION
Our study reveals that objective color scores can be misleading when the values are unrelated to the visual system of the relevant receiver(s) and highlights the importance of considering receiver perception when assessing animal signal content. There are a number of important points about color signal assessment and interpretation that arise from our analyses.
R:G color ratios and luminance
The clearest statistical results found in the present analysis involve the facial R:G ratio of the camera sensors—the original measure used in Dubuc et al. (2009), which varies highly significantly with respect to the timing of the fertile phase. Variation in this facial measure is far greater than that seen in the R:G ratio of the rhesus retinal receptors, whereas the opposite relationship was found for luminance. This discrepancy is likely to be because camera manufacturers routinely ensure even spacing of the sensors (optimal for capturing wide variation in color), whereas primate LW and MW receptors overlap significantly, partly because they arose from gene duplication (Osorio and Vorobyev 2008; Figure 2). This greater spectral separation increases variation when calculating a ratio of 2 sensors values but decreases variation when calculating a summation of the 2 values (as in trichromat primate luminance calculation); as the 2 sensors move further apart, they are more likely to have different values relative to each other in response to a color. Analogously, the larger overlap between trichromatic primate LW and MW cones, compared with those of birds, results in a larger red–green chromatic contrast for the avian compared with the primate visual system (Lovell et al. 2005). Osorio et al. (2004) argue that the large spectral overlap between the LW and MW cones in trichromatic primates might reflect a constraint in order to maintain a strong luminance signal; having widely spaced cones capturing widely different ranges of wavelengths might corrupt the luminance signal. However, possessing overlapping cones comes at the cost of suboptimal color detection (Osorio et al. 2004).
Our results highlight the danger of using objective color measures unrelated to perception because these can detect statistically significant variation that modeling suggests is nonetheless biologically irrelevant while not detecting variation that is relevant. Similarly, recent findings contradict the hypothesis that blue–green coloration of some birds’ eggs may function as a sexually selected signal of female quality because discrimination modeling indicates that most variation in egg coloration between clutches is not perceptible (Cassey et al. 2009). However, objective measures may be preferable to using human assessment, which suffers from high variability in inter and intra-observer reliability (Stevens and Cuthill 2005). Further, visual systems integrate different aspects of vision, such as color and luminance vision, rendering it difficult for human observers to recognize, for example, whether a signal is really varying in its chromatic or achromatic component. As such, objective measures may still be preferable if little information about the receiver's visual system is known (Stevens et al. 2007).
Perceptual differences in color and luminance
The finding that there were perceptual changes in facial luminance but not color during the fertile phase fits well with how changes in such signals occur in the rhesus macaque and other primates. Subtle changes in skin color that occur around the fertile phase but within the wider period of estrous reddening in female primates are caused by estrogen-related increased vascularization and blood flow (Dixson 1998). At the extreme levels of blood flow seen under such circumstances, these changes are unlikely to be related to a change in blood composition (e.g., a change in the proportion of oxygenated to deoxygenated blood) that might involve an actual color change but rather in the amount of blood (i.e., how saturated with blood the area is). The result of these changes appears to be an increased saturation of the same color (i.e., the color of well-oxygenated blood), which makes the area appear darker (reducing luminance).
Some studies have presented a visual validation of the use of the R:G ratio (as measured by digital cameras) to measure blood-related signals by showing that human observer rankings of color variation correlate with variation in R:G (e.g., Bergman and Beehner 2008). In the present study, camera R:G measures also correlated with observer impressions (Dubuc C, unpublished data). This may be because, as R:G varied (imperceptibly) with respect to the fertile period (probably related to slight changes in the proportion of oxygenated to deoxygenated blood associated with increased blood flow) and luminance varied perceptibly with respect to this timing (probably related to increased saturation of blood), a human observer could see variation in luminance that they would rate and that would correlate with variation in R:G (so “validating” the use of R:G as a measure). In addition, the correlation between the R:G and luminance measures may also stem from these 2 components coming from the same 2 inputs (R and G or LW and MW); whereas color is a ratio between the 2, luminance is the addition of these components. Despite this, variation in actual color in the present study would almost certainly be imperceptible to humans. For signals based on subtle changes in blood volume, luminance may be the most important measure of signal change. From the perspective of the rhesus visual system, it makes sense that the signal should be expressed primarily in luminance rather than color, as the large spectral overlap of the rhesus LW and MW cones makes them potentially better at detecting achromatic variation while being suboptimal for discriminating fine gradations in very similar colors (Osorio et al. 2004). In addition, although color measures such as R:G might be especially useful for describing color variation along a green–red axis (e.g., ripening fruit), they may not be well suited to describing variation in color where all colors observed are basically red and blood linked, as is often the case in primate sex skins (Dixson 1998) and in other types of animal signal, such as the gapes of bird nestlings (Kilner 1999).
Rhesus macaque visual signals of ovulation and the fertile phase
The central findings of Dubuc et al. (2009), that facial signal variation contains information about the timing of the fertile period but that hindquarter signal variation does not, are supported by our study. However, our analysis suggests that it is variation in rhesus female facial luminance, not color, that is biologically relevant. Remarkably, the mean changes in luminance that occur for the period of 3 days to 1 day before ovulation appear to be almost exactly in units of rhesus macaque visual perceptibility (e.g., moving from almost exactly 3 JNDs from baseline to almost exactly 5 JNDs from baseline and back again; Figure 3). This pattern is extremely clear and appears visually much more convincing that the plots produced by either set of R:G ratios. Changes in signal color variation specifically in units of JNDs as observed in our study are consistent with fine tuning of the signal to the visual system (or vice versa), a phenomenon that has been suggested before for primate color signals (Changizi et al. 2006).
One slightly puzzling result of our analysis is that the luminance signal lightens on the day of ovulation itself rather than, for example, the day after ovulation. There are several possible explanations for this: one is methodological. Our estimates of the timing of ovulation take the very end of a possible ovulation window as the most likely date of ovulation (see “METHODS”) and may skew our ovulation estimate to be slightly later than may actually be the case. Variation in luminance may actually be very well timed to the date of ovulation and indicate a 3-day peak conception window incorporating the day of ovulation and 2 days before, as in humans (Wilcox et al. 1995). Alternatively, rhesus macaques may be most fertile in the few days before ovulation, with little chance of conception once ovulation has occurred. If so, the key time for signaling fertility may be the few days before ovulation (see Behboodi et al. 1991 for evidence that this may be the case in macaques).
Our analysis also revealed apparent perceptible darkening and lightening periods a few days before and after the fertile period. The darkening seems nonrandom, moving almost exactly from 2 to 3 JNDs from baseline to almost exactly 4 JNDs from baseline (also notably almost exactly 1 JND less than the signal observed during the actual fertile period). Females are likely to be consorted by multiple males during an estrous period, and any signaling strategies that distribute both paternity confusion and assurance across these males are likely to be favored (Nunn 1999). By exhibiting changes in coloration signals that appear to darken and lighten again perceptibly in waves throughout an extended estrous period, females can present smaller scale signals to males consorting outside of the actual fertile period that may provide paternity confusion.
Conclusion
Assessing and presenting animal colors with respect to specific visual systems are still relatively uncommon. Though Sumner and Mollon (2003) presented primate skin and pelage colors in terms of primate receptor quantal catch response and opponency channels, our case study represents, to the best of our knowledge, the first to use visual discrimination threshold modeling to analyze variation in a color signal exhibited by a mammal species. Even for a species with similar trichromatic vision to humans, our analysis gave results that were more meaningful (as they were directly related to the perception of the receiver) and more directly related to the underlying mechanisms by which the color change occurs. They also confirmed other recent analyses suggesting that objective color scores may produce measures that are statistically significant but biologically irrelevant (Cassey et al. 2009). Further, they gave new insight into the function of these signals within the multimale multifemale promiscuous societies within which rhesus macaques live, revealing the presence of apparently perceptually meaningful decreases in signal luminance outside of the fertile period that may be involved in paternity confusion. It is important to note, however, that all results of perceptual modeling should be confirmed by experimental analysis. Another key advantage of discrimination modeling lies in the ability to compare how the same color signals are perceived by different receivers with different visual systems (e.g., Siddiqi et al. 2004). In primates, for example, many New World monkeys and lemur species exhibit color vision polymorphisms (Bradley and Mundy 2008), where different group members have a different number and combination of color receptors and therefore view the same colors differently. Furthermore, wild animal species often have strong selective pressures from predators that have very different visual systems to their own, in this case, trichromatic primates being mainly preyed upon by dichromatic mammalian carnivores and tetrachromatic raptors. Exploration of how color signals are viewed differently by conspecifics and heterospecifics with different visual systems is likely to produce valuable new insights into the varied selective pressures operating on many color signaling systems.
FUNDING
Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1) and Girton College, Cambridge (to M.S.); Natural Sciences and Engineering and the Social Sciences and Humanities, Research Councils of Canada, respectively, to L.J.N.B. and C.D. This publication was made possible by Grant Number CM-20-P40RR003640 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
Acknowledgments
We thank Petroc Sumner for reflectance spectra from http://vision.psychol.cam.ac.uk/spectra/ and Daniel Osorio for advice on modeling and using the program ColourWorker.
References
- Andersson S, Prager M. Quantifying colors. In: Hill GE, McGraw KJ, editors. Bird coloration, vol. I: mechanisms and measurements. Cambridge (MA): Harvard University Press; 2006. pp. 41–89. [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of the cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboodi E, Katz DF, Samuels SJ, Tell L, Hendrickx AG, Lasley BL. The use of a urinary estrone conjugates assay for detection of optimal mating time in the cynomolgus macaque (Macaca fascicularis) J Med Primatol. 1991;20:229–234. [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC. A simple method for measuring colour in wild animals: validation and use on chest patch colour in geladas (Theropithecus gelada) Biol J Linn Soc. 2008;94:231–240. [Google Scholar]
- Bowmaker JK, Dartnall HJA, Lythgoe JN, Mollon JD. The visual pigments of rods and cones in the rhesus monkey Macaca mulatta. J Physiol. 1978;274:329–348. doi: 10.1113/jphysiol.1978.sp012151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BJ, Mundy NI. The primate palette: the evolution of primate coloration. Evol Anthropol. 2008;17:97–111. [Google Scholar]
- Cassey P, Ewen JG, Marshall NJ, Vorobyev M, Blackburn TM, Hauber ME. Are avian eggshell colours effective intraspecific communication signals in the Muscicapoidea? A perceptual modelling approach. Ibis. 2009;151:689–698. [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. Bare skin, blood, and the evolution of primate color vision. Biol Lett. 2006;2:217–221. doi: 10.1098/rsbl.2006.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao CC, Osorio D, Vorobyev M, Cronin TW. Characterization of natural illuminants in forests and the use of digital video data to reconstruct illuminant spectra. J Opt Soc Am A. 2000;17:1713–1721. doi: 10.1364/josaa.17.001713. [DOI] [PubMed] [Google Scholar]
- Dartnall HJA, Bowmaker JK, Mollon JD. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B Biol Sci. 1983;220:115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Darwin C. Sexual selection in relation to monkeys. Nature. 1876;15:18–19. [Google Scholar]
- Davies NB, Brooke Mde L, Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc R Soc Lond B Biol Sci. 1996;263:925–931. [Google Scholar]
- Dixson AF. Primate sexuality: comparative studies of prosimians, monkeys, apes, and human beings. Oxford: Oxford University Press; 1998. [Google Scholar]
- Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. Sexual skin color contains information about the timing of the fertile phase in free-ranging rhesus macaques. Int J Primatol. 2009;30:777–789. [Google Scholar]
- Endler JA. On the measurement and classification of colour in studies of animal colour patterns. Biol J Linn Soc. 1990;41:315–352. [Google Scholar]
- Endler JA, Mielke PWJ. Comparing color patterns as birds see them. Biol J Linn Soc. 2005;86:405–431. [Google Scholar]
- France JT. Overview of the biological aspects of the fertile period. Int J Fertil. 1981;26:143–152. [PubMed] [Google Scholar]
- Gerald MS. Primate colour predicts social status and aggressive outcome. Anim Behav. 2001;61:559–566. [Google Scholar]
- Håstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Nat Acad Sci. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistermann M, Uhrigshardt J, Husung A, Kaumanns A, Hodges JK. Measurement of faecal steroid metabolites in the lion-tailed macaque (Macaca silenus): a non-invasive tool for assessing female ovarian function. Primate Rep. 2001;59:27–42. [Google Scholar]
- Higham JP. The reproductive ecology of female olive baboons (Papio hamadryas anubis) at Gashaka-Gumti National Park, Nigeria. London: Roehampton University; 2006. [Google Scholar]
- Higham JP. Primate coloration—an introduction to the special issue. Int J Primatol. 2009;30:749–751. [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: information content of size and color. Horm Behav. 2008;53:452–462. doi: 10.1016/j.yhbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Higham JP, Semple S, MacLarnon A, Heistermann M, Ross C. Female reproductive signaling, and male mating behavior, in the olive baboon. Horm Behav. 2009;55:60–67. doi: 10.1016/j.yhbeh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF., II Spectral sensitivity of macaque monkeys measured with ERG flicker photometry. Vis Neurosci. 1997;14:921–928. doi: 10.1017/s0952523800011639. [DOI] [PubMed] [Google Scholar]
- Jeffcoate SL. Use of rapid hormone assays in the prediction of ovulation. In: Jeffcoate SL, editor. Ovulation: methods for its prediction and detection. Chichester (UK): Wiley; 1983. pp. 67–82. [Google Scholar]
- Kilner RM. Family conflicts and the evolution of nestling mouth colour. Behaviour. 1999;136:779–804. [Google Scholar]
- Knoblauch K, Neitz M, Neitz J. An urn model of the development of L/M cone ratios in human and macaque retinas. Vis Neurosci. 2006;23:387–394. doi: 10.1017/S0952523806233157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore NE, Stevens M, Maurer G, Kilner RM. Are dark cuckoo eggs cryptic in host nests? Anim Behav. 2009;78:461–468. [Google Scholar]
- Lovell PG, Tolhurst DJ, Párraga CA, Baddeley R, Leonards U, Troscianko J, Troscianko T. Stability of the color-opponent signals under changes of illuminant in natural scenes. J Opt Soc Am. 2005;22:2060–2071. doi: 10.1364/josaa.22.002060. [DOI] [PubMed] [Google Scholar]
- Loyau A, Saint Jalme M, Cagniant C, Sorci G. Multiple sexual advertisements honestly reflect health status in peacocks (Pavo cristatus) Behav Ecol Sociobiol. 2005;58:552–557. [Google Scholar]
- Marty JS, Higham JP, Gadsby EL, Ross C. Dominance, coloration and social and sexual behavior in male drills (Mandrillus leucophaeus) Int J Primatol. 2009;30:807–823. [Google Scholar]
- Nunn C. The evolution of exaggerated sexual swellings in primates and the graded signal hypothesis. Anim Behav. 1999;58:229–246. doi: 10.1006/anbe.1999.1159. [DOI] [PubMed] [Google Scholar]
- Osorio D, Smith AC, Vorobyev M, Buchanan-Smith HM. Detection of fruit and the selection of primate visual pigments for color vision. Am Nat. 2004;164:696–708. doi: 10.1086/425332. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proc R Soc Lond B Biol Sci. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc Biol Sci. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. A review of the evolution of animal colour vision and visual communication signals. Vision Res. 2008;48:2042–2051. doi: 10.1016/j.visres.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Kraft TW, Nunn BJ, Baylor DA. Spectral sensitivity of primate photoreceptors. Vis Neurosci. 1988;1:255–261. doi: 10.1017/s0952523800001917. [DOI] [PubMed] [Google Scholar]
- Setchell JM. Do female mandrills prefer brightly colored males? Int J Primatol. 2005;26:715–734. [Google Scholar]
- Setchell JM, Charpentier MJ, Abbott KM, Wickings J, Knapp LA. Is brightest best? Testing the Hamilton-Zuk hypothesis in mandrills. Int J Primatol. 2009;30:825–844. [Google Scholar]
- Shideler SE, Munro CJ, Tell L, Owiti G, Laughlin L, Chatterton R, Lasley BL. The relationship of serum estradiol and progesterone concentrations to the enzyme immunoassay measurements of urinary estrone conjugates and immunoreactive pregnanediol-3-glucuronide in Macaca mulatta. Am J Primatol. 1990;22:113–122. doi: 10.1002/ajp.1350220205. [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- Stevens M, Cuthill IC. The unsuitability of html-based colour charts for estimating animal colours—a comment on Berggren & Merilä. Front Zool. 2005;2:14. doi: 10.1186/1742-9994-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill IC. Disruptive coloration, crypsis and edge detection in early visual processing. Proc R Soc Biol Sci. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Párraga A, Cuthill IC, Partridge JC, Troscianko T. Using digital photography to study animal coloration. Biol J Linn Soc. 2007;90:211–237. [Google Scholar]
- Stevens M, Stoddard MC, Higham JP. Studying primate color: towards visual system-dependent methods. Int J Primatol. 2009;30:893–917. [Google Scholar]
- Sumner P, Mollon JD. Colors of primate pelage and skin: objective assessment of conspicuousness. Am J Primatol. 2003;59:67–91. doi: 10.1002/ajp.10066. [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B Biol Sci. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SK, Monfort SL, Southers J, Wildt DE. Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces. J Reprod Fertil. 1994;101:213–220. doi: 10.1530/jrf.0.1010213. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation—effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–1522. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- Zuckerman S, van Wagenen G, Gardner RH. The sexual skin of the rhesus monkey. Proc Zool Soc Lond. 1938;108:385–401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.