Abstract
Complex interactions between genes and environment result in a sodium-induced elevation in blood pressure (salt sensitivity) and/or hypertension that lead to significant morbidity and mortality affecting up to 25% of the middle-aged adult population worldwide. Determining the etiology of genetic and/or environmentally-induced high blood pressure has been difficult because of the many interacting systems involved. Two main pathways have been implicated as principal determinants of blood pressure since they are located in the kidney (the key organ responsible for blood pressure regulation), and have profound effects on sodium balance: the dopaminergic and renin-angiotensin systems. These systems counteract or modulate each other, in concert with a host of intracellular second messenger pathways to regulate sodium and water balance. In particular, the G protein-coupled receptor kinase type 4 (GRK4) appears to play a key role in regulating dopaminergic-mediated natriuresis. Constitutively activated GRK4 gene variants (R65L, A142V, and A486V), by themselves or by their interaction with other genes involved in blood pressure regulation, are associated with essential hypertension and/or salt-sensitive hypertension in several ethnic groups. GRK4γ 142V transgenic mice are hypertensive on normal salt intake while GRK4γ 486V transgenic mice develop hypertension only with an increase in salt intake. GRK4 gene variants have been shown to hyperphosphorylate, desensitize, and internalize two members of the dopamine receptor family, the D1 (D1R) and D3 (D3R) dopamine receptors, but also increase the expression of a key receptor of the renin-angiotensin system, the angiotensin type 1 receptor (AT1R). Knowledge of the numerous blood pressure regulatory pathways involving angiotensin and dopamine may provide new therapeutic approaches to the pharmacological regulation of sodium excretion and ultimately blood pressure control.
Keywords: dopamine, dopamine receptors, G protein-coupled receptor kinase 4, sodium transport, essential hypertension
1.1 Introduction
Dopamine is important in the regulation of sodium balance and blood pressure via renal mechanisms [1,2] The affinity of dopamine for its receptors is in the nanomolar range; higher concentrations occupy other GPCRs [1,2]. Circulating dopamine concentrations (picomolar range) are not sufficiently high to activate dopamine receptors, but high nanomolar concentrations can be attained in dopamine-producing tissues (e.g., renal proximal tubule, jejunum). Independent of innervation, renal proximal tubules synthesize dopamine that is not converted to norepinephrine [1,2]. Dietary sodium and intracellular sodium are the major determinants for the renal tubular synthesis/release of dopamine [3–9]; the stimulatory effect of increased dietary sodium on renal dopamine production is impaired in some hypertensive humans [10–12]. Locally generated dopamine, which is secreted preferentially into the renal tubular lumen, and acts in an autocrine/paracrine manner [1,2,13], is responsible for over 50% of incremental sodium excretion, especially when sodium intake is increased. The increase in renal sodium excretion due to dopamine is caused by inhibition of sodium transporter and pump activities, in the short-term, and a decrease in the expression of several sodium transporters, in the long-term. The inhibitory effect of dopamine on sodium pump activity is tissue/cell-specific. Indeed, in alveolar epithelial cells, dopamine stimulates rather than inhibits sodium channel and pump [14–16]. The short-term inhibition of sodium transport by dopamine involves interaction at caveolin-1 rich plasma membrane microdomains followed by their internalization, via scaffolding proteins [17–32]. The long-term inhibition of sodium transport by dopamine may involve the regulation of protein expression [33].
Dopamine can also affect sodium balance by regulating fluid and sodium intake via the “appetite” centers in the brain [34–36] and gastrointestinal transport [37]. Dopamine regulates the secretion/release of other hormones and humoral agents [38–44] that also regulate sodium balance and blood pressure (1). These hormones may interact with dopamine to increase (e.g., atrial natriuretic peptide [45], prolactin [46]) or decrease its inhibitory effect on sodium transport (e.g., angiotensin II [47–50], insulin [51,52]). Oxidative stress and inflammation also impair dopamine receptor function [53–58]. This article reviews the role of dopamine and dopamine receptor subtypes and their regulation by G protein-coupled receptor kinase (GRK4), with especial emphasis on GRK4 type 4 (GRK4), in essential hypertension.
2.1 Renal dopamine receptor subtypes
In mammals, dopamine exerts its actions via two receptor classes, D1-like and D2-like, that belong to the α group of the rhodopsin-like family of GPCRs[1,2,59]. The D1-like receptors, D1 (D1R) and D5 (D5R) subtypes (also called D1AR and D1BR in rodents), stimulate adenylyl cyclases [1,2,60]. The D1R, but not D5R, couples to GO [61]. In contrast, D5R, but not D1R, couples to Gz and Gα12/13 [62,63]. The D1-like receptors are also linked to Gαq [64–67]. The linkage of G protein subunits to the specific D1-like receptor is tissue-specific. In fibroblasts, the D1R couples to Gαq and phospholipase C [68]. More recently, the D5R has also been linked to stimulation of phospholipase C activity of neural tissue (hippocampus, cortex, and striatum) [69]. In neural (striatal) cells, D1R mediated-stimulation of phospholipase C requires the presence of D2R, while D5R, by itself, increases calcium mobilization that is inhibited by D2R [70]. However, in a pituitary adenoma rat cell line, GH4C1, transfected with the D5R, the D5R actually decreases inositol phosphate production [71]. Therefore, the linkage between D1R and D5R to phospholipase C activation is cell-specific.
The D2-like receptors, D2R, D3R, and D4R, couple to G-proteins Gαi and Go, inhibit adenylyl cyclase and calcium channel activities, and modulate potassium channel activity [1,2,60]. There are two isoforms of D2R; postsynaptic D2R effects are mediated by the long isoform, D2LR, while presynaptic D2R effects are mediated by the short isoform, D2SR [60]. There could be seven distinct alternatively spliced D3R variants. The full-length D3R and a shorter receptor isoform, the D3SR, bind to dopamine. There are five other alternatively spliced D3R variants that do not bind dopamine, including D3Rnf, but regulate receptor dimerization [72]. Different numbers of 16 amino acid repeats in the third cytoplasmic loop cause several human D4R isoforms (e.g., D4-2, D4-4, and D4-7) [73]. The role of these D4R isoforms remains to be determined. However, the D4R long (at least one 7 to 10 repeat) has been reported to be associated with higher diastolic and systolic blood pressure [74].
The D3R may also couple to Gαq in renal proximal tubule cells [75]. As stated above, the D1R and D2R heterodimer stimulates phospholipase C but the D2SR can stimulate phospholipase D, independent of D1R [76]; the latter enzyme is inhibited by D5R [57]. These effects need not negate each other because, as mentioned earlier, the D2SR is presynaptic, while the inhibition of phospholipapse D by D5R occurs in renal proximal tubule cells. The D4R may also regulate phospholipase C-coupled D1-like receptor action, e.g., D1-like receptor-mediated grooming [77].
All of the dopamine receptor subtypes are expressed in the renal tubule and renal vasculature. However, dopamine receptors are not distributed evenly along the mammalian nephron. All members of the dopamine receptor family are present in the renal proximal tubule. The medullary thick ascending limb of Henle expresses D1R, D3R, and D5R while the cortical thick ascending limb expresses D3R only. The distal convoluted tubule expresses D1R and D3R, while the collecting duct expresses all members of the dopamine receptor family except D2R [1,78,79].
Dopamine inhibits sodium transport at multiple sites along the renal tubule and acts on multiple targets (NHE1 [80], NHE3 [22,75,81,82], Na/PiIIa[24,31,83,84], Na+/HCO3− cotransporter [30], Cl−/HCO3− exchanger [85], Na+/K+ATPase [17–19,23,27,28,37,50,86–91], and probably NCC [92]. Dopamine, via the D4R, may also inhibit ENaC [93,94] and arginine vasopressin-dependent sodium transport and water permeability [94]. Dopamine stimulates NKCC2 in medullary thick ascending limb, but because Na+/K+ATPase is inhibited, overall transport is decreased [95]. There is tissue specific regulation of sodium transport by dopamine. For example, in pulmonary alveolar cells, dopamine stimulates Na+/K+ATPase [91], and D2LR stimulates Na+/K+ATPase in murine fibroblasts [96]. D1R and D2R, on the one hand, and Na+/K+ATPase, on the other, can also negatively regulate each other in HEK293T cells by direct protein-protein interaction [97]. While the inhibition of Na+/K+ATPase in the kidney by dopamine under conditions of NaCl excess is beneficial, inhibition of Na+/K+ATPase activity in neuronal cells by high concentrations of dopamine can lead to cell death [98]. Inhibition of Na+/K+ATPase activity in vascular smooth muscle cells would increase vascular resistance, as has been reported in the rat tail [99]. Low concentrations of dopamine, however, decreases systemic vascular resistance, probably by other mechanisms [100–102], e.g., opening of potassium channels [103] that is mediated by D5R but not D1R, at least in human coronary arteries [104].
The autocrine/paracrine regulation of renal tubular sodium transport, via D1-like receptors, is mediated by tubular and not by hemodynamic mechanisms [105–108]. Thus, systemically administered dopaminergic drugs may not mimic the autocrine/paracrine function of dopamine. However, D3R may regulate glomerular dynamics [109]. The quantitative contribution of a particular dopamine receptor subtype to renal sodium transport and glomerular dynamics has not been studied. However, the D1R is responsible for ≈80% of D1-like receptor activity in renal proximal tubules [110] while the D5R may be more important in the distal nephron [92,111]. Each of the dopamine receptor subtypes, alone, or via interaction with the other dopamine receptor subtypes or other GPCRs regulate sodium transport in a unique fashion [1,2,78]. Indeed, disruption of any of the dopamine receptor genes in mice results in hypertension, the pathogenesis of which is specific for each subtype [1,78].
3.1 Regulation of dopamine receptor function
As with other GPCRs, dopamine receptor signal transduction is regulated precisely [112–119]. Loss of receptor responsiveness (desensitization) is a mechanism that dampens short-term agonist effects following repeated agonist exposure. At least three families of regulatory molecules contribute to GPCR desensitization: second messenger-dependent protein kinases, GRKs, and arrestins [112–119]. Desensitization of GPCRs involves phosphorylation, sequestration/internalization, and degradation of receptors.
Homologous desensitization, in response to agonist stimulation, occurs via action of a member(s) of the GRK family [112–119]. Heterologous desensitization, mediated by second messenger-dependent kinases, occurs when a decrease in receptor responsiveness is induced by a ligand other than its own specific ligand. The phosphorylation of GPCRs, including the D1R, leads to the binding of a member(s) of the arrestin family, uncoupling of the receptor from its G protein complex, and a decrease in its functional response. The phosphorylated GPCR/β-arrestin complex undergoes endocytosis/internalization via clathrin-coated pits into a series of endosomal units, where the GPCR is dephosphorylated, and recycled back to the plasma membrane. The unrecycled GPCRs are degraded in proteasomes and/or lysosomes.
3.2 G protein-coupled receptor kinase (GRK) and dopamine receptors
There are seven GRKs in humans: GRKs 1 and 7 belong to the opsin kinase family, GRKs 2 and 3 belong to the β-adrenergic receptor kinase (βARK) family, and GRKs 4, 5, and 6 belong to the GRK4 family [116]. The tissue distribution of GRK4 is different from the other GRKs [117]. GRKs 1 and 7 are expressed in rods and cones, respectively. GRKs 2, 3, 5, and 6 are ubiquitously expressed while GRK4 is expressed to a greater extent in the testes and myometrium and to a lesser extent in specific brain areas [119], intestines [120], and the kidney [112,117].
3.3 GRK2 and GRK4 and renal D1R
The D1R (but not D5R), expressed endogenously in human [19,112,121] and rat renal proximal tubule cells [52,122,123], is regulated to a lesser extent by GRK2 and to a greater extent by GRK4 in human kidneys [121], but the converse may be true in rat kidneys [53, 123]. In a human embryonic kidney cell line (HEK293), overexpression of GRK3 also desensitizes the rat D1R [114]; a role for GRK5 in the desensitization of the rat D1R is not settled (113, 114). GRK6 is not be important in the regulation of D1R in the kidney [124] but it is important in the desensitization of the D1R in intestinal crypt cells [120], emphasizing the importance of cell type in D1R regulation.
3.4 GRK4 isoforms and renal dopamine receptors
GRK4 is constitutively active. This may be due to its ability to bind to inactive GαS and Gβ subunits [125]. Unlike the other GRKs, GRK4 has several splice variants. Four GRK4 (GRK4α, β, γ, and δ) splice variants have been reported in humans, five in rats, and one in mice [117,119,121,122,126–128]. Only the GRKα in humans, GRK4A in rats, and the only GRK4 reported in mice are closely homologous (approximately 70%) [119,126,127].
The GRK4 isoform that desensitizes D1R and D3R is cell-specific; GRK4γ in CHO and human renal proximal tubule cells [112,129]. GRK4α also desensitizes D1R in HEK-293 cells [113,114], and D3R in human renal proximal tubule cells [129]. There is also GRK4 isoform-specific regulation of other GPCRs. GRK4α desensitizes the metabotropic glutamate receptor [130], G protein-coupled calcium-sensing receptor [131], GABAB [132,133], luteinizing hormone/human chorionic gonadotropin receptor [119,134], FSH receptor [135], and mutant (Y326A) β2 adrenergic receptor [135].
GRK4α does not desensitize the angiotensin type 1 receptor (AT1R) [137], formyl peptide receptor [138], mGlu4 metabotropic glutamate receptor [139], mGlu5 metabotropic glutamate receptor [140], parathyroid hormone receptor [112,141], wild-type β2 adrenergic receptor [137,142], and m1, m2, m3, m4, and m5 muscarinic receptors [143]. GRK4α is also not linked to Gαq [144]. GRK4β desensitizes the luteinizing hormone/human chorionic gonadotropin receptor [139], and possibly the V2 vasopressin receptor [145]. GRK4δ, in the presence of GRK5 and GRK6, desensitizes the m2 muscarinic receptor [143] and luteinizing hormone/human chorionic gonadotropin receptor [119], but sensitizes the m3 muscarinic receptor [143]. GRK4δ does not desensitize D1R (unpublished data). As mentioned earlier, GRK4γ, especially its gene variants, desensitizes the D1R [112], and D3R [129], and only at high concentrations does GRK4γ minimally desensitize the luteinizing hormone/human chorionic gonadotropin receptor [119]. GRK4γ wild type does not desensitize the parathyroid hormone receptor [122], and AT1R but GRK4 142V and GRK4 486V may actually increase, directly or indirectly, AT1R expression and function [146,147]. GRK4 142V increases AT1R expression in mice on normal salt diet [146], while GRK4 486V increases AT1R expression in mice on high salt diet [147].
3.5 GRK regulation of dopamine receptors other than D1R (Table 1)
Table 1.
G protein-coupled receptor kinases involved in specific dopamine receptor signaling.
| Dopamine Receptor Subtype | G protein-coupled receptor kinase | References |
|---|---|---|
| D1R (in differentiated kidney cells) (in embryonic kidney cells) |
GRK2, GRK4 (GRK4α and GRK4γ in humans#, GRK4E in rats) GRK2, GRK3, GRK4α but not GRK4γ, GRK5* |
53, 112, 121–123 113, 114 |
| (in intestines but not kidney cells) | GRK6 | 120, 124 |
| D2R | GRK2, GRK3, GRK5, GRK6 | 73, 148, 149 |
| D3R (in kidney cells) | GRK2, GRK3, GRK4γ>GRK4α | 129, 151 |
| D4R | GRK2 or GRK3? | 73 |
| D5R | ? |
GRK5 increased agonist-dependent phosphorylation of rat D1R one report (114), but not in another report (113). GRK4α and GRK4γ desensitize the human D1R (112) while GRK4α but not GRKγ desensitizes the rat D1R (113).
?unknown or not definite
The D2R is regulated by GRK2, GRK3, GRK5, and GRK6 [148,149], with D2SR affected to a greater extent than D2LR [73]. However, GRK2 or GRK3, but not GRK5 or GRK6, is involved in the desensitization of the calcium signal mediated by D1R/D2R interaction [150]. The D3R is regulated by GRK2, GRK3 [151], and GRK4 (GRK4γ>GRK4α) [129]. The GRK regulating D4R is not clear but does not seem to involve either GRK2 or GRK3 [73]. The GRK regulating D5R is also not clear but does not seem to involve GRK4 [47]. These studies show that the GRK regulation of dopamine receptor subtypes is GRK isoform-specific.
3.6 GRK and sodium transporters
GRK2 decreases the degradation of ENaC [152,153]. GRK2 and GRK3 phosphorylate and may aid in the internalization of Na+K+ ATPase [154]. It is unclear how this effect of GRK2 on D1R desensitization and decreased internalization of Na+K+ ATPase is modulated [17–19,23,27,28,37,50,86–91]. NKCC1 colocalizes with GRK3 in rodent olfactory epithelia, but its regulation by GRK3 has not been demonstrated [155].
4.1 GRK4 and essential hypertension
Hypertension is the most expensive disease in the USA. It affects 73 million Americans, causes 50% of heart diseases and 75% of strokes, and costs in excess of $69 billion in 2008. Hypertension affects a third of middle-aged adults, but the prevalence is higher (65%) in individuals above 60 years of age [156,157]. About 30% to 50% of essential hypertension is thought to be heritable, but the genetic causes of essential hypertension have been difficult to identify [158]. More than one gene is undoubtedly involved, because Mendelian dominant and recessive traits are not readily discernible in hypertensive subjects, except in those with monogenic forms of hypertension. Indeed, recent genome-wide association studies (GWAS) have been able to identify only 2% of genetic factors believed to influence blood pressure [159–164]. However, the GWAS were not designed to identify predisposing genes engaged in a complex network of gene-gene and gene/environment interactions [165], e.g., the genes (or factors) underlying salt sensitivity, a dietary sodium-induced increase in blood pressure that may or may not be in the hypertensive range.
Several criteria have been suggested to link gene(s) to complex disorders such as salt sensitivity and hypertension, but the definitive evidence is provided by swapping one phenotype for another (i.e., transgenic studies) [166]. Many genes have been proposed to be causal of hypertension. Their gene variants, including those identified in the GWAS, however, have not been shown to produce hypertension in mice. Furthermore, gene overexpression and deletion studies performed in mice must take into account the salt sensitivity of the strain. C57BL/6 mice from Jackson Laboratories have an impaired ability to excrete a NaCl load which results in an increase in blood pressure when their salt intake is increased; others are salt-resistant (e.g., SJL mice) [167]. We have reported recently that the renal D1-like receptor function is impaired in salt-sensitive C57BL/6 Jackson mice. Renal GRK4 expression is increased in salt-loaded C57BL/6 Jackson mice [167]. Deletion of Grk4 in C57BL/6 mice prevents the development of salt-sensitive hypertension [168]. Renal D1-like receptor function is also impaired in the spontaneously hypertensive rat (SHR), a strain with increased expression of GRK4E. Renal cortical silencing of GRK4 attenuates the increase in blood pressure with age in the SHR but not in normotensive Wistar-Kyoto rats whose blood pressures minimally increase with age [121].
The GRK4 locus on human chromosome 4p16.3 is linked to the increase in blood pressure from childhood to adulthood [169] and to hypertension in adults [170]. Interestingly, adolescents with GRK4 65L/142V/A486 haplotype have a greater increase in blood pressure with age than those with the wild-type GRK4 haplotype [171]. We have reported [172–174] with subsequent confirmation by others [175,176] that GRK4 gene variants (65L, 142V, and 486V) are associated with essential hypertension in several ethnic groups: Caucasians, Chinese, Ghanaians, and Japanese. In salt-sensitive hypertensive Japanese the presence of three GRK4 variants impaired the natriuretic effect of a dopaminergic drug and predicted salt-sensitive hypertension correctly in 94% of cases [174]. In Ghanaians, multilocus genotype combinations of angiotensin-converting enzyme insertion/deletion, and GRK4 65L had an estimated predictive accuracy for hypertension of 70% [173], confirming an earlier study [177].
A meta-analysis revealed a significant association of GRK4 486V with hypertension, with an odds ratio of 1.5 (95% CI: 1.2 to 1.9) [117]. One study however, did not find an association of GRK4 486V with the top fifth percentile of diastolic blood pressure of subjects with white European ancestry [178]. However, the authors did not test the association of GRK4 gene variants with hypertension [178]. Another study did not find an association between GRK4 142V and hypertension but did find an association between variants of the promoter region of D1R and hypertension [179]. The discordance between this report in European Caucasians [179] and other reports involving other populations may be a result of the influence of genetic background in the phenotypic expression of a quantitative trait essential hypertension. Interestingly, low renin hypertension is less frequent in the Caucasian (15–20%) [180] than in other populations (40–60% in Japanese) [181]. In our Japanese study, the single best genetic model for low-renin hypertension included only GRK4 A142V, by itself, or GRK4 A142V and CYP11B2, with an estimated predictive accuracy of 78% [174]. Ethnicity may also explain some of the discordances. GRK4 65L and GRK4 142V are less frequent while GRK4 486V is more frequent in Asians than in African-Americans. GRK4 486V is also more frequent in Hispanic and non-Hispanic whites than in African-Americans [182]. Recent GWAS did not identify GRK4 as associated with hypertension [158–164]. This is probably because salt sensitivity and gene-gene interaction were not taken into account. Previous studies have shown that it was critical to assess the association of GRK4 with hypertension, in conjunction with other GRK4 SNPs [174] and genes, e.g., ACE with GRK4 65L [173,177], ADRB2, TH, and GRK4 486V [176]. GRK4 A142V and GRK4 A486V are, moreover, not included in the Affymetrix or Illumina platforms, respectively.
Early in the process of D1R [20,86,183,184] and D3R stimulation [129], D1R and D3R increase their respective activities, in part, by the recruitment of intracellular D1R and D3R to the plasma membrane. This recruitment of D1R and D3R to the plasma membrane requires the presence of GRK4γ wild-type [129,184]. However, as indicated above [117], sustained D1R and D3R stimulation results in desensitization caused by their phosphorylation and internalization. Resensitization occurs by receptor dephosphorylation, caused by protein phosphatase 2A in D1R [183], and recycling to the plasma membrane. Sorting nexins also help in the recycling of GPCRs to the plasma membrane. The GRK4γ wild-type (but not GRK4α wild-type) desensitizes the AT1R and decreases AT1R expression in the kidney [146,147]. Therefore, GRK4 wild-type is necessary for D1R and D3R [129,184] to exert their renal autocrine/paracrine natriuretic function, in part by inhibiting the antinatriuretic effect of AT1R [146,147]. However, GRK4 gene variants constitutively modify, phosphorylate, and internalize D1R [112] and presumably the D3R also, preventing their recycling to the plasma membrane. GRK4 gene variants also increase AT1R expression in mice. This involves GRK4γ 142V on normal salt diet and by GRK4γ486V on high salt diet [146,147]. While GRK4γ 142V transgenic mice are hypertensive even on a normal salt diet [112,146,185], GRK4γ 486V transgenic mice develop hypertension only when stressed by a high salt diet [147,186]. Depending upon the genetic background of the mouse, overexpression of human GRK4γ wild-type converts a salt-sensitive phenotype to a salt-resistant phenotype, while overexpression of human GRK4γ 486V converts a salt-resistant phenotype to a salt-sensitive phenotype [146,186]. These phenotype changes, related to differential actions of human GRK4γ variants and their regulation of D1R and other GPCRs, could be taken as evidence of the “apparent polygenicity” of hypertension.
GRK4γ 65L transgenic mice are normotensive on a normal salt diet (unpublished data) but whether or not some form of stress is needed for the hypertensive phenotype to develop is not known [173,177]. It is known however, that adolescent African-Americans expressing GRK4 65L, when exposed to mental stress, respond with an increase in blood pressure and a decrease in sodium excretion [187].
4.2 Role of other GRKs in hypertension
GRK activity and GRK2 expression are increased in lymphocytes of patients with essential hypertension and SHRs [188]. Overexpression of GRK2 in vascular smooth muscle in mice produces hypertension and impairs the vasodilatory action of β-adrenoceptors [189]. The vasoconstrictor response to angiotensin II is also impaired in these mice, which is at odds with the increased reactivity and sensitivity to angiotensin II in essential hypertension [190]. Interestingly, GRK2 activates the epithelial sodium channel by phosphorylating the C terminus of its β subunit, making it insensitive to the inactivating effects of ubiquitin protein ligases Nedd4 and Nedd2 [191]. Although GRK2 polymorphisms have not been associated with human essential hypertension, increased renal expression of GRK2, which is increased with aging [87], in the insulin/obesity/metabolic syndrome [52,58,123], and by oxidative stress [32,53,58], impairs D1R function in rats. More importantly, increased GRK2 expression (but not GRK5) has been reported in lymphocytes of African-Americans with hypertension [192]. GRK5 overexpression in vascular smooth muscle cells in mice also increases blood pressure. The hypertension in male GRK5 transgenic mice is caused, in part, by decreased β1-adrenergic receptor activity, whereas the high blood pressure in female mice is caused, in part, by increased AT1R activity [193]. The increase in GRK5 expression in hypertension may be secondary not primary; angiotensin II-induced GRK5 up-regulation in the rat aorta may be due to hypertension per se [194].
5.1 GRK4 and pharmacogenomics in essential hypertension
GRK4 polymorphisms may provide predictive pharmacogenetic insight into therapeutic antihypertensive strategies. In hypertensive African- Americans, the GRK4 65L/A142 haplotype is predictive of a poor response to β-adrenergic blockade [195]. Our preliminary studies in hypertensive Japanese suggest that the absolute decrease in blood pressure in response to angiotensin receptor blockers (ARBs) is associated with GRK4 142V [196]. (Interestingly, ARBs also normalize the blood pressure of GRK4γ 142V transgenic mice [146].) The addition of a diuretic to the non-responders of ARBs decreased blood pressure in hypertensive Japanese with the GRK4 486V gene variant. These studies suggest that the pharmacogenetics of GRK4 can be important in guiding the therapy for hypertension.
6.1 Summary
In summary, there is GPCR specificity of GRK4, especially the human GRK4γ isoform, in the regulation of human D1R and D3R (Figure 1). The human GRK4 locus is linked to hypertension and the human GRK4 gene variants, either alone or in conjunction with variants of other genes, are associated with essential hypertension. The ability of humans with salt-sensitive essential hypertension to excrete a chronic sodium load is inversely correlated with the number of human GRK4 allelic variants. Therefore, salt sensitivity may be imparted by the GRK4 gene variants, and this effect seems to be dependent on the number of allelic variants present. Human GRK4γ 142V transgenic mice are hypertensive even on a normal sodium intake while human GRK4γ 486V transgenic mice develop hypertension only when given a high salt diet. Additional genes contribute to the predictive value of GRK4 single nucleotide polymorphisms for salt sensitivity and hypertension, suggesting that epistasis is responsible for the etiology of this complex polygenic disorder. GRK4 gene variants may not only be predictive of hypertension phenotypes (e.g., salt sensitivity, low plasma renin) but may also predict response to antihypertensive drugs.
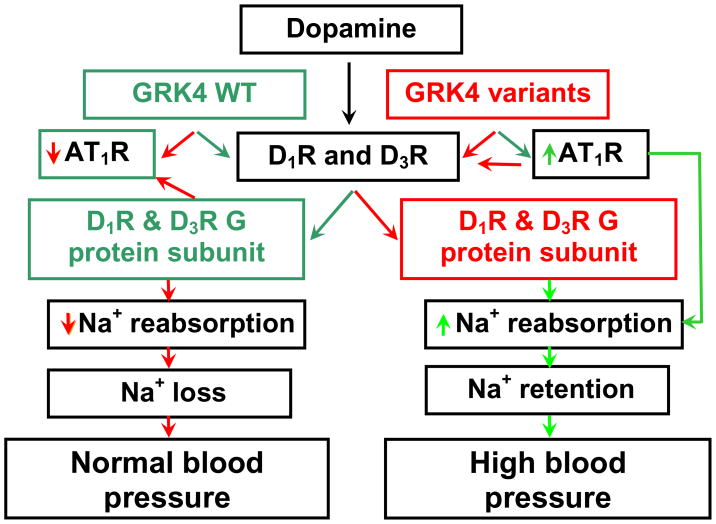
Figure 1.
GRK4 and renal dopamine and angiotensin type 1 receptor interaction During conditions of moderately increased NaCl intake, the renal D1R is stimulated by dopamine produced in the kidney. The D1R or D3R, whose coupling to G protein subunits is regulated by G protein-coupled receptor kinase type 4 (GRK4), inhibits sodium reabsorption in several nephron segments. This results in an increase in sodium excretion and maintenance of normal blood pressure. GRK4 wild-type (GRK4 WT) also negatively regulates AT1R transcription. The decrease in AT1R expression, caused by GRK4 WT, facilitates the inhibitory effect of D1R on renal sodium transport. In essential hypertension, constitutively active variants of GRK4 not only uncouple D1R and D3R from G protein subunits, but also increase AT1R transcription in the kidney. These effects impair the ability of the kidney to excrete the excess sodium load, resulting in sodium retention, and ultimately hypertension.
Green = normal coupling of D1R and D3R to G protein subunits, Red = uncoupling of D1R and D3R from G protein subunits
Green arrows = stimulatory, Red arrows = inhibitory
Acknowledgments
This work was supported in part by grants from the National Institutes of Health, USA (P01HL074940, P01HL068686, R01HL092196, R37HL023081, and R01DK039308) and from the Children’s Research Institute, Children National Medical Center, Washington, DC, USA
Abbreviations
- AT1R
angiotensin type 1 receptor
- D1R
D1 dopamine receptor
- D2R
D2 dopamine receptor
- D3R
D3 dopamine receptor
- D4R
D4 dopamine receptor
- D5R
D5 dopamine receptor
- ENaC
epithelial sodium channel
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- GRK2
G protein-coupled receptor kinase type 2
- GRK3
G protein-coupled receptor kinase type 3
- GRK4
G protein-coupled receptor kinase type 4
- GRK5
G protein-coupled receptor kinase type 5
- GRK6
G protein-coupled receptor kinase type 6
- GWAS
genome-wide association studies
- Na/PiIIa
sodium phosphate cotransporter type Iia
- NCC
sodium chloride cotransporter
- NHE1
sodium hydrogen exchanger type 1
- NHE3
sodium hydrogen exchanger type 3
- NKCC2
sodium potassium 2 chloride cotransporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–69. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–42. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RW, Gill JR, Jr, Yamabe H, Lovenberg W, Keiser HR. Effects of dietary sodium and of acute saline infusion on the interrelationship between dopamine excretion and adrenergic activity in man. J Clin Invest. 1974;54:194–200. doi: 10.1172/JCI107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey RM, VanLoon GR, Baines AD, Ortt EM. Decreased plasma and urinary dopamine during dietary sodium depletion in man. J Clin Endocrinol Metab. 1981;52:903–9. doi: 10.1210/jcem-52-5-903. [DOI] [PubMed] [Google Scholar]
- 5.Maurel A, Spreux-Varoquaux O, Amenta F, Tayebati SK, Tomassoni D, Seguelas MH, Parini A, Pizzinat N. Vesicular monoamine transporter 1 mediates dopamine secretion in rat proximal tubular cells. Am J Physiol Renal Physiol. 2007;292:F1592–8. doi: 10.1152/ajprenal.00514.2006. [DOI] [PubMed] [Google Scholar]
- 6.Pinho MJ, Serrão MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2007;292:F1452–63. doi: 10.1152/ajprenal.00465.2006. [DOI] [PubMed] [Google Scholar]
- 7.Silva E, Gomes P, Soares-da-Silva P. Increases in transepithelial vectorial Na+ transport facilitates Na+-dependent L-DOPA transport in renal OK cells. Life Sci. 2006;79:723–9. doi: 10.1016/j.lfs.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Choi MR, Correa AH, del Valle Turco V, Garcia FA, Fernández BE. Angiotensin II regulates extraneuronal dopamine uptake in the kidney. Nephron Physiol. 2006;104:136–43. doi: 10.1159/000095856. [DOI] [PubMed] [Google Scholar]
- 9.Chen N, Reith ME. Interaction between dopamine and its transporter: role of intracellular sodium ions and membrane potential. J Neurochem. 2004;89:750–65. doi: 10.1111/j.1471-4159.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 10.Damasceno A, Santos A, Serrão P, Caupers P, Soares-da-Silva P, Polónia J. Deficiency of renal dopaminergic-dependent natriuretic response to acute sodium load in black salt-sensitive subjects in contrast to salt-resistant subjects. J Hypertens. 1999;17:1995–2001. doi: 10.1097/00004872-199917121-00033. [DOI] [PubMed] [Google Scholar]
- 11.Sowers JR, Zemel MB, Zemel P, Beck FW, Walsh MF, Zawada ET. Salt sensitivity in blacks. Salt intake and natriuretic substances. Hypertension. 1988;12:485–90. doi: 10.1161/01.hyp.12.5.485. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira A, Bettencourt P, Pestana M, Correia F, Serrão P, Martins L, Cerqueira-Gomes M, Soares-Da-Silva P. Heart failure, aging, and renal synthesis of dopamine. Am J Kidney Dis. 2001;38:502–9. doi: 10.1053/ajkd.2001.26834. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z-Q, Siragy HM, Felder RA, Carey RM. Intrarenal dopamine production and distribution in the rat: physiological control of sodium excretion. Hypertension. 1997;29:228–34. doi: 10.1161/01.hyp.29.1.228. [DOI] [PubMed] [Google Scholar]
- 14.Helms MN, Self J, Bao HF, Job LC, Jain L, Eaton DC. Dopamine activates amiloride-sensitive sodium channels in alveolar type I cells in lung slice preparations. Am J Physiol Lung Cell Mol Physiol. 2006;291:L610–8. doi: 10.1152/ajplung.00426.2005. [DOI] [PubMed] [Google Scholar]
- 15.Bertorello AM, Komarova Y, Smith K, Leibiger IB, Efendiev R, Pedemonte CH, Borisy G, Sznajder JI. Analysis of Na+, K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell. 2003;14:1149–57. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281:L79–85. doi: 10.1152/ajplung.2001.281.1.L79. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Leibiger I, Katz AI, Bertorello AM. Pals-associated tight junction protein functionally links dopamine and angiotensin II to the regulation of sodium transport in renal epithelial cells. Br J Pharmacol. 2009;158:486–9. doi: 10.1111/j.1476-5381.2009.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol. 2008;295:F1117–25. doi: 10.1152/ajprenal.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gildea JJ, Israel JA, Johnson AK, Zhang J, Jose PA, Felder RA. Caveolin-1 and dopamine-mediated internalization of NaKATPase in human renal proximal tubule cells. Hypertension. 2009;54:1010–6. doi: 10.1161/HYPERTENSIONAHA.109.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse MS, Adachi S, Scott L, Holtbäck U, Greengard P, Aperia A, Brismar H. Recruitment of renal dopamine 1 receptors requires an intact microtubulin network. Pflugers Arch. 2003;445:534–9. doi: 10.1007/s00424-002-0899-5. [DOI] [PubMed] [Google Scholar]
- 21.Bacic D, Capuano P, Baum M, Zhang J, Stange G, Biber J, Kaissling B, Moe OW, Wagner CA, Murer H. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol. 2005;288:F740–7. doi: 10.1152/ajprenal.00380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64:2133–41. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khundmiri SJ, Lederer E. PTH and DA regulate Na-K ATPase through divergent pathways. Am J Physiol Renal Physiol. 2002;282:F512–2. doi: 10.1152/ajprenal.00111.2000. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham R, Biswas R, Brazie M, Steplock D, Shenolikar S, Weinman EJ. Signaling pathways utilized by PTH and dopamine to inhibit phosphate transport in mouse renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;296:F355–61. doi: 10.1152/ajprenal.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaral JS, Pinho MJ, Soares-da-Silva P. Regulation of amino acid transporters in the rat remnant kidney. Nephrol Dial Transplant. 2009;24:2058–67. doi: 10.1093/ndt/gfn752. [DOI] [PubMed] [Google Scholar]
- 26.Lanaspa MA, Giral H, Breusegem SY, Halaihel N, Baile G, Catalán J, Carrodeguas JA, Barry NP, Levi M, Sorribas V. Interaction of MAP17 with NHERF3/4 induces translocation of the renal Na/Pi IIa transporter to the trans-Golgi. Am J Physiol Renal Physiol. 2007;292:F230–42. doi: 10.1152/ajprenal.00075.2006. [DOI] [PubMed] [Google Scholar]
- 27.Efendiev R, Chen Z, Krmar RT, Uhles S, Katz AI, Pedemonte CH, Bertorello AM. The 14-3-3 protein translates the NA+, K+-ATPaseα1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem. 2005;280:16272–7. doi: 10.1074/jbc.M500486200. [DOI] [PubMed] [Google Scholar]
- 28.Gomes P, Soares-da-Silva P. Dopamine-induced inhibition of Na+-K+-ATPase activity requires integrity of actin cytoskeleton in opossum kidney cells. Acta Physiol Scand. 2002;175:93–10. doi: 10.1046/j.1365-201X.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JA, Li L, Sun D. The collecting duct, dopamine and vasopressin-dependent hypertension. Acta Physiol Scand. 2000;168:239–4. doi: 10.1046/j.1365-201x.2000.00631.x. [DOI] [PubMed] [Google Scholar]
- 30.Kunimi M, Seki G, Hara C, Taniguchi S, Uwatoko S, Goto A, Kimura S, Fujita T. Dopamine inhibits renal Na+:HCO3- cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000;57:534–43. doi: 10.1046/j.1523-1755.2000.00873.x. [DOI] [PubMed] [Google Scholar]
- 31.Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells? J Am Soc Nephrol. 1998;9:1604–12. doi: 10.1681/ASN.V991604. [DOI] [PubMed] [Google Scholar]
- 32.Banday AA, Lokhandwala MF. Inhibition of natriuretic factors increases blood pressure in rats. Am J Physiol Renal Physiol. 2009;297:F397–402. doi: 10.1152/ajprenal.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Armando I, Luo Y, Pascua A, Villar VA, Asico L, Jones JE, Escano CS, Friedman PA, Jose PA. Dopamine D3 receptors directly regulate NHE3 in renal proximal tubules. J Am Soc Nephrol. 2007;18:597A. [Google Scholar]
- 34.Cocores JA, Gold MS. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med Hypotheses. 2009;73:892–9. doi: 10.1016/j.mehy.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Roitman MF, Schafe GE, Thiele TE, Bernstein IL. Dopamine and sodium appetite: Antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci. 1997;111:606–611. doi: 10.1037//0735-7044.111.3.606. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22(2 Suppl):12–9. doi: 10.1177/0269216307087148. [DOI] [PubMed] [Google Scholar]
- 37.Lucas-Teixeira VA, Hussain T, Serrão P, Soares-da-Silva P, Lokhandwala MF. Intestinal dopaminergic activity in obese and lean Zucker rats: response to high salt intake. Clin Exp Hypertens. 2002;24:383–96. doi: 10.1081/ceh-120004799. [DOI] [PubMed] [Google Scholar]
- 38.Saveanu A, Jaquet P, Brue T, Barlier A. Relevance of coexpression of somatostatin and dopamine D2 receptors in pituitary adenomas. Mol Cell Endocrinol. 2008;286:206–13. doi: 10.1016/j.mce.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Cote TE, Felder R, Kebabian JW, Sekura RD, Reisine T, Affolter HU. D-2 dopamine receptor-mediated inhibition of pro-opiomelanocortin synthesis in rat intermediate lobe. Abolition by pertussis toxin or activators of adenylate cyclase. J Biol Chem. 1986;261:4555–61. [PubMed] [Google Scholar]
- 40.Schteingart DE. Drugs in the medical treatment of Cushing’s syndrome. Expert Opin Emerg Drugs. 2009;14:661–71. doi: 10.1517/14728210903413522. [DOI] [PubMed] [Google Scholar]
- 41.Kok P, Roelfsema F, Frölich M, van Pelt J, Meinders AE, Pijl H. Bromocriptine reduces augmented thyrotropin secretion in obese premenopausal women. J Clin Endocrinol Metab. 2009;94:1176–81. doi: 10.1210/jc.2008-2303. [DOI] [PubMed] [Google Scholar]
- 42.van den Buuse M. Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin Exp Pharmacol Physiol. 1998;25:661–8. doi: 10.1111/j.1440-1681.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 43.Sowers JR, Viosca SP, Windsor C, Korenman SG. Influence of dopaminergic mechanisms on 24-hour secretory patterns of prolactin, luteinizing hormone and testosterone in recumbent men. J Endocrinol Invest. 1983;6:9–15. doi: 10.1007/BF03350554. [DOI] [PubMed] [Google Scholar]
- 44.Carey RM, Sen S. Recent progress in the control of aldosterone secretion. Recent Prog Horm Res. 1986;42:251–96. doi: 10.1016/b978-0-12-571142-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 45.Correa AH, Choi MR, Gironacci M, Aprile F, Fernández BE. Atrial natriuretic factor decreases renal dopamine turnover and catabolism without modifying its release. Regul Pept. 2008;146:238–42. doi: 10.1016/j.regpep.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 46.Ibarra F, Crambert S, Eklöf AC, Lundquist A, Hansell P, Holtbäck U. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005;68:1700–7. doi: 10.1111/j.1523-1755.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 47.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–92. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 48.Zeng C, Asico LD, Wang X, Hopfer U, Eisner GM, Felder RA, Jose PA. Angiotensin II regulation of AT1 and D3 dopamine receptors in renal proximal tubule cells of SHR. Hypertension. 2003;41:724–9. doi: 10.1161/01.HYP.0000047880.78462.0E. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–9. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan F, Spicarová Z, Zelenin S, Holtbäck U, Scott L, Aperia A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;295:F1110–6. doi: 10.1152/ajprenal.90336.2008. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Cui Z, He D, Ren H, Han Y, Yu C, Fu C, Wang Z, Yang C, Wang X, Zhou L, Asico LD, Villar VA, Hopfer U, Mi M, Zeng C, Jose PA. Insulin increases D5 dopamine receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats. Am J Hypertens. 2009;22:770–6. doi: 10.1038/ajh.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banday AA, Fazili FR, Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol. 2007;293:F877–84. doi: 10.1152/ajprenal.00184.2007. [DOI] [PubMed] [Google Scholar]
- 53.Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-Gq/11alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol. 2007;293:F306–15. doi: 10.1152/ajprenal.00108.2007. [DOI] [PubMed] [Google Scholar]
- 54.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–7. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 55.Abílio VC, Silva RH, Carvalho RC, Grassl C, Calzavara MB, Registro S, D’Almeida V, Ribeiro Rde A, Frussa-Filho R. Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology. 2004;47:263–72. doi: 10.1016/j.neuropharm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–8. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 57.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 58.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54:2219–26. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- 59.Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005;142:94–101. doi: 10.1016/j.ygcen.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47:1117–34. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 61.Kimura K, White BH, Sidhu A. Coupling of human D-1 dopamine receptors to different guanine nucleotide binding proteins: evidence that D-1 dopamine receptors can couple to both Gs and Go. J Biol Chem. 1995;270:14672–8. doi: 10.1074/jbc.270.24.14672. [DOI] [PubMed] [Google Scholar]
- 62.Sidhu A, Kimura K, Uh M, White BH, Patel S. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J Neurochem. 1998;70:2459–67. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- 63.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–10. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 64.Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–5. [PubMed] [Google Scholar]
- 65.Jin LQ, Wang HY, Friedman E. Stimulated D1 dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–90. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 66.Vyas SJ, Eichberg J, Lokhandwala MF. Characterization of receptors involved in dopamine-induced activation of phospholipase-C in rat renal cortex. J Pharmacol Exp Ther. 1992;260:134–9. [PubMed] [Google Scholar]
- 67.Liu J, Wang F, Huang C, Long LH, Wu WN, Cai F, Wang JH, Ma LQ, Chen JG. Activation of phosphatidylinositol-linked novel D1 dopamine receptor contributes to the calcium mobilization in cultured rat prefrontal cortical astrocytes. Cell Mol Neurobiol. 2009;29:317–28. doi: 10.1007/s10571-008-9323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu PY, Eisner GM, Yamaguchi I, Mouradian MM, Felder RA, Jose PA. Dopamine D1A receptor regulation of phospholipase C isoform. J Biol Chem. 1996;271:19503–8. doi: 10.1074/jbc.271.32.19503. [DOI] [PubMed] [Google Scholar]
- 69.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–53. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O’Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75:843–54. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White BH, Kimura K, Sidhu A. Inhibition of hormonally induced inositol trisphosphate production in transfected GH4C1 cells: A novel role for the D5 subtype of the dopamine receptor. Neuroendocrinology. 1999;69:209–16. doi: 10.1159/000054421. [DOI] [PubMed] [Google Scholar]
- 72.Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–7. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun. 2006;350:634–4. doi: 10.1016/j.bbrc.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 74.Sen S, Nesse R, Sheng L, Stoltenberg SF, Gleiberman L, Burmeister M, Weder AB. Association between a dopamine-4 receptor polymorphism and blood pressure. Am J Hypertens. 2005;18:1206–10. doi: 10.1016/j.amjhyper.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Pedrosa R, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Gialpha3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC-PKC-mediated event. Am J Physiol Renal Physiol. 2004;287:F1059–66. doi: 10.1152/ajprenal.00139.2004. [DOI] [PubMed] [Google Scholar]
- 76.Senogles SE. D2s dopamine receptor mediates phospholipase D and antiproliferation. Mol Cell Endocrinol. 2003;209:61–9. doi: 10.1016/j.mce.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 77.O’Sullivan GJ, Kinsella A, Grandy DK, Tighe O, Croke DT, Waddington JL. Ethological resolution of behavioral topography and D2-like vs. D1-like agonist responses in congenic D4 dopamine receptor “knockouts”: identification of D4:D1-like interactions. Synapse. 2006;59:107–18. doi: 10.1002/syn.20225. [DOI] [PubMed] [Google Scholar]
- 78.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–50. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 79.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–46. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 80.Lin CY, Varma MG, Joubel A, Madabushi S, Lichtarge O, Barber DL. Conserved motifs in somatostatin, D2-dopamine, and alpha 2B-adrenergic receptors for inhibiting the Na-H exchanger, NHE1. J Biol Chem. 2003;278:15128–35. doi: 10.1074/jbc.M212315200. [DOI] [PubMed] [Google Scholar]
- 81.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol. 2005;289:F249–58. doi: 10.1152/ajprenal.00082.2004. [DOI] [PubMed] [Google Scholar]
- 82.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1064–73. doi: 10.1152/ajpregu.2000.278.4.R1064. [DOI] [PubMed] [Google Scholar]
- 83.Glahn RP, Onsgard MJ, Tyce GM, Chinnow SL, Knox FG, Dousa TP. Autocrine/paracrine regulation of renal Na+-phosphate cotransport by dopamine. Am J Physiol. 1993;264:F618–22. doi: 10.1152/ajprenal.1993.264.4.F618. [DOI] [PubMed] [Google Scholar]
- 84.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol. 2003;285:F1233–43. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 85.Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3- exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–6. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- 86.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA. 1998;95:5573–8. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asghar M, Kansra V, Hussain T, Lokhandwala MF. Hyperphosphorylation of Na-pump contributes to defective renal dopamine response in old rats. J Am Soc Nephrol. 2001;12:226–32. doi: 10.1681/ASN.V122226. [DOI] [PubMed] [Google Scholar]
- 88.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na, K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol. 2005;25:322–7. doi: 10.1016/j.semnephrol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension. 1998;32:1049–53. doi: 10.1161/01.hyp.32.6.1049. [DOI] [PubMed] [Google Scholar]
- 90.Satoh T, Ominato M, Katz AI. Different mechanisms of renal Na-K-ATPase regulation by dopamine in the proximal and distal nephron. Hypertens Res. 1995 Jun;18(Suppl 1):S137–40. doi: 10.1291/hypres.18.supplementi_s137. [DOI] [PubMed] [Google Scholar]
- 91.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol. 2005;33:432–7. doi: 10.1165/rcmb.2005-0297TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Escano CS, Asico L, Li H, Jones JE, Armando I, Jose PA. Up-regulation of renal sodium transporters in distal tubules are preserved in D5 deficient mice treated with losartan. Hypertension. 2008;52:E36. (Abstract) [Google Scholar]
- 93.Saito O, Ando Y, Kusano E, Asano Y. Functional characterization of basolateral and luminal dopamine receptors in rabbit CCD. Am J Physiol Renal Physiol. 2001;281:F114–22. doi: 10.1152/ajprenal.2001.281.1.F114. [DOI] [PubMed] [Google Scholar]
- 94.Sun D, Schafer JA. Dopamine inhibits AVP-dependent Na+ transport and water permeability in rat CCD via a D4-like receptor. Am J Physiol. 1996;271:F391–400. doi: 10.1152/ajprenal.1996.271.2.F391. [DOI] [PubMed] [Google Scholar]
- 95.Aoki Y, Albrecht FE, Bergman KR, Jose PA. Stimulation of Na+-K+-2Cl- cotransport in rat medullary thick ascending limb by dopamine. Am J Physiol. 1996;271:R1561–7. doi: 10.1152/ajpregu.1996.271.6.R1561. [DOI] [PubMed] [Google Scholar]
- 96.Yamaguchi I, Walk SF, Jose PA, Felder RA. Dopamine D2L receptors stimulate Na+/K+-ATPase activity in murine LTK- cells. Mol Pharmacol. 1996;49:373–8. [PubMed] [Google Scholar]
- 97.Hazelwood LA, Free RB, Cabrera DM, Skinbjerg M, Sibley DR. Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase. J Biol Chem. 2008;283:36441–53. doi: 10.1074/jbc.M805520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bagh MB, Maiti AK, Jana S, Banerjee K, Roy A, Chakrabarti S. Quinone and oxyradical scavenging properties of N-acetylcysteine prevent dopamine mediated inhibition of Na+, K+-ATPase and mitochondrial electron transport chain activity in rat brain: implications in the neuroprotective therapy of Parkinson’s disease. Free Radic Res. 2008;42:574–81. doi: 10.1080/10715760802158430. [DOI] [PubMed] [Google Scholar]
- 99.Rashed SM, Songu-Mize E. Regulation of Na+-pump activity by dopamine in rat tail arteries. Eur J Pharmacol. 1995;284:289–97. doi: 10.1016/0014-2999(95)00363-p. [DOI] [PubMed] [Google Scholar]
- 100.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–9. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- 101.Okamura T, Toda N. Comparison of the effect of dopamine in primate arteries and veins. Hypertens Res. 1995;18 (Suppl 1):S35–7. doi: 10.1291/hypres.18.supplementi_s35. [DOI] [PubMed] [Google Scholar]
- 102.Polakowski JS, Segreti JA, Cox BF, Hsieh GC, Kolasa T, Moreland RB, Brioni JD. Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats. Clin Exp Pharmacol Physiol. 2004;31:837–41. doi: 10.1111/j.1440-1681.2004.04095.x. [DOI] [PubMed] [Google Scholar]
- 103.Han G, Kryman JP, McMillin PJ, White RE, Carrier GO. A novel transduction mechanism mediating dopamine-induced vascular relaxation: opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J Cardiovasc Pharmacol. 1999;34:619–27. doi: 10.1097/00005344-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Natarajan A, Han G, Chen SY, Yu P, White R, Jose P. The D5 dopamine receptor mediates large-conductance, calcium- and voltage-activated potassium channel activation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2010;332:640–9. doi: 10.1124/jpet.109.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol. 1989;257:F469–77. doi: 10.1152/ajprenal.1989.257.3.F469. [DOI] [PubMed] [Google Scholar]
- 106.Hegde SS, Jadhav AL, Lokhandwala MF. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension. 1989;13:828–34. doi: 10.1161/01.hyp.13.6.828. [DOI] [PubMed] [Google Scholar]
- 107.Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension. 1990;15:560–9. doi: 10.1161/01.hyp.15.6.560. [DOI] [PubMed] [Google Scholar]
- 108.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–8. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 109.Luippold G, Schneider S, Vallon V, Osswald H, Mühlbauer B. Postglomerular vasoconstriction induced by dopamine D3 receptor activation in anesthetized rats. Am J Physiol Renal Physiol. 2000;278:F570–5. doi: 10.1152/ajprenal.2000.278.4.F570. [DOI] [PubMed] [Google Scholar]
- 110.Sanada H, Xu J, Watanabe H, Jose P, Felder R. Differential expression and regulation of dopamine-1 (D-1) and dopamine-5 (D-5) receptor function in human kidney. Am J Hypertens. 2000;13:156A. (Abstract) [Google Scholar]
- 111.Yao LP, Huque E, Baraniuk JN, Felder RA, Carey RM, Jose PA. Dopamine-1 receptor subtype (D1A and D1B) expression in microdissected rat nephron segments. Pediatr Res. 1997;41:286A. (Abstract) [Google Scholar]
- 112.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–7. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–69. doi: 10.1124/mol.105.019901. [DOI] [PubMed] [Google Scholar]
- 114.Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J Biol Chem. 1996;271:3771–8. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- 115.Gardner B, Liu ZF, Jiang D, Sibley DR. The role of phosphorylation/dephosphorylation in agonist-induced desensitization of D1 dopamine receptor function: evidence for a novel pathway for receptor dephosphorylation. Mol Pharmacol. 2001;59:310–21. doi: 10.1124/mol.59.2.310. [DOI] [PubMed] [Google Scholar]
- 116.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 117.Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, Jose PA. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension. 2008;51:1449–55. doi: 10.1161/HYPERTENSIONAHA.107.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pao CS, Benovic JL. Phosphorylation-independent desensitization of G protein-coupled receptors? Sci STKE. 2002;2002(153):PE42. doi: 10.1126/stke.2002.153.pe42. [DOI] [PubMed] [Google Scholar]
- 119.Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1996;271:6403–10. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 120.Fraga S, Jose PA, Soares-da-Silva P. Involvement of G protein-coupled receptor kinase 4 and 6 in rapid desensitization of dopamine D1 receptor in rat IEC-6 intestinal epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2004;287:R772–9. doi: 10.1152/ajpregu.00208.2004. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–8. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 122.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–9. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 123.Trivedi M, Lokhandwala MF. Rosiglitazone restores renal D1A receptor-Gs protein coupling by reducing receptor hyperphosphorylation in obese rats. Am J Physiol Renal Physiol. 2005;289:F298–304. doi: 10.1152/ajprenal.00362.2004. [DOI] [PubMed] [Google Scholar]
- 124.Xu J, Watanabe H, Felder RA, Jose PA. GRK6 in the kidney in human and rat genetic hypertension. FASEB J. 2001;15:A774. [Google Scholar]
- 125.Keever LB, Jones JE, Andresen BT. G protein-coupled receptor kinase 4γ interacts with inactive Gαs and Gα13. Biochem Biophys Res Commun. 2008;367:649–55. doi: 10.1016/j.bbrc.2007.12.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Premont RT, Macrae AD, Aparicio SAJR, Kendall HE, Welch JE, Lefkowitz RJ. The GRK4 subfamily of G protein-coupled receptor kinases: alternative splicing, gene organization and sequence conservation. J Biol Chem. 1999;274:29381–9. doi: 10.1074/jbc.274.41.29381. [DOI] [PubMed] [Google Scholar]
- 127.Virlon B, Firsov D, Cheval L, Reiter E, Troispoux C, Guillou F, Elalouf JM. Rat G protein-coupled receptor kinase GRK4: identification, functional expression, and differential tissue distribution of two splice variants. Endocrinology. 1998;139:2784–95. doi: 10.1210/endo.139.6.6078. [DOI] [PubMed] [Google Scholar]
- 128.Sallese M, Mariggiò S, Collodel G, Moretti E, Piomboni P, Baccetti B, De Blasi A. G protein-coupled receptor kinase GRK4. Molecular analysis of the four isoforms and ultrastructural localization in spermatozoa and germinal cells. J Biol Chem. 1997;272:10188–9. doi: 10.1074/jbc.272.15.10188. [DOI] [PubMed] [Google Scholar]
- 129.Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, Keever L, Arnaldo FB, Wang Z, Luo Y, Felder RA, Jose PA. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–34. doi: 10.1074/jbc.M109.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggio S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and β-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–42. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- 131.Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, Quarles LD. Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol. 2005;19:1078–87. doi: 10.1210/me.2004-0450. [DOI] [PubMed] [Google Scholar]
- 132.Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M. Phosphorylation-independent desensitization of GABAB receptor by GRK4. EMBO J. 2003;22:3816–24. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kanaide M, Uezono Y, Matsumoto M, Hojo M, Ando Y, Sudo Y, Sumikawa K, Taniyama K. Desensitization of GABAB receptor signaling by formation of protein complexes of GABAB2 subunit with GRK4 or GRK5. J Cell Physiol. 2007;210:237–45. doi: 10.1002/jcp.20863. [DOI] [PubMed] [Google Scholar]
- 134.Munshi UM, Peegel H, Menon KM. Palmitoylation of the luteinizing hormone/human chorionic gonadotropin receptor regulates receptor interaction with the arrestin-mediated internalization pathway. Eur J Biochem. 2001;268:1631–9. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- 135.Lazari MF, Liu X, Nakamura K, Benovic JL, Ascoli M. Role of G protein-coupled receptor kinases on the agonist-induced phosphorylation and internalization of the follitropin receptor. Mol Endocrinol. 1999;13:866–78. doi: 10.1210/mend.13.6.0289. [DOI] [PubMed] [Google Scholar]
- 136.Menard L, Ferguson SS, Barak LS, Bertrand L, Premont RT, Colapietro AM, Lefkowitz RJ, Caron MG. Members of the G protein-coupled receptor kinase family that phosphorylate the β2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35:4155–60. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- 137.Oppermann M, Diverse-Pierluissi M, Drazner MH, Dyer SL, Freedman NJ, Peppel KC, Lefkowitz RJ. Monoclonal antibodies reveal receptor specificity among G-protein-coupled receptor kinases. Proc Natl Acad Sci USA. 1996;93:7649–54. doi: 10.1073/pnas.93.15.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rane MJ, Prossnitz ER, Arthur JM, Ward RA, McLeish KR. Deficient homologous desensitization of formyl peptide receptors stably expressed in undifferentiated HL-60 cells. Biochem Pharmacol. 2000;60:179–87. doi: 10.1016/s0006-2952(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 139.Iacovelli L, Capobianco L, Iula M, Di Giorgi Gerevini V, Picascia A, Blahos J, Melchiorri D, Nicoletti F, De Blasi A. Regulation of mGlu4 metabotropic glutamate receptor signaling by type-2 G-protein coupled receptor kinase (GRK2) Mol Pharmacol. 2004;65:1103–10. doi: 10.1124/mol.65.5.1103. [DOI] [PubMed] [Google Scholar]
- 140.Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 141.Flannery PJ, Spurney RF. Domains of the parathyroid hormone (PTH) receptor required for regulation by G protein-coupled receptor kinases (GRKs) Biochem Pharmacol. 2001;62:1047–58. doi: 10.1016/s0006-2952(01)00749-3. [DOI] [PubMed] [Google Scholar]
- 142.Simon V, Robin MT, Legrand C, Cohen-Tannoudji J. Endogenous G protein-coupled receptor kinase 6 triggers homologous β-adrenergic receptor desensitization in primary uterine smooth muscle cells. Endocrinology. 2003;144:3058–66. doi: 10.1210/en.2002-0138. [DOI] [PubMed] [Google Scholar]
- 143.Tsuga H, Okuno E, Kameyama K, Haga T. Sequestration of human muscarinic acetylcholine receptor hm1-hm5 subtypes: effect of G protein-coupled receptor kinases GRK2, GRK4, GRK5 and GRK6. J Pharmacol Exp Ther. 1998;284:1218–26. [PubMed] [Google Scholar]
- 144.Picascia A, Capobianco L, Iacovelli L. A De Blasi Analysis of differential modulatory activities of GRK2 and GRK4 on Gαq-coupled receptor signaling. Methods Enzymol. 2004;390:337–53. doi: 10.1016/S0076-6879(04)90021-3. [DOI] [PubMed] [Google Scholar]
- 145.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol. 2004;286:F216–224. doi: 10.1152/ajprenal.00307.2003. [DOI] [PubMed] [Google Scholar]
- 146.Wang Z, Chen S, Asico LD, Escano C, Villar VM, Lu Q, Zeng C, Jones JE, Armando I, Felder RA, Jose PA. AT1R dysregulation is crucial in the hypertension of human GRK4γ-142V transgenic mice. 2009 Experimental Biology meeting abstracts; D478 802, FASEB J. 2009;23:802.7. (Abstract) [Google Scholar]
- 147.Wang Z, Asico L, Wang X, Escano C, Jose P. Human G protein-coupled receptor kinase type 4γ (GRK4γ) 486V-promoted salt sensitivity in transgenic mice is related with increased AT1 receptor (AT1R) J Am Soc Nephrol. 2007;18:148A. (Abstract) [Google Scholar]
- 148.Namkung Y, Dipace C, Javitch JA, Sibley DR. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284:15038–51. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ito K, Haga T, Lameh J, Sadée W. Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem. 1999;260:112–9. doi: 10.1046/j.1432-1327.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- 150.So CH, Verma V, O’Dowd BF, George SR. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol Pharmacol. 2007;72:450–62. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]
- 151.Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-beta-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–80. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- 152.Sanchez-Perez A, Kumar S, Cook DI. GRK2 interacts with and phosphorylates Nedd4 and Nedd4-2. Biochem Biophys Res Commun. 2007;359:611–5. doi: 10.1016/j.bbrc.2007.05.134. [DOI] [PubMed] [Google Scholar]
- 153.Arthur JW, Sanchez-Perez A, Cook DI. Scoring of predicted GRK2 phosphorylation sites in Nedd4-2. Bioinformatics. 2006;22:2192–5. doi: 10.1093/bioinformatics/btl381. [DOI] [PubMed] [Google Scholar]
- 154.Kimura T, Allen PB, Nairn AC, Caplan MJ. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell. 2007;18:4508–18. doi: 10.1091/mbc.E06-08-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Menco BP. The fine-structural distribution of G-protein receptor kinase 3, beta-arrestin-2, Ca2+/calmodulin-dependent protein kinase II and phosphodiesterase PDE1C2, and a Cl−-cotransporter in rodent olfactory epithelia. J Neurocytol. 2005;34:11–36. doi: 10.1007/s11068-005-5045-9. [DOI] [PubMed] [Google Scholar]
- 156.Stason WB. Hypertension: a policy perspective, 1976–2008. J Am Soc Hypertens. 2009;3:113–8. doi: 10.1016/j.jash.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 157.Sander GE. High blood pressure in the geriatric population: treatment considerations. Am J Geriatr Cardiol. 2002;11:223–32. doi: 10.1111/j.1076-7460.2002.00032.x. [DOI] [PubMed] [Google Scholar]
- 158.Harrison M, Maresso K, Broeckel U. Genetic determinants of hypertension: an update. Curr Hypertens Rep. 2008;10:488–95. doi: 10.1007/s11906-008-0091-1. [DOI] [PubMed] [Google Scholar]
- 159.Harrap SB. Blood pressure genetics: time to focus. J Am Soc Hypertens. 2009;3:231–7. doi: 10.1016/j.jash.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 160.Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:667–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, Zhou J, Lashley K, Chen Y, Christman M, Rotimi C. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5:e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, Shi X, Pan L, Rampersaud E, Shen H, Kim JD, Subramanya AR, Steinle NI, Parsa A, Ober CC, Welling PA, Chakravarti A, Weder AB, Cooper RS, Mitchell BD, Shuldiner AR, Chang YP. From the Cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA. 2009;106:226–31. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–34. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 165.Moore JH, Williams SM. New strategies for identifying gene-gene interactions in hypertension. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- 166.Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–9. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 167.Escano CS, Armando I, Wang X, Asico L, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009 Sep; doi: 10.1152/ajpregu.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Armando I, Jones JE, Escano C, Asico L, Premont RT, Jose PA. [P-194] Deletion of the GRK4 gene decreases blood pressure and reverses salt sensitivity in mice. Meeting; New Orleans, LA: American Society of Hypertension; 2008. [Google Scholar]
- 169.Chen W, Li S, Srinivasan SR, Boerwinkle E, Berenson GS. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: the Bogalusa Heart Study. Hypertension. 2005;45:954–9. doi: 10.1161/01.HYP.0000161881.02361.11. [DOI] [PubMed] [Google Scholar]
- 170.Allayee H, Dominguez KM, Aouizerat BE, Krauss RM, Rotter JI, Lu J, Cantor RM, de Bruin TW, Lusis AJ. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–8. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 171.Zhu H, Lu Y, Wang X, Treiber FA, Harshfield GA, Snieder H, Dong Y. The G protein-coupled receptor kinase gene affects blood pressure in young normotensive twins. Am J Hypertens. 2006;19:61–6. doi: 10.1016/j.amjhyper.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 172.Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping essential hypertension SNPs using a homogenous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–40. [PubMed] [Google Scholar]
- 173.Williams SM, Ritchie MD, Phillips JA, III, Addy JH, Kpodonu J, Wong L-J, Felder RA, Jose PA, Moore JH. Identification of multilocus genotypes that associate with high-risk and low-risk for hypertension. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 174.Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–60. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 175.Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, Morris BJ. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–36. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 176.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–54. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 177.Williams SM, Addy JA, Phillips JA, III, Dai M, Kpodonu J, Afful J, Jackson H, Joseph K, Eason F, Murray MM, Epperson P, Aduonum A, Wong L-J, Jose PA, Felder RA. Combinations of variations in multiple genes are associated with hypertension. Hypertension. 2000;36:2–6. doi: 10.1161/01.hyp.36.1.2. [DOI] [PubMed] [Google Scholar]
- 178.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 179.Staessen JA, Kuznetsova T, Zhang H, Maillard M, Bochud M, Hasenkamp S, Westerkamp J, Richart T, Thijs L, Li X, Brand-Herrmann SM, Burnier M, Brand E. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–50. doi: 10.1161/HYPERTENSIONAHA.107.109611. [DOI] [PubMed] [Google Scholar]
- 180.Grant FD, Romero JR, Jeunemaitre X, Hunt SC, Hopkins PN, Hollenberg NH, Williams GH. Low-renin hypertension, altered sodium homeostasis, and an alpha-adducin polymorphism. Hypertension. 2002;39:191–6. doi: 10.1161/hy0202.104273. [DOI] [PubMed] [Google Scholar]
- 181.Sugimoto K, Hozawa A, Katsuya T, Matsubara M, Ohkubo T, Tsuji I, Motone M, Higaki J, Hisamachi S, Imai Y, Ogihara T. αl-Adducin Gly460Trp polymorphism is associated with low renin hypertension in younger subjects in the Ohasama study. J Hypertens. 2002;20:1779–84. doi: 10.1097/00004872-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 182.Lohmueller KE, Wong LJ, Mauney MM, Jiang L, Felder RA, Jose PA, Williams SM. Patterns of genetic variation in the hypertension candidate gene GRK4: ethnic variation and haplotype structure. Ann Hum Genet. 2006;70:27–4. doi: 10.1111/j.1529-8817.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- 183.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–9. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 184.Gildea JJ, Yatabe J, Sasaki M, Jose PA, Felder RA. G-protein coupled receptor kinase 4 (GRK4) polymorphisms block receptor recruitment to cell membranes. Hypertension. 2006;48:e85. (Abstract) [Google Scholar]
- 185.Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4γ A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–92. doi: 10.1152/ajpheart.00944.2006. [DOI] [PubMed] [Google Scholar]
- 186.Wang Z, Asico LD, Escano CS, Felder RA, Jose PA. Human G protein-coupled receptor kinase type 4γ (hGRK4γ) wild-type prevents salt sensitivity while its variant, hGRK4γ486V, promotes salt sensitivity in transgenic mice: role of genetic background. Hypertension. 2006;48:e27. [Google Scholar]
- 187.Zhu H, Lu Y, Wang X, Snieder H, Treiber FA, Harshfield GA, Dong Y. The G protein-coupled receptor kinase 4 gene modulates stress-induced sodium excretion in black normotensive adolescents. Pediatr Res. 2006;60:440–2. doi: 10.1203/01.pdr.0000238250.64591.44. [DOI] [PubMed] [Google Scholar]
- 188.Feldman RD. G-Protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension. 2000;35:38–42. doi: 10.1161/01.hyp.35.1.38. [DOI] [PubMed] [Google Scholar]
- 189.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–58. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 190.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 191.Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc Natl Acad Sci USA. 2004;101:11886–9. doi: 10.1073/pnas.0402178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension. 2009;54:71–6. doi: 10.1161/HYPERTENSIONAHA.108.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation. 2005;112:1145–53. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- 194.Ishizaka N, Alexander RW, Laursen JB, Kai H, Fukui T, Oppermann M, Lefkowitz RJ, Lyons PR, Griendling KK. G protein-coupled receptor kinase 5 in cultured vascular smooth muscle cells and rat aorta. Regulation by angiotensin II and hypertension. J Biol Chem. 1997;272:32482–8. doi: 10.1074/jbc.272.51.32482. [DOI] [PubMed] [Google Scholar]
- 195.Bhatnagar V, O’Connor DT, Brophy VH, Schork NJ, Richard E, Salem RM, Nievergelt CM, Bakris GL, Middleton JP, Norris KC, Wright J, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS AASK Study Investigators. G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. 2009;22:332–8. doi: 10.1038/ajh.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanada H, Yatabe J, Yatabe MS, Yokokawa H, Williams S, Bartlett J, Wang Z, Felder R, Jose PA. G Protein-coupled receptor type 4 gene variants and response to antihypertensive medication. Circulation. 2009;120:S1087. (Abstract 5413) [Google Scholar]