At homeostasis, complex regulatory networks maintain the ongoing production of all blood cell types in a normal individual.1 During this process, very few HSCs are actively dividing and instead lie dormant, or quiescent, in the bone marrow. In response to injury to the hematopoietic system, such as from irradiation or chemotherapy, HSCs can temporarily exit their quiescent state and generate a larger pool of progenitor cells, thus increasing production of the needed mature blood cells. Some of the proteins that regulate of this choice between quiescence and proliferation in HSCs have already been identified.1,2 Two recent studies3,4 now expand on this small list of players by reporting that interferon (IFN)-α stimulates the turnover and proliferation of HSCs in vivo. This intriguing feedback mechanism between the immune system and the tip of the hematopoietic hierarchy may lead to a new strategy for leukemia treatment.
Type I interferons (IFN-α and -β) are secreted cytokines that are produced by a variety of immune and non-immune cells in response to microbial infections or recognition of tumor cells.5 Upon engaging their receptors, type I interferons induce of a set of genes that inhibit viral replication and clear infected cells. Because of their ability to regulate the immune system, recombinant type I interferons have been used clinically to treat viral infections, solid tumors, myeloproliferative disorders, hematopoietic neoplasms and autoimmune diseases such as multiple sclerosis. For most of these diseases, the mechanisms of action and the precise cellular targets of IFNs are still largely unknown, but IFN-α treatment of cancer cells generally results in growth arrest, by activating negative regulators of proliferation such as p53.6
Now Essers et al. and Sato et al. report a new role for IFN signaling within the most primitive cells of the hematopoietic system. The two groups came to similar conclusions starting from very different observations. In the course of using a mouse model commonly employed in the study of HSC function, Essers et al. found that high levels of IFNα induced HSC proliferation and further characterized this surprising observation. Sato et al. were studying the immune modulatory effects of IFNs and other cytokines on immune effector cells, when they found that mice lacking a component of the IFN signaling pathway had an unexpected imbalance of proliferation within the HSC pool. Both groups tracked their observations to a direct effect of IFNα on the proliferation state of HSCs.
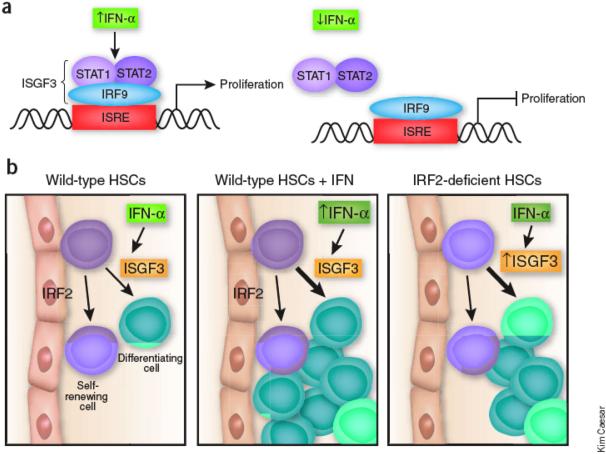
Sato et al. studied mice that were genetically deficient for a negative regulator of type I interferon signaling, interferon response factor 2 (IRF2). 4, 7 The investigators discovered that these mice had a greater proportion of HSCs that were proliferating instead of quiescent as would be found in normal mice. Chronic proliferation can impair HSC function, such as their ability to repopulate the bone marrow of irradiated mice.2 Sato et al. found that the IRF2-deficient HSCs were unable to restore hematopoiesis in irradiated mice, indicating that this cell population was no longer fully functional. However, if type I IFN signaling was disabled in the HSCs, however, the ability to repopulate the bone marrow could be restored in IRF2-deficient HSCs. This observation suggests that IRF2 normally suppresses IFN signaling in wild type HSCs, maintaining these cells mostly in a dormant state (Figure 1).
Figure 1. Role of low-level and induced IFNα signaling within HSCs.
A) Hypothetical target gene linking IFNα signaling to HSC proliferation. The upper panel illustrates IFNα-induced proliferation via the ISGF3 complex and the lower panel depicts endogenous level (uninduced) IFNα signaling in which IRF2 acts on the same hypothetical gene to constitutively supress expression. B) Outcome of HSC proliferation in the presence of endogenous IFNα signaling, induced IFNα signaling (↑IFNα) and in the IRF2 knockout. Arrows indicate decisions of the stem cell to divide to yield mostly progeny commmitting to differentiate (green cells) versus dividing to self-renew and maintain the stem cell pool (purple cells).
Furthermore, both groups found that high levels of IFN〈 directly induced wild-type HSCs to exit quiescence and transiently proliferate in vivo. Since most cell types stop proliferating in response to IFNα5, the wiring of this signaling pathway must be fundamentally different in HSCs. Signaling by type I IFNs activates a complex called interferon-stimulated gene factor 3 (ISGF3), which is composed of Stat1, Stat2 and IRF9 (Figure 1). IRF2 lacks the domain required for Stat protein interaction and so interferes with IFN signaling and IRF9-mediated transcription. Essers et al. found that Stat1 was required for the IFNα-mediated exit from dormancy, demonstrating that this unusual effect on proliferation was mediated by a canonical IFN signaling component. How IFNα signals are perceived differently in HSCs compared to other cell types is yet to be determined.
A remaining mystery from these studies is why an important anti-viral signal should influence HSC activity and hematopoiesis. The induced proliferation of HSCs could be a means to replace the immune cells turned over in the process of viral clearance. However, both groups show that one outcome of the enhanced HSC proliferation is ultimately the decreased production of mature cells. So, it is unlikely that this feedback mechanism is in place simply to amplify cells at the top of the hematopoietic hierarchy. One aspect not investigated by either group is whether the proliferating HSCs are mobilized and/or trafficked differently as compared to non-induced HSCs. Previous studies have illustrated that similar signals cause the preferential migration of HSCs into peripheral tissues, where they participate in immune regulation by differentiating into antigen presenting cells.8 Therefore, the HSC proliferation induced by IFNs may serve to generate immune effector cells from circulating HSCs for rapid deployment against infections or tumor cells.
In contrast, the potential clinical value of these findings is quite clear. IFNα has long been used as a therapy for cancer, particularly for chronic myelogenous leukemia (CML), and the discovery of its proliferative effect on quiescent HSCs provides an exciting new interpretation for recent patient data. Strikingly, a handful of CML patients that were first treated with IFNα and then switched to imatinib treatment – a molecularly targeted therapy directed against BCR-ABL, the hallmark of CML – experienced persistent remission, perhaps cure, upon drug discontinuation. In contrast, patients from the same study, and from other clinical cohorts, who achieved remission after imatinib treatment but were not previously treated with IFNα, often relapsed upon imatinib discontinuation.9,11 Persistent CML-initiating cells, or CML stem cells, which are protected from imatinib killing by their quiescent status are likely responsible for the re-growth of the disease.10 Thus, the emerging possibility to explain the stable remission in the patients previously treated with IFNα could be that the exposure to IFNα had induced the CML stem cells to exit quiescence and proliferate such that upon imatinib treatment, they have become vulnerable to the effect of imatinib rather than remaining protected. Although these results need to be confirmed in larger studies, they raise the tantalizing possibility of an effective targeting strategy for drug-resistant quiescent cancer stem cells in patients suffering from CML and other hematologic malignancies.11 These new insights into the role of IFN signaling in normal HSC quiescence may spark further interest in manipulating this pathway to kick quiescent leukemia stem cells into a more vulnerable state.
Footnotes
“Waking up the Sleeping Lion: Immune Regulation of Hematopoietic Stem Cell Proliferation”
Hematopoietic stem cells (HSCs) are predominantly found resting in the bone marrow microenvironment. Two recent studies identify interferon-α as an unexpected signal that has profound and direct effects on the turnover and proliferation state of HSCs. The findings hint at a new strategy for type I interferon treatment against hematopoietic cancers.
References
- 1.Wilson A, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 2.Jude CD, Gaudet JJ, Speck NA, Ernst P. Leukemia and hematopoietic stem cells: balancing proliferation and quiescence. Cell Cycle. 2008;7:586–91. doi: 10.4161/cc.7.5.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, et al. IRF-2 protects quiescent HSCs from type-I interferon-dependent exhaustion. Nature Medicine. 2009 doi: 10.1038/nm.1973. this issue. [DOI] [PubMed] [Google Scholar]
- 5.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 6.Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–23. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 7.Hida S, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13:643–55. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 8.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alimena G, et al. Imatinib mesylate therapy in chronic myeloid leukemia patients in stable complete cytogenic response after interferon-alpha results in a very high complete molecular response rate. Leuk Res. 2008;32:255–61. doi: 10.1016/j.leukres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105:1862–66. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- 11.Krause D, Van Etten RA. Bedside to bench: interfering with leukemic stem cells. Nature Medicine. 2008;14:495–495. doi: 10.1038/nm0508-494. [DOI] [PMC free article] [PubMed] [Google Scholar]