A polymorphism within the catechol-O-methyltransferase (COMT) gene results in valine (Val) to methionine (Met) substitution, with the Met158 allele having lower enzymatic activity giving increased synaptic dopamine in prefrontal cortical regions where the gene is heavily expressed.1 There is mixed evidence linking this increased dopamine to enhanced cognitive performance and more ‘efficient’ underlying cortical activity in Met158 carriers.2,3 Few studies have examined possible neuroanatomic effects, most only at a lobar volumetric level, and none has included a pediatric population, nor longitudinal data that afford an opportunity to detect age-related gene effects.4–8 Here we demonstrate a ‘dosage’ effect of the Met158 allele, with a stepwise increase in cortical thickness of the right inferior frontal and superior temporal gyrus with increasing numbers of Met158 allele, and that this genotype effect is developmentally stable.
We genotyped 206 unrelated children and adolescents using standard methods,9 and acquired 464 T1-weighted spoiled gradient recalled neuroanatomic scans on the same 1.5 T GE scanner (for methodological details see Shaw et al.10). Following linear registration to standardized space and correction for nonuniformity artifacts, MR volumes were tissue segmented, and the inner and outer cortical surfaces were then extracted using deformable models, blurred, and cortical thickness was measured in native space at 40 960 vertices. There were 41 Val158 allele homozygotes, 113 Met158 heterozygotes, and 52 Met158 homozygotes. All had at least one neuroanatomic image (mean age 11.1 years, s.d. 3.9 years); 136 (66%) had at least two (mean age 13.1, s.d. 4 years); 79 (38%) had at least three scans (mean age 14.9, s.d. 3.9 years); and 43 (21%) had four or more scans (mean age 17.1 s.d. 3.9 years). Most (54%) were singleton births; the remainder was twin births (one child chosen at random per twin set). The proportion of singleton births did not differ significantly by genotype (χ(2)2 = 2.3, P = 0.31). There were 19 male children (46%) in the Val158Val, 63 (56%) in Val158−Met, and 35 (67%) in Met158Met group (χ(2)2 = 4.2, P = 0.12). The majority of children were Caucasian (N= 177, 86%) and the genotype groups did not differ significantly on ethnic composition (white versus others: χ(2)2 = 4.0, P = 0.13).
In mixed model regression analyses, genotype group was treated as a linear ordered factor (that is, Val158Val = 0, Val158Met = 1, Met158Met = 2) as there were no significant quadratic relationships throughout the cortex. Analyses showed no significant interaction between genotype and sex, nor between genotype and age terms implying that genotype effects were stable across the age range, and the final model thus included age and gender as covariates. Thus, the ith cortical thickness of the jth individual was modeled as
where dj is a random effects term modeling within-person dependence; the intercept and β terms are fixed effects, and eij represents the residual error.
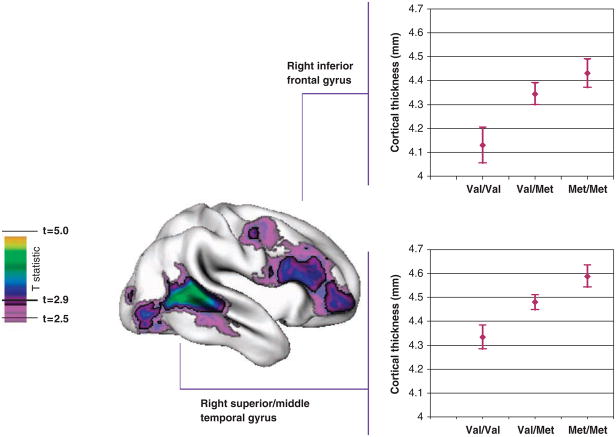
Met158 alleles were associated with a linear increase in cortical thickness in the right inferior frontal gyrus with a mean stepwise increase (β) of 0.14mm, s.e. = 0.04mm for each increase in the number of Met158 alleles (t = 3.4, P = 0.0008) and in the right superior and similarly in the right superior/middle temporal gyrus (β = 0.13mm, s.e. = 0.03mm, t = 3.9, P = 0.0001; Figure 1). This survived adjustment for multiple comparisons using the false discovery rate procedure with P = 0.05. The pattern of results held when IQ, sex, age, birth status were controlled, and for a Caucasian-only sample.
Figure 1.
T statistics are projected onto a brain template (right lateral view) that shows the regions with a linear increase in cortical thickness where Val158Val < Val158Met < Met158Met. Regions in pale purple had a significant linear trend at an unadjusted P < 0.01 (corresponding to t = 2.5) and regions in bold colors were significant following adjustment for multiple comparisons (false discovery rate-adjusted P < 0.05, t > 2.9). The graphs show the mean thickness (± s.e.) by genotype of the regions showing the Met158 ‘dosage’ effects, the right inferior frontal gyrus (top) and right superior/middle temporal gyrus (bottom).
IQ, as determined from age appropriate versions of the Wechsler intelligence scales showed a nonsignificant stepwise increase with genotype: Val158Val mean IQ of 112.7, s.d. 15; Val158Met mean 113.4, s.d. 14; Met158Met mean 114.2, s.d. 14 (β = 0.78, s.e. = 1.5, t = 0.5, P = 0.60).
The Met158 allele ‘dose’ effect on right inferior frontal cortical thickness is interesting as meta-analysis finds that among cognitive tests, possession of Met158 alleles is most robustly, if modestly (effect size of 0.06), linked with increased general intelligence (or ‘g’) in healthy subjects.3 The effect size translates to an increase of 0.9 IQ points with Met158 alleles, which is in line with our IQ findings. While ‘g’ is likely supported by a distributed brain network, there does appear to be a specific link with right inferior frontal cortex.11 This region is also implicated in working memory and response inhibition that some but not all find to be sensitive to COMT genotype, especially in children.3,12 The temporal cortical findings were unexpected, although the area is richly interconnected by the arcuate fiasciculus with the prefrontal cortex, others have reported greater temporal gray matter volume in Met158 homozygotes in adults,8 and patterns of temporal cortical gene expression have yet to be explored.
We thus demonstrate a robust Met158 genotype effect on cortical morphology in a right prefrontal cortical region intimately linked to key aspects of cognition, which may thus prove a suitable neuroanatomic endophenotype for future studies.
References
- 1.Tunbridge EM, Weickert CS, Kleinman JE, et al. Cereb Cortex. 2007;17:1206. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- 2.Tunbridge EM, Harrison PJ, Weinberger DR. Biol Psychiatry. 2006;60:141. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Barnett JH, Scoriels L, Munafo MR. Biol Psychiatry. 2008;64:137. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Cerasa A, Gioia MC, Labate A, et al. Neuroreport. 2008;19:405. doi: 10.1097/WNR.0b013e3282f5f784. [DOI] [PubMed] [Google Scholar]
- 5.Zinkstok J, Schmitz N, van Amelsvoort T, et al. Neurosci Lett. 2006;405:34. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi T, Hashimoto R, Mori T, et al. Brain. 2006;129(Part 2):399. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 7.Ho BC, Wassink TH, O’Leary DS, et al. Mol Psychiatry. 2005;10:229. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- 8.Taylor WD, Zuchner S, Payne ME, et al. Psychiatry Res. 2007;155:173. doi: 10.1016/j.pscychresns.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobb AJ, Addington AM, Sidransky E, et al. Am J Med Genet B Neuropsychiatr Genet. 2005;134:67. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 10.Shaw P, Greenstein D, Lerch J, et al. Nature. 2006;440:676. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 11.Duncan J, Seitz RJ, Kolodny J, et al. Science. 2000;289:457. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 12.Diamond A, Briand L, Fossella J, et al. Am J Psychiatry. 2004;161:125. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]