Abstract
Human brain development is created through continuing complex interactions of genetic and environmental influences. The challenge of linking specific genetic or environmental risk factors to typical or atypical behaviors has led to interest in using brain structural features as an intermediate phenotype. Twin studies in adults have found that many aspects of brain anatomy are highly heritable, demonstrating that genetic factors provide a significant contribution to variation in brain structures. Less is known about the relative impact of genes and environment while the brain is actively developing. We summarize results from the ongoing National Institute of Mental Health child and adolescent twin study that suggest that heritability of different brain areas changes over the course of development in a regionally specific fashion. Areas associated with more complex reasoning abilities become increasingly heritable with maturation. The potential mechanisms by which gene–environment interactions may affect heritability values during development is discussed.
Human structural, functional, and behavioral brain development emerges as an ongoing dialogue between a child’s genetic heritage and his or her environment. Understanding how these factors interact at different points during development may help to identify how and when to intervene to help children grow to their fullest potential. However, establishing the links between specific risk factors and developmental outcomes has proven to be extremely challenging. Research of complex neurodevelopmental disorders such as schizophrenia, autism, and attention-deficit/hyperactivity disorder (ADHD) has shown that a particular clinical syndrome may be associated with a wide variety of genetic risk factors, suggesting that there may be multiple routes from nucleotide to behavior (Abrahams & Geschwind, 2008; Owen, Craddock, & O’Donovan, 2005; Samaco, Hogart, & LaSalle, 2005; Walsh et al., 2008). Even disorders arising from a well-characterized genetic abnormality such as Rett syndrome or fragile X can show broad variability in the resultant phenotype, speaking to the complexity of the interactions with other genes or environmental factors that affect how a given genetic feature is expressed (Dykens & Hodapp, 2007; Hessl et al., 2001; Hoffbuhr, Moses, Jerdonek, Naidu, & Hoffman, 2002; Loesch et al., 2003; Moretti & Zoghbi, 2006). Relating behavioral differences to specific environmental factors is also not straightforward. The effects of environmental toxins such as prenatal alcohol exposure depend not only on the timing and quantity of the exposure, but also on the genotype of both fetus and mother (Warren & Li, 2005). Individuals exposed to traumatic environments typically show a range of responses, raising the question of what factors provide resilience as well as vulnerability (Curtis & Cicchetti, 2003; Yehuda, Flory, Southwick, & Charney, 2006).
Brain Structural Features as Endophenotypes
One approach to disentangling these complex interactions is to use stepping stones such as brain structure to help bridge the gap between genetic and environmental risk factors and behavior (Figure 1). Genes do not code for behaviors, but for the building blocks of the cells whose interactions eventually give rise to those behaviors; conversely, the translation of environmental input into persistent behavioral changes occurs through alterations in brain systems and even structures. The advent of brain imaging revolutionized the neuroscience of behavior by making it possible to noninvasively study the brain in vivo. As an example, cortical thickness can be measured at a level of resolution high enough to detect differences in regions across the cortex that are also known to be functionally and cytoarchitecturally heterogeneous. The association of a particular behavioral feature with thickness in a specific cortical area may be a clue that the mechanisms involved in the development of that cortical region are also relevant to that behavior. In addition, finding that specific genetic polymorphisms affect a cortical region directs us to the functions of associated gene products.
Figure 1.
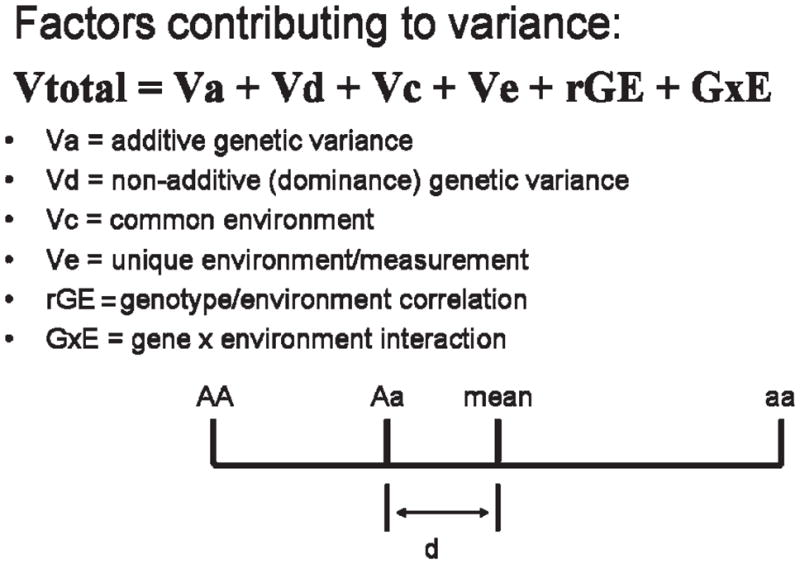
Factors contributing to variance. Dominance effects: AA and aa are homozygous alleles. If the genotypic effects were completely additive, the phenotype of the heterozygous allele Aa would be the mean value between them. The degree to which the heterozygote departs from this is the degree of dominance (d).
The first landmark report of brain structural differences associated with a psychiatric disorder described increased ventricular volume in individuals with schizophrenia (Johnstone, Crow, Frith, Husband, & Kreel, 1976). Since then, a large body of observations has accumulated relating differences in brain structure and function to typical and atypical aspects of behavior, and work in this area is accelerating as techniques improve. In the following discussion we will be focusing on brain structural imaging, but it should be noted that there is increasing interest in the exploration of similar consideration for other brain measures such as functional magnetic resonance imaging (fMRI; Menzies et al., 2007; Pearlson & Calhoun, 2007).
To take schizophrenia as an example, structural abnormalities have been reported in many areas of the brain. Abnormalities such as enlarged ventricles and decreased cortical gray matter in the frontal and mediotemporal lobes are found relatively frequently, whereas changes in other brain regions, such as decreased volume of the thalamus and cerebellum, are more variable (Pearlson & Calhoun, 2007; Shenton, Dickey, Frumin, & McCarley, 2001; van Haren, Bakker, & Kahn, 2008). Some of the disparity is likely due to changes in imaging methodology inherent in the technical advances of the past 3 decades. In addition, it has become apparent that most findings are relatively subtle, and that many studies are underpowered (Meda et al., 2008; van Haren et al., 2008). Heterogeneity of patient populations may also be a contributing factor. Clinically, individuals with schizophrenia can have very different presentations, depending on the relative severity of different types of symptoms, and an ongoing debate concerns how best to account for the imperfect correspondence between the clinical diagnostic entity and the underlying neurobiology (Hyman, 2007). An additional complication arises when considering the stage of the disorder. Results from several longitudinal studies have indicated that decreases in gray matter volume become more pronounced over the course of the disorder. It is not known to what extent the progressive gray matter loss is a fundamental aspect of the disease process, is due to treatment with antipsychotic medications, or occurs as a response to the stress and potential environmental hardships associated with having a chronic severe psychiatric illness. A notable finding has been that individuals who develop schizophrenia during childhood appear to have a much more rapid and severe loss of cortical gray matter than those with the more typical onset in early adulthood, raising the additional question of how disease-related factors interact with normal brain developmental processes (Thompson, Vidal, et al., 2001).
Such considerations are not limited to schizophrenia; they are also applicable for neuroimaging studies in other neurodevelopmental disorders such as autism and ADHD.
The question thus arises regarding how to disentangle “state” differences related to factors such as age and disease stage, environmental factors, medication, or comorbidities, from “trait” genetic heterogeneity that may indicate different genetic risk factors, potentially associated with different disorder subtypes or causal pathways.
One approach has been to search for endophenotypes, simpler characteristics that are associated with the upstream causes of a complex phenotype such as a psychiatric disorder but whose links to genetic factors are potentially easier to parse (Gottesman & Gould, 2003). Several requirements are necessary for a trait to be an effective endophenotype. It must be not only associated with the syndrome of interest but also present independent of disease state. In addition it should be present in healthy individuals at increased genetic risk for the disorder, and variance of the trait must be measurably affected by genetic differences, that is, it must be heritable. Heritability is broadly defined as the amount of variation due to genetic factors. It is worth recalling that the heritability of a trait refers only to the extent to which genetic factors affect variation in the trait, and not whether genes play a role in producing the trait itself.
Preliminary results support the exploration of brain structural features as endophenotypes for neurodevelopmental disorders. Two studies in autism found limbic structures in unaffected first-degree relatives were more similar to affected family members than healthy controls (Dalton, Nacewicz, Alexander, & Davidson, 2007; Rojas et al., 2004). Children with ADHD and their unaffected siblings both had decreases in right pre-frontal gray and matter and left occipital gray and white matter compared with healthy controls; however, cerebellar abnormalities were found only in the children with ADHD (Durston et al., 2004). Specific areas of the cortex were different in healthy monozygotic twins of individuals with schizophrenia compared to unrelated controls, suggesting some abnormalities in cortical thickness were related to disease state and other to genetic liability (Cannon et al., 2002; Gogtay et al., 2003). A recent meta-analysis concluded that there was evidence of decreased gray matter volume and hippocampal abnormalities in first-degree relatives of individuals with schizophrenia (Boos, Aleman, Cahn, Pol, & Kahn, 2007). The scope of the conclusions was limited, however, by the few brain structures in which enough data was available for adequate analysis. The findings were also criticized on the basis that the available twin data suggest the heritability of the hippocampus is relatively low (McDonald, Dineen, & Hallahan, 2008), highlighting the need to establish the heritability of a trait being considered as an endophenotype.
Twin Modeling
Fisher (1918), Wright (1968), and others in the field of quantitative genetics derived sophisticated methods of estimating variance components from correlations of traits in family members with different degrees of relatedness. These techniques make it possible to parcellate variance in the absence of more precise data regarding what the specific operative genetic and environmental factors actually are. Twin studies are one of the most powerful study designs for this purpose. The classic twin model assumes that the amount of genetic material shared by two family members is 100% in monozygotic (MZ) twins and 50% in dizygotic (DZ) twins. When twins are raised together, the environment is also assumed to be identical for both individuals. Increases in phenotypic similarity between MZ twins compared to DZ is ascribed to genetic factors. Similarity because of shared environmental effects (C) will increase the similarity between DZ twins. Nonadditive genetic effects will tend to increase the similarity in MZ twins relative to DZ, providing an estimate of dominance (D). The residual variance (E) is a combination of environmental factors unique to the individual combined with measurement error.
A twin study including MZ and DZ twins who have been raised together can therefore provide estimates of A, C, or D, and E. C and D cannot be estimated simultaneously because these measures are negatively confounded with each other; shared environmental effects increase the correlation between DZ twins, whereas dominance effects increase the correlation between MZ twins (Evans, Gillespie, & Martin, 2002). These effects cannot be differentiated without additional data from twins reared apart, in which the effects of shared environment can be more directly modeled. Inclusion of broader kinships, such as children of twins, allows further estimation of additional factors such genetic and environment correlation. Sophisticated methodology such as structural equation modeling (Figure 2) provides the means to create more flexible models, for example, including data additional family members such as siblings, or modeling interactions of variance components with factors such as age. It also makes it possible to test whether particular variance components significantly improve the fit of the overall model.
Figure 2.
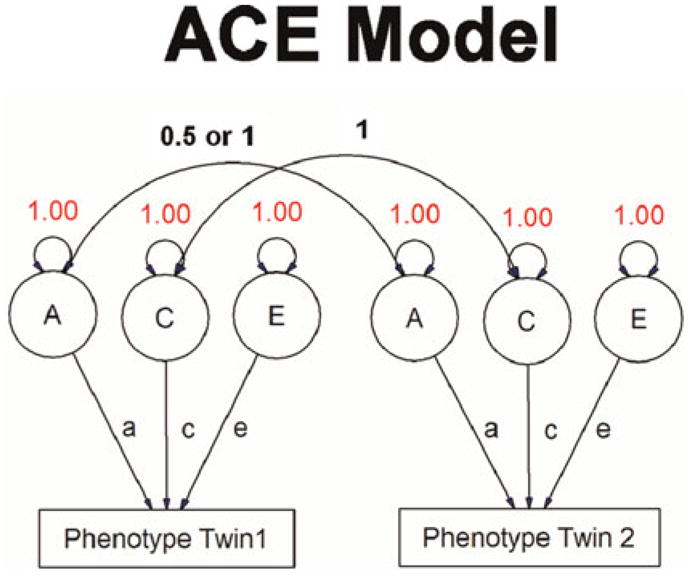
The ACE model. The rectangles contain the observed variables, and the circles contain the latent variables. Single- and double-headed arrows indicate the causal and correlational (variance is correlation with self) relationships, respectively. [A color version of this figure can viewed online at journals.cambridge.org/dpp]
Although the twin study design has proven highly useful in partitioning contributors to variance of complex traits, it relies on some key assumptions that have been questioned. The “equal environments assumption” is that that the environment for MZ and DZ twins can be treated as identical. Some researchers have objected to this based on the observation that identical twins may elicit different social responses than nonidentical twins. Several studies have attempted to determine to what extent the equal environments assumption is valid, finding that, in general, the discrepancy between social environments does not seem to play a significant role, although its potential impact may need to be looked at individually for specific traits (Derks, Dolan, & Boomsma, 2006; Kendler & Gardner, 1998; Plomin, Willerman, & Loehlin, 1976). Chorionic differences can be thought of as another type of environmental factor, and there has been some evidence that chorionic type may influence variance within MZ twins for some phenotypes (Jacobs et al., 2001), although not all (Hur & Shin, 2008; Loos, Beunen, Fagard, Derom, & Vlietinck, 2001).
Recent advances in the field of genetics have shown that there may be limitations regarding a second assumption of the twin model, which is that MZ twins actually have identical genetic material. Examples of genetic differences have been described (Machin, 1996), and more recently epigenetic features such as methylation status have been shown to become increasingly divergent between MZ twin pairs with age (Fraga et al., 2005). More recently, researchers found a significant incidence of differences in copy number variations in MZ twin pairs, providing evidence for the presence of somatic mosaicism and the possible contribution of posttwinning structural variation in chromosomal architecture to phenotypic differences between identical twins (Bruder et al., 2008). Such work speaks to the potential utility of integrating modern genome-wide scans with quantitative statistical methods to begin identifying specific sources of variation.
Heritability of Brain Anatomy
There have now been several studies exploring heritability of different aspects of brain structure (Baare et al., 2001; Posthuma et al., 2000; Tramo et al., 1998; Wright, Sham, Murray, Weinberger, & Bullmore, 2002). Results in adult populations have consistently shown that variation in most brain volumes is highly driven by genetic factors. Topological features such as gyrification have been less studied, but available reports indicate that cortical folding has strong environmental influences (White, Andreasen, & Nopoulos, 2002). A study of cortical gray matter density in 10 MZ and 10 DZ adult twin pairs found regional variation in heritability, with highest values in frontal and temporal areas (Thompson, Cannon, et al., 2001). An interesting MRI study of brain volumes in an extended kinship of baboons also found similar areas to be the most heritable (Rogers et al., 2007).
The finding that differences in anatomy between individuals are more because of genetic influences in some brain regions and environmental influences in others raises questions regarding implications for the associated cognitive functions. In the study by Thompson, Cannon, et al. (2001), the same regions that were highly heritable were also those that correlated most strongly with cognitive ability. A multivariate analysis of IQ and brain gray and white matter volumes in a sample of 24 MZ pairs, 31 DZ pairs, and 25 additional siblings found that the association between IQ and gray and white matter volumes was because of genetic rather than environmental factors (Posthuma et al., 2002). A second study by the same group using voxel-based morphometry described more focal heritable regions of both gray and white matter, which appeared to be affected by common genetic factors with IQ (Hulshoff Pol et al., 2006).
These results demonstrate that functionally relevant effects of genetic variation can be detected in brain structure, an important step toward supporting the validity of brain anatomy as an intermediate phenotype between genes and behavior. The regional variation of heritability is also intriguing regarding implications of the relative balance of genetic and environmental factors for specific cognitive functions. In the studies described above, primary motor and sensory cortices fell within regions shaped most strongly by environmental factors, possibly congruent with the role of these regions in adapting to the varying sensory experiences and motor activities of daily life. By contrast, regions of association cortex which integrate information from other brain regions and support higher cognitive activities such as language and executive function were those that more clearly showed effects of genetic variation.
The observation of a high degree of heritability of brain structure is reinforced by the preliminary findings regarding the effects of genetic polymorphisms on brain structure within healthy populations (Glahn, Thompson, & Blangero, 2007; van Haren et al., 2008). Brain-derived neurotrophic factor (BDNF) is a gene that has been linked with susceptibility to schizophrenia, and has also shown effects on brain morphometric features in healthy controls (Agartz et al., 2006; Ho et al., 2006). The disrupted in schizophrenia 1 (DISC1) gene, another susceptibility gene for schizophrenia (Porteous, Thomson, Brandon, & Millar, 2006), was recently shown to be associated with decreased gray matter volumes in the prefrontal and anterior cingulate regions in both patients with schizophrenia and healthy volunteers (Szeszko et al., 2007). However, studies of effects of single genetic polymorphisms are often negative (van Haren et al., 2008). One of the reasons for this may be lack of attention to the role of interaction with the environment in gene expression. Another consideration is that genes do not act in isolation, particular in the generation of complex traits such as brain volumes, and so statistical methods that are able to model the effects of multiple genes simultaneously may prove more informative.
An interesting question is whether the heritability values reported in healthy individuals are similar in individuals with neurodevelopmental disorders. The difficulty of amassing a suitable sample of affected individuals and family members necessary for this type of analysis is considerable, and only a few studies have attempted to assess this. The limited data available suggests that relative contributions of genetic and environmental factors may be different for some brain structures in affected twins. The most common study design is comparison of MZ twins who are discordant for the disorder with healthy pairs. Because the genetic contribution is hypothesized to be the same within MZ twin pairs, increased variation within the discordant pair suggests the impact of additional environmental or disease-related factors, whereas brain differences found in both twins of the affected pair compared with the healthy twins imply a genetic risk factor independent of disease state. Studies have reported increased environmental contributions to the volumes of the lateral ventricles (Suddath, Christison, Torrey, Casanova, & Weinberger, 1990; van Haren et al., 2004) and the hippocampus (van Erp et al., 2004) in twins discordant for schizophrenia.Hulshoff Pol and colleagues (2004) studied gray and white matter volumes, and found that gray matter was different between the affected twin and their sibling, whereas white matter was decreased in both twins compared to controls, suggesting that white matter differences may be a clearer correlate of genetic vulnerability. A study of twins discordant for autism also found several structures to be similarly affected in both twins, whereas cerebellar volumes were only abnormal in the affected individuals (Kates et al., 2004). The recent findings of epistatic modifications and copy number variation within MZ twin pairs may make interpretation of brain differences in discordant pairs more complex.
Are the relative roles of genetic and environmental influences on brain structure during childhood and adolescence static? A recent meta-analysis found that heritability increased for a range of cognitive and behavioral features, including IQ, externalizing behaviors, anxiety, and depressive symptoms, although not ADHD (Bergen, Gardner, & Kendler, 2007). The post-pubertal onset of schizophrenia has raised the question of whether its manifestation could be related to developmental changes in brain gene expression (Cannon et al., 2003). Studies directly studying developmental changes in gene expression in animal models have found marked changes during both pre- and postnatal development. An intriguing study by Stead and colleagues (2006) compared gene expression across different regions of the brain in mice at different developmental stages. They found that developmental stage explained more of the variance in gene expression of different regions than any other factor, speaking to the importance of considering developmental effects on gene expression. Similar data from human brain development has been relatively sparse, in part due to the rarity of appropriate postmortem tissue samples from healthy pediatric subjects. However, a gradually accumulating body of literature has provided evidence of postnatal changes in genes, including dopamine transporter, BDNF, and its receptor tyrosine kinase B, among others (Webster, Herman, Kleinman, & Shannon Weickert, 2006; Weickert et al., 2007).
The noninvasive nature of MRI has now made it possible to quantify genetic and environmental influences on brain development during childhood and adolescence through the study of pediatric twins. A challenging aspect of large-scale, multicenter or longitudinal MRI studies is avoiding potential biases introduced by differences in equipment at different sites or different times during the study. Measurements such as the volume of subcortical nuclei or the thickness of the cerebral cortex are dependent on both the resolution of the image and the clarity of the contrast between gray matter, white matter, and cerebrospinal fluid, and thus methodologic differences can introduce variation in measurement results (Filippi et al., 1997). For example, in many older MRI studies, brain images were acquired in slices that were 3–5 mm thick. Because 3–5 mm is also the depth of the cerebral cortex, studies done at this resolution would have limited ability to detect changes in cortical thickness, and may even result in different volumes of brain compartments due to partial volume effects at borders between tissue types. More subtle confounds can arise from image distortions specific to each individual MRI scanner, which can result in measurement differences despite otherwise similar image parameters (Jovicich et al., 2006). A strength of the NIMH investigation has been the stability of the MRI platform and acquisition parameters throughout the duration of the study.
Although several studies are currently underway, as yet there have been few published reports of MRI results from pediatric subjects, and the NIH study is the only to date to have had sufficient sample size across a broad enough age range to explore the issue of effects of age on the heritability of different brain regions. We therefore will be concentrating on results from this study.
The NIMH Pediatric Twin Study
Healthy pediatric MZ and DZ twins ages 4–24 years are recruited nationwide as part of a larger longitudinal study of normal brain development (Giedd et al., 1999). Siblings of twin pairs are included in the study as they are able to add to the power to detect shared environmental or dominance effects (Posthuma & Boomsma, 2000). Following a telephone screen, families are brought to NIH where participants receive physical examination and interview, participate in a neurocognitive testing, provide blood to confirm zygosity and for further genetic analysis, and undergo an MRI. Participants return for follow-up cognitive testing and MRI at approximately 2-year intervals.
We first studied brain structures including volumes for lobar gray and white matter, caudate and lateral ventricle, and area of the corpus callosum in 90 MZ twin pairs, 38 same-gender DZ twin pairs, and 158 singletons (average age = 11.5, SD = 3.5; Wallace et al., 2006). All brain regions with the exception of the lateral ventricles and cerebellum were significantly heritable, with genetic factors responsible for between 70 and 90% of variance, similar to previous reports in adults. When we then looked at the interaction of age with heritability, we found that variance due to genetic effects increased with age in both gray and white matter. However, in white matter the environmental variance decreased, whereas in gray matter it increased, leading to a decrease in the heritability ratio. These findings suggested that heritability indeed changes during the age span within our study, and that effects are specific for different types of brain tissue.
We next analyzed heritability of cortical thickness in an expanded pediatric sample (214 MZ twins, 94 same-gender DZ twins, 64 siblings of twins, and 228 singletons, average age = 11.08, SD = 3.5; Lenroot, Schmitt, et al., 2007). Cortical thickness measurements were obtained and at each of 40,062 individual vertices using an automated method developed by colleagues at the Montreal Neurologic Institute (Lerch & Evans, 2005). Variance parameters were then estimated with structural equation modeling both for each individual vertex and for the average thickness in 54 separate cortical regions (Kabani, Le Goualher, MacDonald, & Evans, 2001).
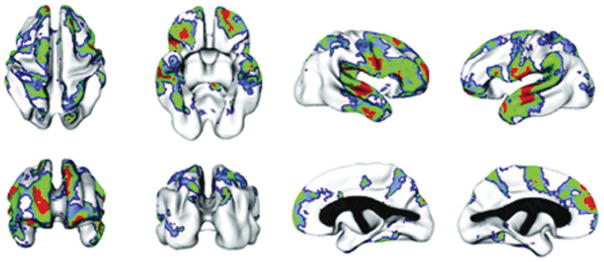
When parameters were calculated with the effects of age accounted for in the model, we found significant genetic effects in the dorsal prefrontal and superior temporal regions, areas also reported as heritable in the earlier study done with a different methodology in adults (Figure 3; Thompson, Cannon, et al., 2001).
Figure 3.
The regions of significant heritability. Voxels are color coded for the level of significance following applications of the FDR threshold. Red voxels are significant at p ≤ .05, green voxels are significant at p = .05–.10, and blue voxels are significant at p = .10–.15, corresponding to uncorrected p values of .002, .016, and .042, respectively.
Additional regions under strong genetic influence in our sample included the superior parietal lobes, orbitofrontal cortex, and inferior surfaces of the temporal lobes. However, despite the increased power, the magnitude of heritability estimates was smaller in the pediatric sample than in the previous adult study. These disparities raised the question of whether they were because of the larger sample size in the pediatric study, methodologic considerations, or age differences.
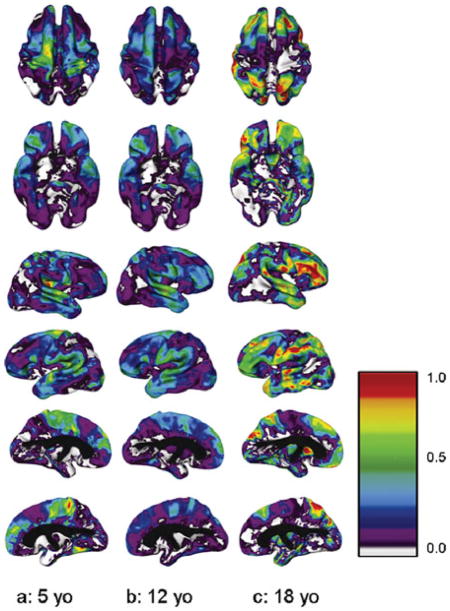
When we looked at how age interacted with heritability values in the pediatric sample, we found that the pattern depended on the region of the cortex. Regions such as the primary motor and sensory cortices that were not highly heritable in adults were significantly heritable in younger children but became progressively less so with time. Conversely, areas that were strongly heritable in adults were less so earlier in development. Instead, heritability in these regions gradually increased over childhood and adolescence. In the oldest ages heritability in the frontal and temporal regions was of comparable magnitude to that reported in the adult study, suggesting that the lower heritability seen when the sample was taken as a whole may have related to the lower average age of the sample (Figure 4).
Figure 4.
Age as a continuous variable: heritability (a2) at ages 5, 12, and 18 years for superior, inferior, right, and left cortical surfaces. Values at each age were derived from the modeled interaction of age as a continuous variable with estimates of genetic and environmental variance components. The color bar shows the scale of heritability values from 0.0 to 1.0.
Examination of how individual variance components changed with age showed that the strongest trend was a decrease in total variance across the cortex, primarily due to decreases in environmental variance. An opposite trend was seen in some areas, in that genetic variance increased in the dorsolateral prefrontal and superior parietal lobes, while variance due to environmental factors also increased in portions of the primary motor and sensory cortices.
Areas in which nongenetic factors were the chief contributors to variance were extensive. It is not possible with this study design to separate true unique environmental influences from any other nongenetic sources of variance, including error, making interpretation more difficult. However, it is intriguing that these areas included those regions associated with primary motor and sensory functions, whereas stronger genetic effects were seen more prominently in regions of association cortex.
Potential Mechanisms for Changes in Heritability With Age
Current theories describe the creation of cortical areas as occurring through the establishment of a series of genetically controlled anchor points which serve as loci for overlapping gradients of growth factors (Grove & Fukuchi-Shimogori, 2003). Characteristics of specific cortical areas develop over time in response to the local combination of growth factors and activation. It has been argued that primary motor and sensory cortices may serve as core anchor regions whose earliest development is strongly genetically determined (Rosa & Tweedale, 2005). The pattern observed here of genetic effects predominating in these core regions early in childhood may be consistent with their relatively early development, followed by a mature state in which a high degree of plasticity reflects their roles as the direct intermediaries with the external environment.
The reason for a decrease in total variance over development, particularly that due to environmental factors, is not obvious. It may seem more logical that variance because of environmental factors should increase with age as opportunities for exposure to unique environmental events extend. For the early-developing cortical regions in which environmental variance does increase during the age range of our study, this may be the case. However, for the later developing areas, the heritable phenotype is something that itself is being created over time. As described earlier, there is an increasing body of literature regarding changes in gene expression continuing over the course of postnatal development. If genes with particularly strong effects come on line, they may potentially substantially impact the variance because of genetic factors. The interaction of genetic with environmental factors over time may also increase heritability.
One mechanism that has been suggested for this is gene–environment correlation (rGE). rGE occurs when the same genes affect both a trait and relevant features of the environment, and also acts to increase the amount of variance ascribed to genetic factors (Kendler & Baker, 2007; Scarr & McCartney, 1983). rGE can be divided into three types. The passive form of rGE occurs when parents provide both genes and early environmental conditions for their children. The active form concerns the tendency for individuals to seek environments that reinforce genetic predispositions The third type, evocative/reactive rGE, refers to the way in which genetically driven behaviors can result in the creation of specific environmental responses (Plomin, DeFries, & Loehlin, 1977). The relative prominence of these factors is likely to change over development, and may increase as children become more independent and able to choose their own environments.
Other models of gene–environment (G×E) interactions include experience-expectant and experience-dependent processes, outlined by Greenough and colleagues as mechanisms by which environmental factors affect brain development (Andersen, 2003; Greenough, Black, & Wallace, 1987). Experience-expectant refers to the integration of environmental stimuli into normal patterns of brain development, the classic example being the effects of monocular visual deprivation on the developing visual cortex as described by Hubel and Wiesel (1998). Specific times during the development of a particular neural system when certain types of environmental stimulation must occur for normal development to take place are called critical periods. Experience-dependent processes are defined in contrast as means by which unique environmental factors may affect the developing nervous system in distinctive ways. In this case the brain may have particular sensitivities to environmental factors such as a trauma at specific developmental stages, resulting in differing effects on the long-term trajectory depending on when an event occurred. The prolonged developmental course of the human brain suggests that these processes may continue to play a role in reaching the mature phenotype well after birth.
Canalization refers to the frequently observed robustness of mature phenotypes against minor genetic or environmental perturbations during development, as in the example of compensatory growth (Flatt, 2005; Schmalhausen, 1949; Tanner, 1963; Waddington, 1942). G×E interactions have been proposed as one path by which canalization may occur. For example, genetic determinants of plasticity in response to the environment may constrain structures to develop along a heritable trajectory from an undifferentiated beginning to a genetically determined mature state (Garlick, 2002). Repetitive patterns of activity may also sculpt plastic developing structures. An example of this was described by Zelditch, Lundrigan, and Garland (2004), who found that variance in murine skull morphometry decreased during early postnatal development. They hypothesized that high initial variance decreased as initial unorganized immature patterns of motor activity took on the predictable characteristics of adulthood. Such a process is reminiscent of those thought to underlie activity-dependent changes in the cerebral cortex.
This model would suggest that heritability values in mature individuals may be different for those whose environment was deficient in the necessary environmental features. Studies regarding the effects of deficient environmental conditions on the heritability of brain structures in adults are not to our knowledge currently available, and identifying and directly measuring relevant environmental factors is highly challenging. However, one might speculate that if an environmental feature during development was an integral component of developing a mature heritable phenotype, departure from the optimal range for family members would result in an increase in variance due to their shared environment. Some indirect evidence for this may be found in the IQ literature. As previously mentioned, IQ increases in heritability over childhood and adolescence, and shared environmental effects are typically nonsignificant (Plomin, Fulker, Corley, & DeFries, 1997). Studies of heritability of cognition have found that there is a G×E interaction such that shared environmental factors become more prominent relative to genetic factors as socioeconomic conditions worsen (Harden, Turkheimer, & Loehlin, 2007; Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003).
Delineating how genetic and environmental factors interact during development is essential to improve our understanding of causes of psychopathology (Thapar, Harold, Rice, Langley, & O’Donovan, 2007). The familiar “stress-diathesis” framework arose from the recognition that individuals possess varying degrees of vulnerability or resilience to environmental stressors. Twin studies have supported the role of G×E interaction in several different disorders. For example, studies have found that adverse life events are more likely to result in depression in both adults and adolescent girls who are also at genetic risk (Kendler et al., 1995; Silberg, Rutter, Neale, & Eaves, 2001). Children are more likely to exhibit antisocial behavior if they have both a genetic risk and a history of adverse early experiences, compared to children with either risk factor separately (Cadoret, Cain, & Crowe, 1983).
Such findings are consistent with a growing body of work on the impact of interactions of specific genetic polymorphisms with specific environmental risk factors (Moffitt, Caspi, & Rutter, 2006). Emerging data from large-scale studies that measure genotypes, relevant environmental factors, and behavioral or health outcomes are indicating that identifying specific G×E interactions is possible. One of the first examples was the finding that maltreated children were more likely to develop behavioral problems if they also had a specific functional polymorphism in the gene encoding a neurotransmitter-metabolizing enzyme, monoamine oxidase A (Caspi et al., 2002). The effects of polymorphism in the promoter region for the gene encoding the serotonin receptor have also been demonstrated to contribute to G×E interactions (Caspi et al., 2003; Kaufman et al., 2004; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005).
In the twin model described earlier, the effects of this type of interaction would be subsumed under genetic effects, and it is likely that such G×E interactions over time are contributing to the increase in heritability of some brain regions. Identifying which environmental conditions affect outcome for individuals with specific genetic variations will be a major advance toward the ultimate goal of knowing how to tailor interventions to facilitate healthy brain development.
Conclusion
Our studies to date have demonstrated regionally specific changes in heritability. Previous studies have shown that the nonlinear characteristics of brain developmental trajectories only become prominent when longitudinal data and large samples are available (Giedd et al., 1999; Lenroot, Gogtay, et al., 2007), and that trajectories may be more informative regarding neurodevelopmental differences between groups than cross-sectional measures (Shaw et al., 2006). Longitudinal analyses will be necessary to determine whether these trajectories themselves are heritable, and our group and others have embarked on following pediatric twins over time to be able to address such questions. Another implication of the findings by ourselves and others that the brain continues to show dynamic changes over the life span is that heritability may also continue to change after early adolescence. Studies of age effects on heritability at the other end of the life span are also sparse. A longitudinal study in elderly male twins described ongoing strong genetic effects despite age-related changes in cortical features such as increased size of the lateral ventricles, supporting that age-related changes during senescence may also be genetically mediated (Pfefferbaum, Sullivan, & Carmelli, 2004).
The contrast between findings of increased heritability of cortical thickness with age seen here and decreased heritability of lobar gray matter volumes reported in a subset of this population (Wallace et al., 2006) appears to be driven chiefly by differences in the interaction of environmental variance with age, which increased for gray matter volume but decreases for cortical thickness. Cortical gray matter volume is affected both by the thickness of the cortex and its surface area, which are determined by different types of cell division during the original formation of the cortex (Rakic, 1988). The surface area is also dependent on factors such as gyrification, which have been found to increase during this age range (Sowell et al., 2002). Although developmental changes in both cortical thickness and surface topology during childhood and adolescence have been reported (Gogtay et al., 2004; Sowell et al., 2002), little is known about how longitudinal changes in these measurements may relate to each other.
It will also be important to begin to look more closely at how the interactions between brain regions are affected by genetic and environmental factors. Behaviors arise from the interactions of many different brain regions, and a variety of neuropsychiatric disorders have demonstrated the presence of impaired functional or structural connections between brain regions (Friston, 2005; Minshew & Williams, 2007; Rich et al., 2008). Studies have found measures of brain connectivity such as EEG coherence to be highly heritable (van Beijsterveldt, Molenaar, de Geus, & Boomsma, 1998) and to show developmental changes (Thatcher, North, & Biver, 2007; van Baal, Boomsma, & de Geus, 2001). Resting state fMRI studies have also found increased correlations between activity in different brain regions with maturation (Fair et al., 2008). In a previous study of a subset of this population, we found that variance decreased and strength of intracortical correlations increased in several of the same association areas that demonstrate increasing heritability in the current study (Lerch et al., 2006). It is tempting to speculate that increasing heritability in these late-developing regions may be also be related to the increasing functional integration of these areas in maturing cortical networks.
Following on the question of the relative roles of genes and environment during brain development is determining which specific genetic and environmental factors are most responsible. Multivariate analyses begin to open the way to asking whether the same factors are affecting different components of brain structure or function. For example, a principal components analysis of a cross-sectional sample within our data has shown that a single genetic factor appears to be responsible for 60% of the variance in cortical thickness, and that regions that are functionally and/or structurally connected also tend to be affected by similar genetic factors (Schmitt et al., 2008). A next step will be to determine if or how these factors change with age.
The increased heritability of later maturing areas such as the prefrontal cortex, superior temporal gyri, and superior parietal lobes parallels the increase of heritability in cognitive features such as IQ and language abilities associated with these areas (Plomin et al., 1997). One implication is that studies using brain morphometry as an endophenotype to link genes and behavior will need to consider age in the analysis, as brain structural elements may be suitable at one developmental stages but not another. Our findings are also congruent with evidence from other fields that different brain regions may be particularly sensitive to environmental factors at specific periods during childhood and adolescence.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institutes of Mental Health.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Sedvall GC, Terenius L, Kulle B, Frigessi A, Hall H, et al. BDNF gene variants and brain morphology in schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B:513–523. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, et al. Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Archives of General Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Bruder CE, Piotrowski A, Gijsbers AA, Andersson R, Erickson S, de Stahl TD, et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. American Journal of Human Genetics. 2008;82:763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret RJ, Cain CA, Crowe RR. Evidence for gene–environment interaction in the development of adolescent antisocial behavior. Behavioral Genetics. 1983;13:301–310. doi: 10.1007/BF01071875. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: Contributions of genes, environment, and their interactions. Schizophrenia Bulletin. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century: Theoretical and methodological considerations in examining the biological contributors to resilience. Development and Psychopathology. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, Boomsma DI. A test of the equal environment assumption (EEA) in multivariate twin studies. Twin Research and Human Genetics. 2006;9:403–411. doi: 10.1375/183242706777591290. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM. Three steps toward improving the measurement of behavior in behavioral phenotype research. Child and Adolescent Psychiatric Clinics of North America. 2007;16:617–630. doi: 10.1016/j.chc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biological Psychology. 2002;61:33–51. doi: 10.1016/s0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, van Waesberghe JH, Horsfield MA, Bressi S, Gasperini C, Yousry TA, et al. Interscanner variation in brain MRI lesion load measurements in MS: Implications for clinical trials. Neurology. 1997;49:371–377. doi: 10.1212/wnl.49.2.371. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1918;52:399–433. [Google Scholar]
- Flatt T. The evolutionary genetics of canalization. Quarterly Review of Biology. 2005;80:287–316. doi: 10.1086/432265. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Disconnection and cognitive dysmetria in schizophrenia. American Journal of Psychiatry. 2005;162:429–432. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- Garlick D. Understanding the nature of the general factor of intelligence: The role of individual differences in neural plasticity as an explanatory mechanism. Psychological Review. 2002;109:116–136. doi: 10.1037/0033-295x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Human Brain Mapping. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Greenstein D, Giedd JN, Lenane M, et al. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. American Journal of Psychiatry. 2003;160:569–571. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annual Reviews in Neuroscience. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents’ cognitive aptitude. Behavioral Genetics. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, et al. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001;108:E88. doi: 10.1542/peds.108.5.e88. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O’Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Archives of General Psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbuhr KC, Moses LM, Jerdonek MA, Naidu S, Hoffman EP. Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:99–105. doi: 10.1002/mrdd.10026. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20:401–412. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Brans RG, van Haren NE, Schnack HG, Langen M, Baare WF, et al. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biological Psychiatry. 2004;55:126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, et al. Genetic contributions to human brain morphology and intelligence. Journal of Neuroscience. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur YM, Shin JS. Effects of chorion type on genetic and environmental influences on height, weight, and body mass index in South Korean young twins. Twin Research and Human Genetics. 2008;11:63–69. doi: 10.1375/twin.11.1.63. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Can neuroscience be integrated into the DSM-V? Nature Reviews Neuroscience. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Van Gestel S, Derom C, Thiery E, Vernon P, Derom R, et al. Heritability estimates of intelligence in twins: Effect of chorion type. Behavioral Genetics. 2001;31:209–217. doi: 10.1023/a:1010257512183. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: A validation study. NeuroImage. 2001;13:375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. American Journal of Psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Twin studies of adult psychiatric and substance dependence disorders: Are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychological Medicine. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. American Journal of Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: A replication. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2007 doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Bui QM, Taylor AK, Pratt C, Epstein J, et al. Effect of fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. American Journal of Medical Genetics. Part A. 2003;122A:13–23. doi: 10.1002/ajmg.a.20214. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. The influence of zygosity and chorion type on fat distribution in young adult twins consequences for twin studies. Twin Research. 2001;4:356–364. doi: 10.1375/1369052012524. [DOI] [PubMed] [Google Scholar]
- Machin GA. Some causes of genotypic and phenotypic discordance in monozygotic twin pairs. American Journal of Medical Genetics. 1996;61:216–228. doi: 10.1002/(SICI)1096-8628(19960122)61:3<216::AID-AJMG5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- McDonald C, Dineen B, Hallahan B. Meta-analysis of brain volumes in unaffected first-degree relatives of patients with schizophrenia overemphasizes hippocampal deficits. Archives of General Psychiatry. 2008;65:603–605. doi: 10.1001/archpsyc.65.5.603. [DOI] [PubMed] [Google Scholar]
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, et al. A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophrenia Research. 2008;101:95–105. doi: 10.1016/j.schres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, et al. Neurocognitive endophenotypes of obsessive–compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene–environment interactions in psychopathology: Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Current Opinion in Genetics and Development. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: Genes at last? Trends in Genetics. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Calhoun V. Structural and functional magnetic resonance imaging in psychiatric disorders. Canadian Journal of Psychiatry. 2007;52:158–166. doi: 10.1177/070674370705200304. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype–environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Plomin R, Fulker D, Corley R, DeFries J. Nature, nurture, and cognitive development from 1 to 16 years: A parent–offspring adoption study. Psychological Science. 1997;8:442–447. [Google Scholar]
- Plomin R, Willerman L, Loehlin JC. Resemblance in appearance and the equal environments assumption in twin studies of personality traits. Behavioral Genetics. 1976;6:43–52. doi: 10.1007/BF01065677. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1—An emerging role in psychosis and cognition. Biological Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behavioral Genetics. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Neale MC, Hulshoff Pol HE, Baare WEC, Kahn RS, et al. Multivariate genetic analysis of brain structure in an extended twin design. Behavioral Genetics. 2000;30:311–319. doi: 10.1023/a:1026501501434. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: Impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Kochunov P, Lancaster J, Shelledy W, Glahn D, Blangero J, et al. Heritability of brain volume, surface area and shape: An MRI study in an extended pedigree of baboons. Human Brain Mapping. 2007;28:576–583. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. American Journal of Psychiatry. 2004;161:2038–2044. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Tweedale R. Brain maps, great and small: Lessons from comparative studies of primate visual cortical organization. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360:665–691. doi: 10.1098/rstb.2005.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schmalhausen II. Factors of evolution: The theory of stabilizing selection. Chicago: University of Chicago Press; 1949. [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, et al. Identification of genetically mediated cortical networks: A multivariate study of pediatric twins and siblings. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Neale M, Eaves L. Genetic moderation of environmental risk for depression and anxiety in adolescent girls. British Journal of Psychiatry. 2001;179:116–121. doi: 10.1192/bjp.179.2.116. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, et al. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: Maturation in perisylvian cortices. Cerebral Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, et al. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs post-partum. Journal of Neuroscience. 2006;26:345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. New England Journal of Medicine. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Hodgkinson CA, Robinson DG, Derosse P, Bilder RM, Lencz T, et al. DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biological Psychology. 2007 doi: 10.1016/j.biopsycho.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. The regulation of human growth. Child Development. 1963;34:817–847. doi: 10.1111/j.1467-8624.1963.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harold G, Rice F, Langley K, O’Donovan M. The contribution of gene–environment interaction to psychopathology. Development and Psychopathology. 2007;19:989–1004. doi: 10.1017/S0954579407000491. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Biver CJ. Development of cortical connections as measured by EEG coherence and phase delays. Human Brain Mapping. 2007;28 doi: 10.1002/hbm.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van Baal GC, Boomsma DI, de Geus EJ. Longitudinal genetic analysis of EEG coherence in young twins. Behavioral Genetics. 2001;31:637–651. doi: 10.1023/a:1013357714500. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Genetic and environmental influences on EEG coherence. Behavioral Genetics. 1998;28:443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Archives of General Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Bakker SC, Kahn RS. Genes and structural brain imaging in schizophrenia. Current Opinion in Psychiatry. 2008;21:161–167. doi: 10.1097/YCO.0b013e3282f4f25b. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, Ribchester T, et al. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biological Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot RK, Viding E, Ordaz S, Rosenthal MA, et al. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Warren KR, Li TK. Genetic polymorphisms: Impact on the risk of fetal alcohol spectrum disorders. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2005;73:195–203. doi: 10.1002/bdra.20125. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expression Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, et al. Postnatal alterations in dopaminergic markers in the human pre-frontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cerebral Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: Methods and preliminary results. NeuroImage. 2002;17:256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution and the genetics of populations. I. Genetic and biometric foundations. Chicago: University of Chicago Press; 1968. [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Annals of the New York Academy of Sciences. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Lundrigan BL, Garland T., Jr Developmental regulation of skull morphology. I. Ontogenetic dynamics of variance. Evolution & Development. 2004;6:194–206. doi: 10.1111/j.1525-142X.2004.04025.x. [DOI] [PubMed] [Google Scholar]