Abstract
Colorectal cancers (CRC) develop on the basis of a deficient DNA mismatch repair (MMR) system in about 15% of cases. MMR-deficient CRC lesions show high level microsatellite instability (MSI-H) and accumulate numerous mutations located at coding microsatellite loci that lead to the generation of immunogenic neopeptides. Consequently, the host’s antitumoral immune response is of high importance for the course of the disease in MSI-H CRC patients. Accordingly, immune evasion mediated by impairment of HLA class I antigen presentation is frequently observed in these cancers.
In the present study, we aimed at a systematic analysis of alterations affecting HLA class II antigen expression in MSI-H CRC. HLA class II antigens are expressed by only two-thirds of MSI-H CRCs. The mechanisms underlying the lack of HLA class II antigens in a subset of MSI-H CRCs still remained unknown. We here screened HLA class II-regulatory genes for the presence of coding microsatellites and identified mutations of the essential regulator genes RFX5 in 9 (26.9%) out of 34 and CIITA in 1 (2.9%) out of 34 MSI-H CRCs. RFX5 mutations were related to lack of or faint HLA class II antigen expression (p=0.006, Fisher’s exact test). Transfection with wild type RFX5 was sufficient to restore interferon gamma (IFN-γ) -inducible HLA class II antigen expression in the RFX5-mutant cell line HDC108. We conclude that somatic mutations of the RFX5 gene represent a novel mechanism of loss of HLA class II antigen expression in tumor cells, potentially contributing to immune evasion in MSI-H CRCs.
Keywords: CIITA; colorectal cancer; HLA class II antigens; immune evasion, microsatellite instability; RFX5
Introduction
Colorectal cancers (CRCs) develop through two major pathways of genetic instability. About 85% of CRCs show chromosomal instability and follow the adenoma-carcinoma model of tumorigenesis 1. On the other hand, about 15% of CRCs display an association of tumorigenesis with deficiency of DNA mismatch repair (MMR) and are hallmarked by high level microsatellite instability2,3 (MSI-H). MSI-H CRCs represent a cancer type with a well characterized molecular pathogenesis. DNA MMR deficiency induces insertion/deletion mutations in coding microsatellite sequences that trigger frameshifts of the respective reading frame. These frame shift mutations may lead to the inactivation of affected genes and result in the generation of novel peptide sequences at the carboxyterminus of the respective mutant proteins.
As a consequence, MSI-H CRCs are characterized by a pronounced immunogenicity4–6 that is associated with several pathological features such as an enhanced infiltration with lymphocytes or a high frequency of Crohn’s like lesions at the tumor site7–9. Moreover, MSI-H CRCs frequently are deficient for the expression of HLA class I antigens as a result of immunoselection. MSI-H CRC represents an ideal model tumor for studying defects of antigen presentation, because mutational targets can be predicted by bioinformatics on the basis of the human genome databases. This approach has led to the identification of mutational targets involved in HLA class I antigen processing and presentation pathway, including B2M, TAP1, and TAP210,11.
In the present study, we aimed at a systematic analysis of alterations affecting HLA class II antigen expression in MSI-H CRC. HLA class II antigens are expressed by only two-thirds of MSI-H CRCs12, and expression is associated with an improved prognosis12–14. The mechanisms underlying the absence of HLA class II antigens in a subset of MSI-H CRCs still remain unknown.
HLA class II antigen expression is inducible by interferon-gamma15 (IFN-γ) and controlled by several regulatory proteins including the master regulator class II transactivator (CIITA) and the RFX-complex consisting of the regulatory factor 5 (RFX5), the regulatory factor X-associated protein (RFXAP) and the regulatory factor X-associated ankyrin-containing protein16 (RFXANK).
We here screened HLA class II regulatory genes for the presence of coding microsatellites and identified mutations of the essential regulator gene RFX5 as a novel mechanism leading to loss of HLA class II antigen expression.
Material and Methods
CRC lesions
Consecutive CRC samples were collected from the Institute of Pathology, Westpfalz-Klinikum Kaiserslautern, Germany. Informed consent was obtained from all patients included in the study. Thirty-four MSI-H CRC lesions were identified in a collection of 326 consecutive CRC lesions using the standard NCI/ICG-HNPCC marker panel2 and CAT2517. Detailed information about tumor specimens is provided in Supplementary Table 1.
Cell lines
The MSI-H CRC cell lines COLO60H, HCT116, RKO and Vaco457 were obtained from ATCC (Manassas, VA, USA) or the German Cancer Research Center (DKFZ, Heidelberg, Germany). Cell line HDC10818 was used for transfection experiments and was kindly provided by Prof. Dr. M. Schwab, Division of Tumor Genetics, DKFZ Heidelberg. The MSI-H CRC cell line K073 was established in house. Cell line Vaco6 was a kind gift of J.K.V. Wilson, M.D. (Howard Hughes Medical Institute, Cleveland, OH, USA). Cells were grown at 37°C, in a 5% CO2 humidified atmosphere in RPMI1640 or DMEM/HAMS-F12 medium supplemented with 10% FCS, 1% L-Glutamine (all cell culture reagents were obtained from PAA, Cölbe, Germany).
Antibodies and cytokines
The HLA-DR, DQ, DP-specific mAb LGII-612.14 was developed and characterized as described19. MAb W6/32 recognizes a determinant expressed by B2M-associated HLA-A, -B, -C, -E, and -G heavy chains 20,21. HCA-2, which recognizes a determinant expressed by B2M-free HLA-A (excluding -A24), -B7301, and -G heavy chains, and HC-10, which recognizes a determinant expressed by B2M-free HLA-A3, -A10, -A28, -A29, -A30, -A31, -A32, -A33, and-B (excluding -B5702, -B5804, and -B73) heavy chains were developed and characterized as described22–24. Biotinylated anti-mouse IgG xenoantibodies and FITC-labeled goat anti-mouse IgG antibodies were purchased from Vector (Burlingame, Canada) and from Dianova (Hamburg, Germany) respectively. IFN-γ was obtained from PromoCell (Heidelberg, Germany).
Immunohistochemistry (IHC)
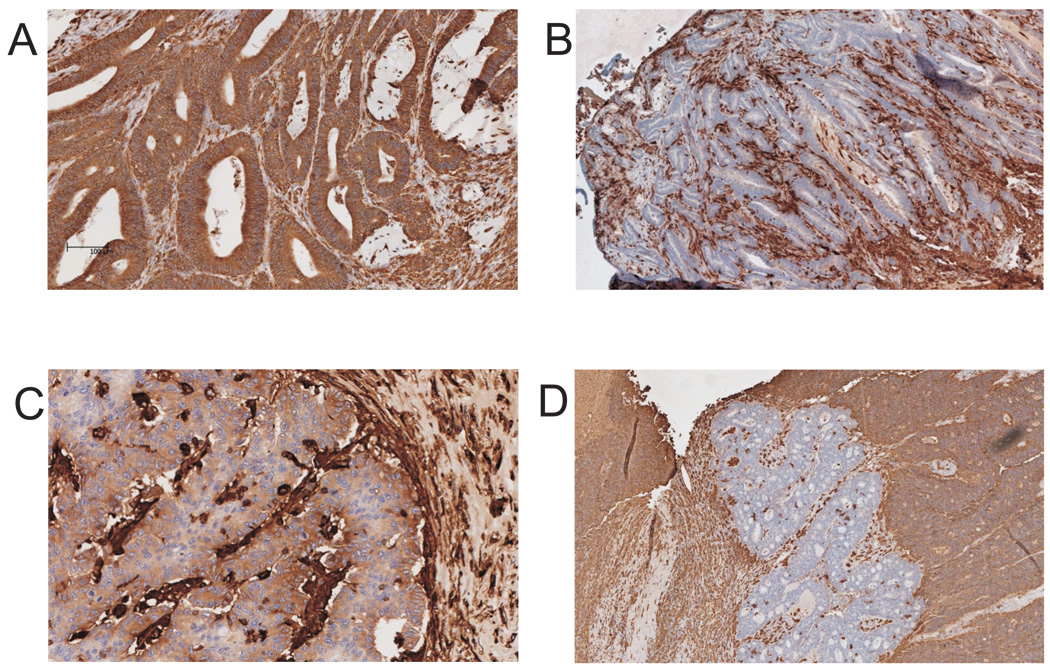
IHC was performed on paraffin sections (2 µm) following standard procedures. Briefly, after deparaffinization and rehydration, slides were boiled in 10 mM citrate buffer (pH 6.0) for 15 min and then allowed to cool for 30 min for antigen retrieval. Endogen peroxidase activity was blocked with 0.6% H2O2 (v/v in methanol), and sections were then incubated in 10% horse serum (v/v in PBS) for 30 min at room temperature to prevent nonspecific antibody binding. Tissue sections were then incubated with an optimal amount of the HLA-DR, -DQ, -DP antigen-specific mAbs LGII-612.14, HC-10, or HCA-2 at room temperature for two hours. After washing and incubation with an optimal amount of biotinylated anti-mouse IgG xenoantibodies for 30 min at room temperature, AB reagent was applied according to the manufacturer’s instructions (Vectastain Elite ABC kit, Vector, Burlingame, Canada). Antigen detection was performed by a color reaction with 3,3-di-amino-benzidine (DAB+ chromogen; DakoCytomation, Glostrup, Denmark). Tissue sections stained for HLA class II antigens were scored as 2, 1 and 0, when the membranous staining of tumor cells was strong and homogeneous, faint and patchy and non detectable, respectively. In addition, lesions with clear-cut focal areas with complete lack of staining surrounded by positively-stained tumor tissue were scored as “regional loss” of HLA class II antigen expression. Representative examples of the staining patterns are presented in Figure 1. Tumor-infiltrating lymphocytes in each slide served as an internal positive control for the staining. Where present in the section, normal colonic epithelium was also evaluated for HLA class II antigen expression. HLA class I antigen expression status (scored as described previously 11) is provided in Supplementary Table 1.
Figure 1.
Patterns of HLA class II antigen expression in MSI-H CRC lesions. Immunohistochemical staining of MSI-H CRC lesions with mAb LGII-612.14 directed against HLA class II antigen. Positively stained cells are brown; nuclei were counterstained with Hematoxylin (blue). Three staining patterns were observed: strong and homogeneous HLA class II antigen surface expression (A), lack of HLA class II antigen surface expression (B), and faint and patchy HLA class II antigen surface expression (C). In some specimens, clear cut focal areas without detectable expression of HLA class II were surrounded by tumor tissue strong expression of HLA class II antigens (D).
Isolation of genomic DNA
Genomic DNA was isolated from microdissected formalin-fixed paraffin-embedded tissue samples using the DNeasy tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions.
COBRA assay for the detection of CIITA promoter IV methylation
DNA (1 µg or 500 ng) was bisulfite-converted using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions. One-fourth of a conversion reaction was used as PCR template. Methylation analysis was performed using enzymatic digest with BstUI to assess methylation at two specific sites in the CIITA promoter IV. The analyzed sites are located in two central regions within a CpG island of about 500 bp surrounding exon 1 of CIITA transcript IV. These regions (GM1 and GM2) have been described as relevant for the regulation of CIITA expression25. Region GM1 ranges from nt 197–391, and GM2 from nt 510–664 in the genomic region covering the CIITA gene (reference assembly of chomosome 16, gi:51511732, position 10878556 – 10926341). Primers for methylation analysis and PCR conditions were used as described 25. The PCR products were digested with BstUI (New England Biolabs, Frankfurt, Germany). DNA from the cell line HCT116 was included as a positive control for CIITA promoter IV methylation in each assay. Following digestion, the PCR products were visualized on 2% agarose TAE gels and analyzed for the presence of additional fragments.
Analysis of frameshift mutations
Genes known to be involved in the regulation of HLA class II expression (CIITA, RFX5, RFXAP, RFXANK, STAT1α, IRF1) were searched for the presence of cMS with a minimum length of 7 nucleotides. Two cMS, a C7 repeat located in exon 11 of CIITA (NM_000246) and a C7 repeat located in exon 3 of RFX5 (NM_000449) were identified and analyzed for the presence of frameshift mutations by direct sequencing following PCR amplification. The primer sequences used included sense 5’-GACTCTATGTCGGCCTGCT-3’ and antisense 5’-GACTCTATGTCGGCCTGCT-3’ for CIITA and sense 5’-CGGGATGGCAGAAGATGA-3’ and antisense 5’-CAGGACTTGGAGATGTGATGA-3’ for RFX5. PCR products were purified with Exo SAPit (USB, Staufen, Germany). The sequencing reaction was performed with the Big Dye Terminator v1.1 kit (Applied Biosystems, Darmstadt, Germany) and the obtained products were analyzed on an ABI3100 genetic analyzer (Applied Biosystems, Darmstadt, Germany). All detected mutations were verified in a second independent PCR and sequencing reaction.
IFN-γ treatment and fluorescence-activated cell sorter (FACS) analysis
Before treatment with IFN-γ, cells were seeded in T75 flasks and allowed to become adherent over night. The next day, cells were treated with 500IU/ml IFN-γ. After 48h, cells were harvested and processed for subsequent experiments. Control samples were prepared for each cell line by seeding and harvesting the cells as described above, but omitting IFN-γ treatment. Cells (2×105) were washed once in PBS containing 1% (v/v) FCS and then incubated with mAb LGII-612.14 for 30 min on ice. After washing three times in PBS 1% FCS, cells were incubated for 30 min on ice and in the dark with FITC-labeled goat anti-mouse IgG antibodies. Cells were then washed three times, fixed with 1% (w/v) paraformaldehyde in PBS and analyzed the next day. For each cell line, a negative control was prepared by incubation with secondary antibody only. The mean fluorescence intensity (FI) of stained cells was determined using FACSCalibur (BD Biosciences, Franklin Lakes, New Jersey, USA) and CellQuest pro software (BD Biosciences). Analysis of HLA class I antigen expression was performed using the mAB W6/32. Cells were harvested and stained without prior IFN-γ treatment, following identical staining procedures as described above.
Transfection experiments
Wild type RFX5 RNA was amplified from Vaco457cells using primers containing restriction sites appropriate for cloning into pcDNA3.1/His A vector (Invitrogen, Karlsruhe, Germany). The sequences of the primers used were sense 5 ’-TACAAGCTTAAAGCCGGGATGGCAGAAGATGAG-3 ’ and antisense 5 ’-TACTCTAGAAAACTGTATCATGGGGGTGTTGC-3’ (gene-specific regions are underlined). HDC108 cells were transfected with pcDNA3.1/His A containing wild type RFX5 or with the empty vector using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. Stably transfected cells were selected for by resistance to G418 (PAA, Cölbe, Germany, 1000 µg/ml final concentration).
Statistical analysis
Fisher’s exact test was performed to determine potential correlation between RFX5 mutation status and HLA class II antigen expression status in clinical MSI-H CRC lesions. P values lower than 0.05 were considered statistically significant.
Results
Expression of HLA class II antigens in MSI-H CRC tissues
The expression of HLA class II antigens in 34 surgically removed MSI-H CRC lesions was determined by immunohistochemical staining with the HLA-DR, -DQ, -DP-specific mAb LGII-612.14. Thirteen (38.2%) out of 34 lesions displayed a strong and homogenous surface staining pattern (score 2), and 13 (38.2%) a faint and patchy staining pattern (score 1); on the other hand no staining (score 0) was detected in the remaining 8 (23.5%) lesions. Representative staining patterns are shown in Figure 1. In 22 (64.7%) out of the 34 samples, normal colonic epithelium adjacent to the lesion was present on the slide. Tumor-adjacent normal epithelium was always stained by the HLA class II antigen-specific mAb LGII-612.14 with a membranous pattern of expression. In 6 MSI-H CRC lesions with positive staining for HLA class II antigens (5 lesions with score 2, and 1 lesion with score 1), clear-cut focal areas with lack of HLA class II antigen expression were detected (a representative example is given in Figure 1D).
To investigate the mechanisms underlying regional lack of HLA class II antigen expression regional microdissection was performed in 4 out of the 6 lesions showing focal absence of HLA class II antigen expression.
Analysis of CIITA promoter IV methylation
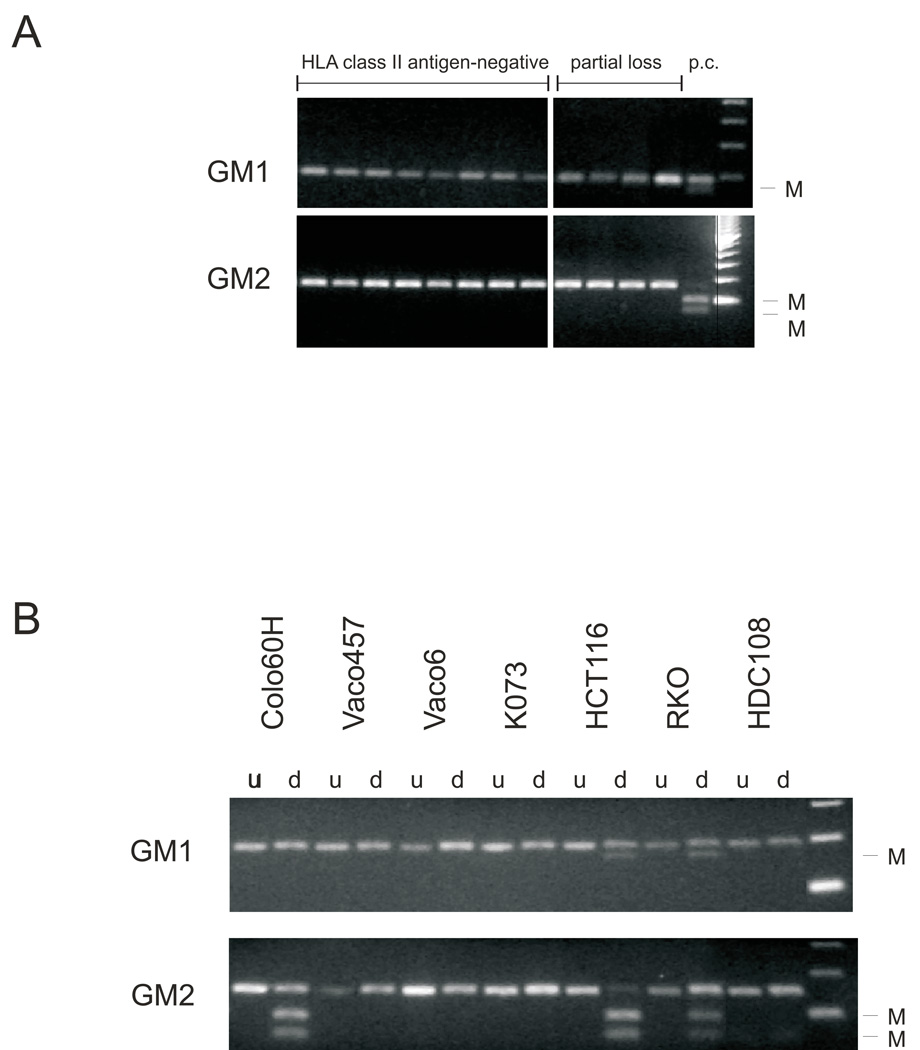
Analysis of CIITA promoter IV methylation in regions stained and not stained for HLA class II antigen expression detected no promoter methylation in all regionally microdissected samples. Similarly no methylation of CIITA promoter IV was detected in 8 MSI-H CRC lesions without detectable HLA class II antigen expression and in 12 with a faint and patchy staining (Figure 2A). These results imply that CIITA promoter IV methylation does not play a major role in the differential HLA class II antigen expression in MSI-H CRC lesions.
Figure 2.
Analysis of CIITA promoter IV methylation in MSI-H CRC lesions (A) and cell lines (B). Two regions (GM1 and GM2) of the promoter were analyzed for each sample. Methylation was analyzed by enzymatic digest with BstUI following bisulfite treatment and PCR amplification. Additional bands indicate methylation (M). Cell line HCT116 which is methylated in both regions (Satoh et al. 2004) was used as a positive control (P.c.).
d - digested with BstUI, u - undigested.
Analysis of RFX5 and CIITA cMS
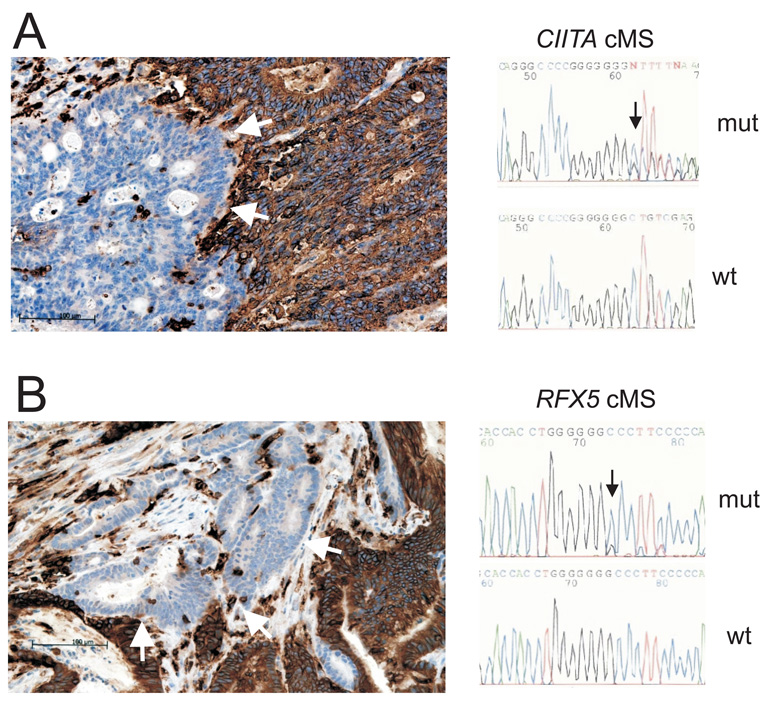
As a consequence of MMR deficiency, MSI-H CRCs accumulate truncating frameshift mutations in cMS which may abrogate protein function and thus contribute to alterations of cellular pathways. In order to identify potential targets of MMR deficiency in HLA class II antigen presentation pathway, we performed a database search26 for cMS with a length of 7 nucleotides or longer in genes, encoding proteins involved in the regulation of HLA class II antigen expression. Two cMS, a C7 repeat located in exon 11 of CIITA and a C7 repeat in exon 3 of RFX5 were identified. Mutation analysis of the CIITA and RFX5 C7 cMS in 4 regionally microdissected specimens identified an insertion mutation in the C7 repeat of CIITA (c.1962_1963insC; p.Gly655ArgfsX94) in a focal area without detectable staining by mAb LGII-612.14. This mutation was not detected in the surrounding tumor tissue that was strongly stained for HLA class II antigens (Figure 3A). Furthermore a one base pair deletion in the C7 repeat located in exon 3 of the RFX5 gene (c.56delC; p.Pro19GlnfsX28) was identified in a regionally microdissected area lacking HLA class II antigen expression and was not detected in the surrounding tumor tissue strongly expressing HLA class II antigens (Figure 3B).
Figure 3.
Mutations of CIITA and RFX5 in MSI-H CRC with regional lack of HLA class II antigen expression. Cells positive for HLA class II antigen expression are brown, negative areas (blue) are indicated by arrows (left panel). cMS mutation analysis of microdissected tissue from HLA class II antigen-negative regions revealed deletion or insertion mutations in CIITA (A) or RFX5 (B) genes. The surrounding HLA class II antigen-positive tissue has retained wild type cMS length (right panel).
As a next step we analyzed the C7 repeats of RFX5 and CIITA for the presence of mutations in the remaining 30 CRC lesions without performing regional microdissection. Base pair deletions in the coding C7 repeat located in exon 3 of the RFX5 gene were found in 8 of the 30 tumors analyzed, corresponding to a total RFX5 cMS mutation frequency of 9 (26.5%) out of 34. The presence of RFX5 mutations significantly correlated with lack of or faint and patchy HLA class II antigen expression. In contrast, mutations in the RFX5 gene were not detected in the MSI-H CRC lesions with strong HLA class II antigen expression (9 out of 21 (42.9%) vs. 0 out of 13 (0%); p=0.006; Fishers’ Exact Test, two-tailed). No additional CIITA mutations were identified in the lesions; therefore, the overall mutation frequency in the CIITA C7 repeat was 1 (2.9%) out of 34.
HLA class II antigen expression in MSI-H CRC cell lines
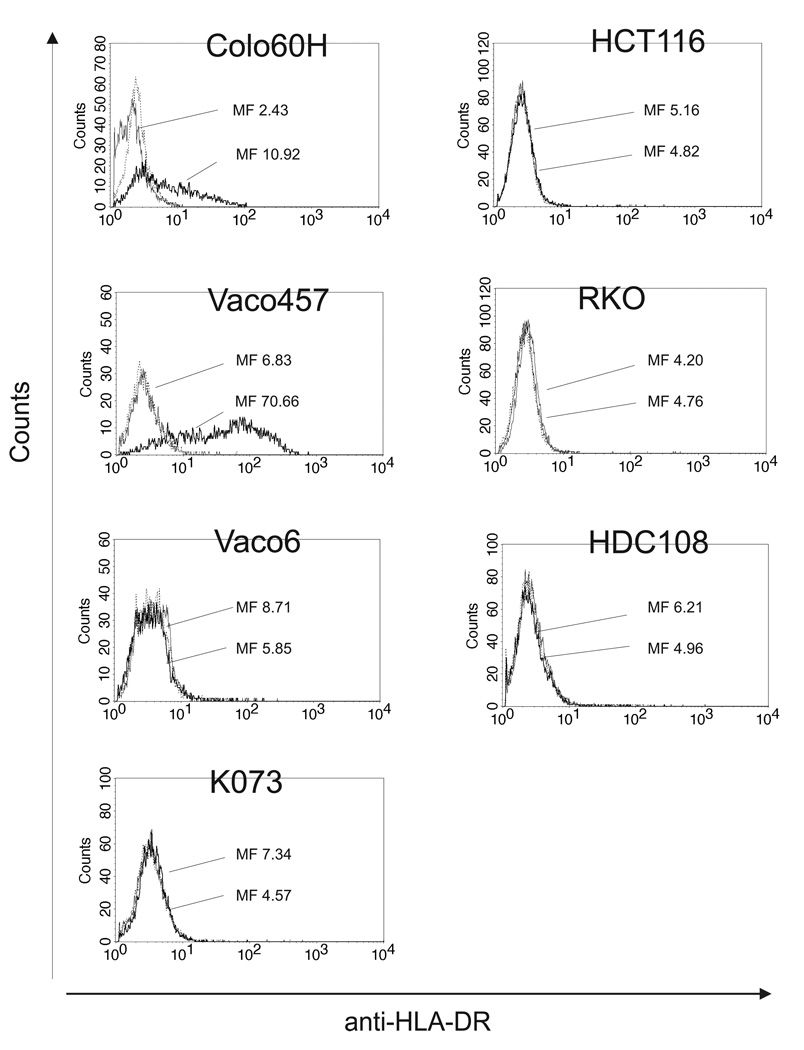
We evaluated the MSI-H CRC cell lines Colo60H, HCT116, HDC108, K073, RKO, Vaco6 and Vaco457 for HLA class II antigen expression under basal conditions and following incubation with IFN-γ using HLA class II antigen-specific antibody LGII-612.14. HLA class II antigens were not detected on any of the cell lines under basal conditions. Following 48 h incubation with IFN-γ at 37 °C, only Colo60H and Vaco457 expressed HLA class II antigens (Figure 4).
Figure 4.
Differential HLA class II antigen expression by MSI-H CRC cell lines. Cells were stained with HLA-DR-specific mAb LGII-612.14. Mean fluorescence (MF) is given for cells under basal conditions (shaded gray) and following 48 h incubation at 37 °C with IFN-γ (500 IU/ml) (bold black line). Negative controls are indicated by thin black lines.
As a first step to elucidate if the lack of HLA class II antigen expression was a consequence of molecular alterations in HLA class II regulatory genes, we analyzed methylation in the CIITA promoter IV in the cell lines HCT116, HDC108, K073, RKO and Vaco6. CIITA promoter IV methylation was present in the cell lines HCT116 and RKO in the two promoter regions analyzed. In addition, we also observed partial methylation in one out of two analyzed promoter regions (GM2) in the cell line Colo60H; this may contribute to the reduced susceptibility to modulation by IFN-γ. Methylation of the CIITA promoter IV was not detected in the two fragments analyzed in the HDC108, K073 and Vaco6 cell lines (Figure 4), suggesting that other mechanisms underlie the lack of HLA class II antigen induction by IFN-γ.
As observed for the MSI-H CRC lesions, sequencing of CIITA and RFX5 cMS revealed the presence of frameshift mutations in these genes. Specifically, one heterozygous insertion (c.1962_1963insC; p.Gly655ArgfsX94) and one heterozygous deletion (c.1962delC; p.Pro654Profs32X) mutation in the CIITA C7 coding repeat were identified in the cell lines K073 and Vaco6, respectively. The cell line HDC108 harbored a homo- or hemizygous one base pair deletion (c.56delC; p.Pro19GlnfsX28) in the C7 cMS of RFX5. In addition a point mutation leading to a non-conservative amino-acid exchange (c.64G>A; p.Ala22Thr) of unknown significance for protein function was detected in RFX5 in the cell line RKO (Table 1).
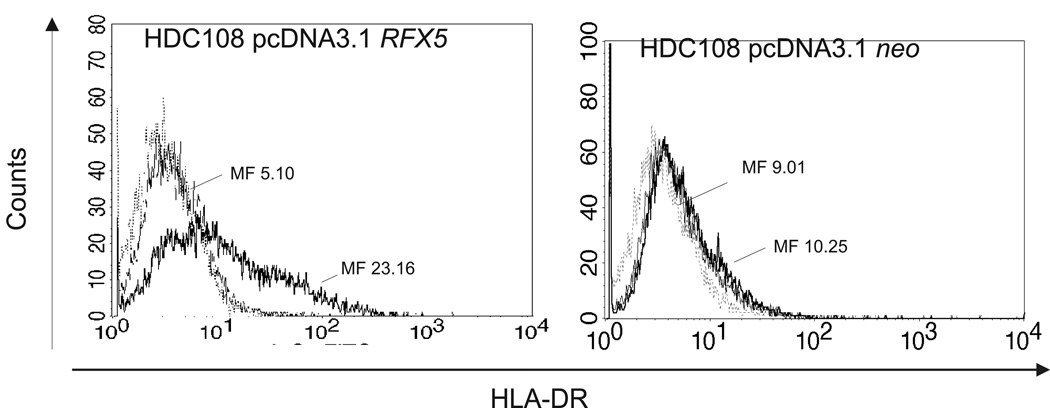
To determine whether transfection with wild type RFX5 could restore IFN-γ-induced expression of HLA class II antigens on HDC108 cells, cells were transfected with a wild type RFX5 cDNA and analyzed for HLA class II antigen expression. As shown in Figure 5, HDC108 cells transfected with wild type RFX5 cDNA were stained by the HLA class II antigen-specific mAb HB-104 following incubation with IFN-γ (500 IU/ml). In contrast, no staining was detected of HDC108 cells transfected with an empty vector. In summary these data suggest that frameshift mutations of CIITA and RFX5 may lead to lack of HLA class II antigen expression in MSI-H CRC lesions.
Figure 5.
Restoration of HLA class II antigen expression in HDC108 cells by stable transfection with wild type RFX5 (left) and incubation with IFN-γ (500 IU/ml) for 48 h at 37 °C. Cells transfected with an empty vector (right) were used as a control. Cells were stained with HLA-DR-specific LGII-612.14. Mean fluorescence (MF) is given for IFN-γ-treated cells (bold black line) and untreated cells (shaded gray). Negative controls are indicated by thin black lines.
Discussion
In the present study, we show that somatic mutations affecting HLA class II-regulatory genes may lead to loss of HLA class II antigen expression by MSI-H CRC cells. Lack of HLA class II antigen expression had previously been reported to occur in about one third of MSI-H CRC lesions12. In accordance with this, in the present study lack of HLA class II antigen expression was observed in 23.5% of MSI-H CRC lesions. In contrast, normal tumor-adjacent colonic epithelium showed HLA class II antigen expression in 100% of the tissues analyzed, suggesting that HLA class II antigen expression-inducing factors were present in the tumor environment. The lack of HLA class II antigen expression in clear-cut areas with surrounding positive tissue further argued in favor of defects which interfere with the ability of colon cancer cells to express HLA class II antigens.
The analysis of cMS in CIITA and RFX5, two molecules essential for the initiation of transcription of HLA class II antigens27,28, demonstrated a high frequency of RFX5 mutations in MSI-H CRC lesions. These mutations were base pair insertions or deletions at cMS, which are particularly prone to structural alterations in MSI-H cells due to DNA MMR deficiency29. The high frequency of frameshift mutations in RFX5 (42.9% in tumors with lack of or faint HLA class II antigen expression) provides evidence that the RFX5 gene may be a common mutational target of MMR deficiency in MSI-H colorectal tumorigenesis, representing a novel mechanism interfering with HLA class II antigen expression in cancer.
Frameshift mutations located in cMS result in shifts of the translational reading frame and may impair protein function. The functional relevance of mutations affecting regulators of HLA class II antigen expression is supported by the following observations: RFX5 and CIITA mutations were correlated with a loss of HLA class II antigen expression at the protein level. They were present only in lesions or tumor regions with lack of or faint staining for HLA class II antigen. Transfection with wild type RFX5 partially restored the IFN-γ inducibility of HLA class II antigen expression in RFX5-mutant cell line HDC108. In addition, evidence for the functional relevance of RFX5 and CIITA alterations for HLA class II antigen expression comes from the literature. In Bare Lymphocyte Syndrome, germline mutations of these genes lead to the abrogation of HLA class II antigen expression on antigen presenting cells and a severe clinical phenotype27,28. The occurrence of mutations in two independent genes that are associated with the same clinical phenotype lends further support to the hypothesis that impairment of HLA class II antigen expression is of biological relevance in MSI-H CRC.
Hypermethylation of the CIITA promoter IV has been described as a cause of HLA class II antigen deficiency in cancer30,31, being related to lack of HLA-DR antigen induction by IFN-γ in MSI-H CRC cell lines HCT116 and RKO25. We analyzed seven MSI-H CRC cell lines and found methylation of the CIITA promoter IV in the cell lines Colo60H, HCT116, and RKO. However, in contrast to the observation of CIITA promoter IV methylation in clinical gastric cancer lesions by Satoh et al.25, CIITA promoter IV methylation was not detected in any of the clinical MSI-H CRC lesions. This suggests that methylation of CIITA promoter IV at the two sites analyzed by COBRA may be a cell culture phenomenon in MSI-H CRC cell lines, but is unlikely to represent a common mechanism leading to lack of HLA class II antigen expression of MSI-H CRC cells in vivo.
Whether additional mechanisms may interfere with HLA class II antigen expression in MSI-H CRCs remains to be elucidated. Abolishment of IFN-γ upstream signaling that might result in loss of HLA class II antigen expression is uncommon in MSI-H CRC32, and was not observed in the cell lines analyzed in the present study (data not shown). Other alterations of the HLA class II antigen presentation pathway have been described in hematological malignancies and solid tumors, including modification of CIITA histone acetylation, histone methylation, or posttranscriptional modification of HLA class II antigens33–35. A potential contribution of these mechanisms in a subset of MSI-H CRCs cannot be excluded. Nevertheless, coding microsatellite mutations of HLA class II-regulatory genes are likely to have a central role in impairment of HLA class II antigen expression in MSI-H CRC. This hypothesis is supported by the abrogation of tumor-relevant pathways by coding microsatellite mutations, which represent a pivotal driving force of DNA MMR deficient cancers36.
In analogy to the impairment of HLA class II antigen expression following frameshift mutations in RFX5, impairment of HLA class I antigen expression is a frequent event in MSIH CRC37 and predominantly mediated by frameshift mutations of the beta2-microglobulin gene10,11,38 likely reflecting immunoselective pressure. Accordingly, the outgrowth of MSI-H CRC cells that lack HLA class II antigen expression is compatible with the possibility that HLA class II antigen-deficient tumor cells may be selected for during MSI-H colorectal tumorigenesis. Although the exact mechanisms of such a selection process are not known at present, it may be speculated that tumor-infiltrating CD4+ T cells may recognize MSI-H CRC cells that express frameshift-derived tumor-specific antigens on the cell surface via HLA class II antigens. This hypothesis is in line with the report of a HLA-DR-restricted CD4+ T cell response in an MSI-H CRC patient5.
In summary, the present study provides evidence that RFX5 is a relevant mutational target of DNA MMR deficiency in MSI-H CRC, thus describing a novel mechanism underlying loss of HLA class II antigen expression in cancer. Moreover, our results support the hypothesis that MSI-H CRC cells showing absence of HLA class II antigen expression may be selected for during tumorigenesis. Further functional studies will have to clarify the role of HLA class II antigen expression for the susceptibility of tumor cells towards different ways of antitumoral immune response in MSI-H CRC.
Statement of novelty
In the present study, we describe a novel mechanism leading to loss of HLA class II antigen expression in human cancer, thereby suggesting a novel strategy of tumors to evade immune response of the host. The results are important for a better understanding of the interaction between tumor and immune cells, and, in particular, for translational therapeutic concepts like the development of immune therapies against highly immunogenic tumors like microsatellite unstable colorectal cancer.
Supplementary Material
Acknowledgements
The excellent technical assistance of Carina Konrad and Irina Voehringer is gratefully acknowledged. This work was supported in part by funding of the Deutsche Krebshilfe (German Cancer Aid) and by PHS grants PO1 CA109688 (SF), and RO1 CA104947 (SF) awarded by the National Cancer Institute.
Abbreviations
- B2M
Beta2-Microglobulin
- CIITA
Class II Transactivator
- CRC
colorectal cancer
- MSI-H
high level microsatellite instability
- HLA
human leukocyte antigen
- IFN-γ
interferon gamma
- MMR
mismatch repair
- RFX5
Regulatory Factor X 5
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 2.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 3.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 4.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 5.Saeterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A, Gaudernack G. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidoboni M, Gafà R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M, Dolcetti R. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckowitz A, Knaebel HP, Benner A, Bläker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–1753. doi: 10.1038/sj.bjc.6602534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/s0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 11.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 12.Løvig T, Andersen SN, Thorstensen L, Diep CB, Meling GI, Lothe RA, Rognum TO. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer. 2002;87:756–762. doi: 10.1038/sj.bjc.6600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita M, Tanaka K, Kawanishi H, Tsuji M, Ookusa T, Takada H, Okamura A, Hioki K. Immunohistochemically demonstrated expression of HLA-DR antigen in colorectal adenocarcinomas and its relation to clinicopathological features. J Surg Oncol. 1995;59:233–238. doi: 10.1002/jso.2930590407. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MD, Dent OF, Young JP, Wright CM, Barker MA, Leggett BA, Bokey L, Chapuis PH, Jass JR, Macdonald GA. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer. 2009;125:1231–1237. doi: 10.1002/ijc.24484. [DOI] [PubMed] [Google Scholar]
- 15.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 16.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 17.Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, Dondog B, Pawlita M, Dippold W, Wagner R, Gebert J, von Knebel Doeberitz M. T25 repeat in the 3' untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005;65:8072–8078. doi: 10.1158/0008-5472.CAN-04-4146. [DOI] [PubMed] [Google Scholar]
- 18.Brüderlein S, van der Bosch K, Schlag P, Schwab M. Cytogenetics and DNA amplification in colorectal cancers. Genes Chromosomes Cancer. 1990;2:63–70. doi: 10.1002/gcc.2870020112. [DOI] [PubMed] [Google Scholar]
- 19.Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods. 1993;161:239–256. doi: 10.1016/0022-1759(93)90300-v. [DOI] [PubMed] [Google Scholar]
- 20.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 21.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 22.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA- C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 23.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–188. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 24.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 25.Satoh A, Toyota M, Ikeda H, Morimoto Y, Akino K, Mita H, Suzuki H, Sasaki Y, Kanaseki T, Takamura Y, Soejima H, Urano T, et al. Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-gamma-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene. 2004;23:8876–8886. doi: 10.1038/sj.onc.1208144. [DOI] [PubMed] [Google Scholar]
- 26.Woerner SM, Yuan YP, Benner A, Korff S, v Knebel Doeberitz M, Bork P. SelTarbase, a database of human mononucleotide microsatellite mutations and their potential impact to tumorigenesis and immunology. doi: 10.1093/nar/gkp839. www.seltarbase.org, release 200807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 28.Steimle V, Durand B, Barras E, Zufferey M, Hadam MR, Mach B, Reith W. A novel DNA-binding regulatory factor is mutated in primary MHC class II deficiency (bare lymphocyte syndrome) Genes Dev. 1995;9:1021–1032. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- 29.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 30.Holling TM, van Eggermond MC, Jager MJ, van den Elsen PJ. Epigenetic silencing of MHC2TA transcription in cancer. Biochem Pharmacol. 2006;72:1570–1576. doi: 10.1016/j.bcp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz MK, Gebert J, Dihlmann S. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer. 2007;46:1080–1089. doi: 10.1002/gcc.20493. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto Y, Toyota M, Satoh A, Murai M, Mita H, Suzuki H, Takamura Y, Ikeda H, Ishida T, Sato N, Tokino T, Imai K. Inactivation of class II transactivator by DNA methylation and histone deacetylation associated with absence of HLA- DR induction by interferon-gamma in haematopoietic tumour cells. Br J Cancer. 2004;90:844–852. doi: 10.1038/sj.bjc.6601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holling TM, Bergevoet MW, Wilson L, Van Eggermond MC, Schooten E, Steenbergen RD, Snijders PJ, Jager MJ, Van den Elsen PJ. A role for EZH2 in silencing of IFN-gamma inducible MHC2TA transcription in uveal melanoma. J Immunol. 2007;179:5317–5325. doi: 10.4049/jimmunol.179.8.5317. [DOI] [PubMed] [Google Scholar]
- 35.Meissner M, Whiteside TL, van Kuik-Romein P, Valesky EM, van den Elsen PJ, Kaufmann R, Seliger B. Loss of interferon-gamma inducibility of the MHC class II antigen processing pathway in head and neck cancer: evidence for post-transcriptional as well as epigenetic regulation. Br J Dermatol. 2008;158:930–940. doi: 10.1111/j.1365-2133.2008.08465.x. [DOI] [PubMed] [Google Scholar]
- 36.Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark. 2006;2:69–86. doi: 10.3233/cbm-2006-21-208. [DOI] [PubMed] [Google Scholar]
- 37.Dierssen JW, de Miranda NF, Mulder A, van Puijenbroek M, Verduyn W, Claas FH, van de Velde CJ, Jan Fleuren G, Cornelisse CJ, Corver WE, Morreau H. High-resolution analysis of HLA class I alterations in colorectal cancer. BMC Cancer. 2006;6:233. doi: 10.1186/1471-2407-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.