Abstract
In an effort to define the antigenic mechanism that contributes to beneficial therapeutic outcomes in patients with polycythemia vera (PV), we screened a human testis cDNA library with serological cloning derived from sera of three PV patients who had undergone therapeutic-induced remission. As a result, we identified a novel antigen, MPD5, which belongs to the group of cryptic antigens with unconventional genomic intron/exon structure. Moreover, MPD5 elicited IgG antibody responses in a subset of PV patients who had benefited from a variety of therapies—including IFN-α, Hydroxyurea, Imatinib mesylate, Anagrelide, and phlebotomy—but not in untreated PV patients or healthy donors, suggesting that MPD5 is a PV-associated, therapy-related antigen. In the granulocytes of PV patients who are responsive to therapy, upregulated MPD5 expression may serve to enhance immune responses. These findings provide new insight into the mechanism underlying regulation of the self-antigen repertoire that elicits anti-tumor immune responses in patients with myeloproliferative diseases, indicating the potential of these self-antigens as targets of novel immunotherapy.
Keywords: tumor antigen, unconventional antigen, IgG antibody responses, polycythemia vera, SEREX
INTRODUCTION
Interferon-α (IFN-α) therapy induces remission of polycythemia vera (PV)(1), a myeloproliferative disease (MPD)(2), suggesting that the anti-self-antigen immunity plays an important role in controlling the disease due to autoimmunity promoting effect of IFN-α. However, the self-antigens eliciting an anti-PV immunity remained poorly defined(3). By applying SEREX (serological analysis of tumor antigens by screening cDNA library with patients’ sera) to detect antigens that elicit immunity contributed to remission of MPDs(4), we have identified three broadly immunogenic antigens, CML66(5), CML28(6,7) and MPD6(3). Most of these tumor antigens identified to date are conventional antigens—encoded by genes with genomic exon-intron organization. In contrast, some tumor antigens are unconventional cryptic antigens (i.e., encoded by introns of genes or the untranslated regions [UTRs] of mRNAs) that can elicit T cell responses(8). However, it was incompletely clear whether unconventional antigens elicit humoral anti-tumor immune responses(3).
Screening testis cDNA library with SEREX is a useful approach to identify aberrantly expressed antigens based on the rationale that testis, an immune-privileged site, does not normally present its self-antigens to the immune system(8). In this report we have identified—for the first time—a novel unconventional SEREX antigen, MPD5, that specifically reacted to serum samples from three PV patients who experienced IFN-α- or other therapeutic-induced remission.
MATERIALS AND METHODS
Serum samples
In accordance with pre-approved IRB protocols and guidelines at Temple University, New York-Presbyterian Hospital, and Baylor College of Medicine (BCM), serum samples were obtained from healthy donors, PV patients whose disease had undergone remission in response to IFN-α or other therapies, and untreated PV patients. Specifically, at BCM, we enrolled: 8 PV patients receiving IFN-α treatment and 10 PV patients receiving other treatments; at New York-Presbyterian Hospital, we enrolled: 12 PV patients receiving IFN-α treatment, 24 PV patients receiving other treatments, and 6 PV patients who had not yet received any treatment. The therapeutic regimens other than IFN-α for the 34 patients with PV included imatinib mesylate (16 patients), hydroxyurea (12 patients), hydroxyurea plus Anagrelide (three patients), Anagrelide plus phlebotomy (one patient), phlebotomy (one patient), and thalidomide (one patient).
Human testis cDNA library screening by SEREX
The library screening and DNA sequencing were performed, as previously described(3).
In vitro transcription and translation (TNT)
TNT with plasmid containing MPD5 cDNA and a control were performed as reported(9).
Bioinformatic analyses
Sequence analyses were performed in the NCBI/NIH website as reported(3,10).
Northern blot and Southern blot
Multiple tissue Northern blots (Ambion, Inc., Austin, TX) were hybridized with probes of the MPD5, NEK6 intron 1 region (outside MPD5) (Fig. 1A), as reported previously(5). The specificity of the NEK6 intron 1 region probe was confirmed by Southern blot hybridization of the NEK6 intron 1 fragment, as previously described(9).
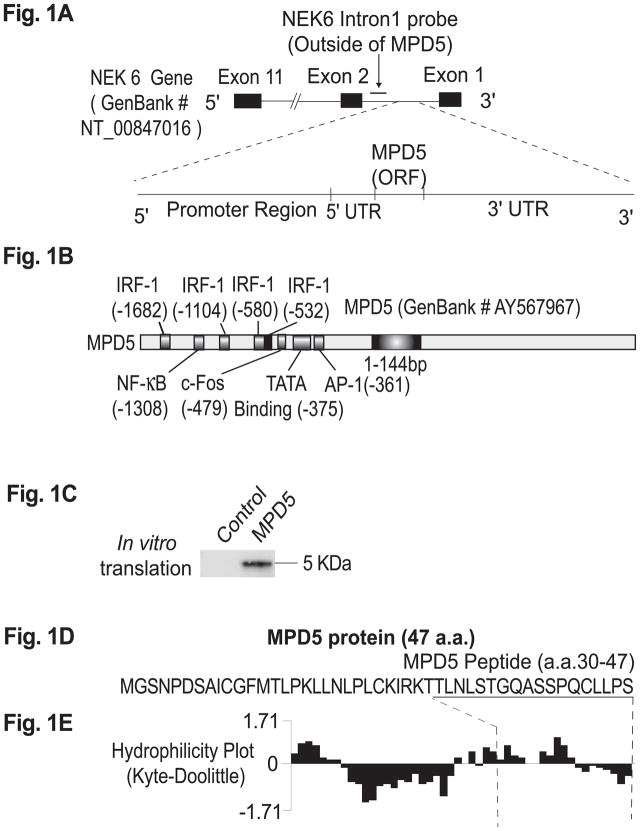
Fig. 1. The novel PV-associated SEREX antigen, MPD5, is an unconventional antigen.
A. A schematic representation of the location of the unconventional antigen, MPD5, in the complementary (i.e., antisense) strand within the intron 1 region of the NEK6 (NIMA-6) gene (GenBank accession number: NT_00847016). B. The structure of MPD5 promoter and the structure of MPD5 mRNA transcript. MPD5 has an ORF of 144 bp (GenBank accession number: AY567967). Four interferon regulatory factor 1 (IRF-1) binding sites—including NF-KB, AP1, and other transcription factor binding sites—were found in the 2kb region of the MPD5 promoter. These results suggest that MPD5 transcription may be regulated by interferon-α stimulation. C. The in vitro transcription and translation product of MPD5. D. The protein sequence of MPD5. E. The hydrophilicity index plot of MPD5, analyzed by the Kyte-Doolittle method, suggests that there are hydrophilic regions within the N-terminal and C-terminal regions of MPD5.
Semi-quantitative reverse transcription (RT)-PCR and PCR cloning
Human IFN-α (1× 105 units/100μl) was purchased from PBL Biomedical Laboratories (Piscataway, NJ). Stimulation of K562 cells, a human myeloid leukemia cell line, with IFN-α was executed (1000 units/ml) for the indicated time points(11). RT-PCR and PCR cloning were performed, as described(9). A sense primer (PV1ORF5) specific to the 5′ sequence of MPD5 (5′-AACAGCAGCCCTTTCTCTCCA GTA-3′) and an antisense primer (PV1ORF3) (5′-TAATACGCAGCAGAGCTGGATT G-3′) specific to the 3′ sequence of MPD5 were used for PCR. The PCR products (290 bp) were cloned into pCRII-TOPO vector (Invitrogen). As a control, PCRs were performed for β-actin with a sense primer, HB-actin5 (5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′), and an antisense primer, HB-actin3 (5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′). The signals of PCR products were normalized as relative densitometric units via comparison to β-actin signals amplified in the same cDNA preparations(9). PCR for the amplification of ISG15 was performed with a sense primer, ISG5 (5′-GAGAGGCAGCGAATTCATCT-3′), and an antisense primer, ISG3 (5′-AAGGGGGACCCTGTCCTG-3′), as a positive control for the genes upregulated by IFN-α stimulation, as reported (11).
Quantitative RT-PCR
Quantitative RT-PCR—used to determine MPD5 mRNA in total RNA isolated from peripheral blood granulocytes via TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems, AB, Foster City, CA)—was performed on the ABI PRISM 7000 System (AB,)(3). The primers and TaqMan probe for MPD5 were synthesized by AB, including: MPD5-EX1F (forward primer), 5′GCTGAGATTGCGCCACTGTA3′; MPD5-EX1R (reverse primer), 5′CCTCTCCT AGGCTCCAAGTGT3′; and MPD5-EX1M1 (probe labeled with FAM), 5′TCCAGCCTGGG ATTC3′. Used as the reference standard and labeled with VIC fluorophore (targeted center of sequence: 606 bp), the 18S ribosomal RNA were also purchased from AB. The amount of RNA representing the linear range of response for MPD5 and 18S was employed (i.e., typically, 100 ng - 0.01 ng RNA/reaction). PCR without RT was also executed for control. Threshold cycles (CT) at the reporter signal were normalized with the reference gene, 18S (h18S), and ΔCT [ΔCT = CTX − CTR, the difference in threshold cycles for the target gene X and the reference (R)] was calculated(2). Mean ΔCT was compared among groups via ANOVA(12).
Peptide synthesis and peptide ELISA
An MPD5-specific peptide was synthesized according to the sequence of MPD5, from aa 30 to aa 47 (N-TLNLSTGQASSPQCLLPS-C), at Sigma-Genosys (The Woodlands, TX). ELISAs were performed, as previously described(5).
Transcriptional clonality assay
The genotypes for exonic polymorphisms of 5 X-chromosome genes (i.e., MPP1, IDS, G6PD, BTK, and FHL1) of three PV patients were determined(2).
RESULTS
A novel PV-associated SEREX antigen, MPD5, is an unconventional antigen
SEREX screening of human testis expression cDNA library was performed using diluted sera collected from 3 PV patients who responded to IFN-α therapy; these patients were selected, based on two criteria: (1) the patients’ diseases had undergone remission, as judged by conversion from monoclonal to polyclonal hematopoiesis(2) (not shown); and (2) improved blood counts(1, 2). A screening of 1× 106λ phage clones led to the identification of two identical clones.
The open reading frame (ORF) in the clone (1517 bp) encoded 47 amino acids with the molecular size of 5 kD, herein referred to as MPD5 (GenBank accession: AY567967). The antisense sequence of MPD5 was identical to a 5.1 kb cDNA clone (GenBank accession: AL832574; not shown). Both MPD5 and the 5.1 kb clone were located in intron 1 of NEK6 (i.e., NIMA [never in mitosis gene a]-related kinase 6) (GenBank accession: NT_008470.16). A member of the serine/threonine kinase family, NEK6 is a homolog of histone H3 kinase, which is required for cell cycle progression through mitosis(13). The 5.1 kb cDNA was encoded on the same strand as the sense-strand of the NEK6 gene; whereas, whereas MPD5 was encoded in the complementary strand within the intron 1 region of the NEK6 gene (Fig. 1A). Notably, there were no long ORFs found in the sense and complementary sequences of this 5.1 kb cDNA clone. The function of the 5.1 kb cDNA is unclear; however, our identification of the MPD5 and 5.1 kb clones in the cDNA library suggest that the region in intron 1 of NEK6 is transcriptionally active on both strands. This argument is further supported by our in silico analysis on the promoter regions for MPD5 transcription, which showed several transcription factors including interferon regulatory factor-1 (IRF-1) binding sites(14) and sites for NF-κB(15), c-fos, and AP-1 (Fig. 1B).
The 5kD ORF of MPD5 was confirmed by TNT, depicted in Fig. 1C. The MPD5 protein sequence (Figs. 1D) was not identical to sequences in the NCBI databases or the SEREX database (http://www2.licr.org/CancerImmunomeDB/), although a segment in the 3′ untranslated region of MPD5 showed homology to a SEREX clone HOM-HD2-117 (the SEREX database, not shown). The following features support our contention that MPD5 is a human protein (Fig. 1D): (1) the start codon and the surrounding sequence contain the Kozak consensus for optimal translation(16); (2) the frequencies of amino acid (a.a.) codon usage, including a.a. residues with multiple codons, are identical to those used in human proteins; (3) analysis using the NCBI conserved domain database demonstrates that the a.a. sequence of MPD5—specifically, aa 7 to aa 43—has a high homology (i.e., 49%) with the GTPase Dbl-homologous (DH) domain (Protein family database: http://pfam.wustl.edu/, pfam00621); and (4) the MPD5 sequence encoded several sites from various posttranslational modifications, including cyclin interaction, protein kinase A phosphorylation site, and N-glycosylation site. Cumulatively, these MPD5 features correspond to the characteristics previously reported in short protein-encoding ORFs(17). Furthermore, in the Kyte-Doolittle hydrophilicity plot of the MPD5 peptide (Fig. 1E), the two hydrophilic regions suggest the potential for antibody binding epitopes(10).
The expression of MPD5 transcripts is elevated in tumors and PV
Applying our reported strategy(5,6), the Northern blot shows that the expression of MPD5 in 10 normal tissues (e.g., the brain [N1], colon [N5], prostate [N8], testes [N9], and ovary [N10], etc.) was negligible (Fig. 2A). However, MPD5 expression was significantly upregulated in a variety of tumor cells (Fig. 2B), including acute T cell leukemia (T1), Burkitt’s lymphoma (T2, T4, and T7), uterine carcinoma (T6), osteosarcoma (T8), histiocytic lymphoma (T9), and cervical adenocarcinoma (T10). Since the MPD5 is located on the antisense strand of intron 1 within the NEK6 gene (Fig. 1A), the Northern hybridization signals in tumor tissues (Fig. 2B) resulting from hybridization might have been attributable to contaminated genomic DNA in the RNA preparation. In order to exclude this possibility, we generated a 3.6 kb NEK6 intron 1 (intron 1) region (the lower panel in Fig. 2B) that has no overlap with the MPD5 region (Fig. 1A). Hybridization of the Northern blot with the intron 1 probe did not yield any observable signals, suggesting that the MPD5 signals (Fig. 2B) did not result from contaminated genomic DNA. In contrast, the Southern blot hybridization on agarose gel—used to analyze the intron 1 fragment with a special intron 1 probe—showed a strong signal (lower panel, Fig 2B). This suggests that the intron 1 probe is capable of detecting potentially contaminated genomic DNA in tumor tissue RNA preparations (Fig. 2B). In sum, our findings indicate the detection of upregulated MPD5 expression in tumor cells.
Fig. 2. The expression of MPD5 transcripts is elevated in tumors and PV.
A. The expression of MPD5 transcripts in normal tissues. Lanes N1 to N10 indicate various normal tissues in the brain (N1), liver (N2), placenta (N3), small intestine (N4), colon (N5), thymus (N6), spleen (N7), prostate (N8), testis (N9), and ovary (N10), respectively. The transcript sizes are indicated as kilobases (kb). B. Expression of MPD5 transcripts in tumor cells, as detected by Northern blot. Lanes T1 to T10 indicate various tumor cells in acute T cell leukemia (Jurkat cells) (T1), Burkitt’s lymphoma (CA46) (T2), breast cancer (MDA-MD-453) (T3), Burkitt’s lymphoma (Namalwa) (T4), epidermal carcinoma (A-431) (T5), uterine carcinoma (MES-SA) (T6), Burkitt’s lymphoma (Raji) (T7), osteosarcoma (MG-63) (T8), histiocytic lymphoma (U-937) (T9), and cervical adenocarcinoma (Hela S3) (T10), respectively. In the lower panel, the specificity of the NEK6 intron 1 probe (outside of MPD5) was confirmed by Southern blot of an agarose gel analyzing a NEK6 intron 1 fragment that was located outside the MPD5 region and had no overlap with MPD5. C. The expression of MPD5 transcripts in the granulocytes from PV patients and healthy donors, as detected by quantitative RT-PCR. The expression levels of MPD5 transcripts are expressed as the ΔCT (MPD5-18S). Low ΔCT values indicate higher expression of the specific gene. The groups, whose expression of MPD5 transcripts is statistically different from that of healthy donors (p<0.05), are marked with an *. D. The MPD5 expression in K562 cells stimulated with IFN-α, as detected by semi-quantitative RT-PCR (the left panel). RT-PCR of β-actin served as a housekeeping control for IFN-α stimulation. The RT-PCR of ISG15 was used as a positive control for IFN-α stimulation. In the right panel, the densitometric units were calculated by normalizing the densities of the PCR products of MPD5 and ISG15 with those of β-actin in the same sample.
We subsequently examined MPD5 expression in granulocytes via quantitative PCR, with 18S as the normalizer(2), to calculate MPD5 expression levels in PV patients receiving IFN-α therapy (n=10), PV patients receiving other treatments (Hydroxyurea, Imatinib mesylate, Anagrelide, or Phlebotomy; n=16), PV patients receiving no treatment (n=6), and healthy donors (n=12). Of note, as previously reported(2), low ΔCT values indicate higher expression of a specific gene. As shown in Fig. 2C, MPD5 was not expressed in the granulocytes of healthy donors (mean ± 2 × standard deviations [2SD] of the ΔCT [MPD5-18S] = 28.5 – 31.5). Conversely, MPD5 expression levels in the granulocytes of PV patients receiving IFN-α (mean ± 2SD of the ΔCT [MPD5-18S] = 24 – 27) and those of PV patients receiving other treatments (mean ± 2SD of the ΔCT [MPD5-18S] = 25 – 27.5) were higher than those of untreated PV patients or healthy donors (mean ± 2SD of the ΔCT [MPD5-18S] = 28.5 – 31.5) (p<0.05). Notwithstanding, MPD5 expression levels in the granulocytes of PV patients receiving IFN-α were not higher than those of patients receiving other treatments (p>0.05). It is also noteworthy that the discrepancy between the numbers of patients and healthy controls in Fig. 2C and that in Fig. 3 resulted from the limited volumes of some blood samples, in which high quality RNAs could not be prepared but the sera could be prepared for performing the experiments presented in Fig. 3.
Fig. 3. MPD5 peptide specifically reacts to sera from PV patients responding to therapies.
IgG antibody responses to the C-terminal antigenic epitope (from aa 30 to aa 47) of MPD5 were detected by peptide ELISA. These experiments were repeated three times, and representative results are shown. The mean plus three standard deviations (SD) of the OD405 peptide ratios over the coating control (derived from 23 healthy donors) was calculated as the upper limit of the normal range of MPD5 peptide antibody responses (the mean + 3SD = 1.38). The groups with detection rates of IgG antibody responses to MPD5 peptide that are statistically higher than those of healthy donors (the chi-square goodness-of-fit test; p<0.05) are marked with an *.
We further hypothesized that MPD5 expression in tumors can be upregulated either by direct IFN-α stimulation associated with IFN-α therapy or, potentially, through the activation of IRF-1 using other therapies(14). This hypothesis was supported by our in silico mapping of IRF-1 binding sites (G(A)AAAG/CT/CGAAAG/CT/C) (14) in the MPD5 promoter region (Fig. 1B). In Fig. 2D, the results of semi-quantitative RT-PCR showed that MPD5 transcripts in K562 myeloid leukemia cells were upregulated by IFN-α stimulation, even though the upregulation scale (nearly 100%) of MPD5 was not as high as that (200%) of IFN-α-stimulated gene-15 (ISG15) (Fig. 2D), which was employed as a positive control for the IFN-α-stimulated genes(11). Of note, the amplification of MPD5 transcripts by RT-PCR did not result from genomic DNA potentially contaminated in RNA prepared from stimulated K562 cells; this conclusion is based on the following: (1) the RNA were treated with RNase-Free DNase before reverse transcription and (2) the PCR involving primers located in the NEK6 intron 1 region outside of MPD5 did not result in any amplified products. However, the results suggest that a transcriptional mechanism in K562 cells could be one of the regulatory programs implicated in the upregulation of MPD5 expression induced by IFN-α or IRF-1 activation associated with other therapies(14). Of note, the limited number of PV patients prevented similar IFN-α stimulation performed with PV cells.
MPD5 peptide specifically reacts to sera from PV patients who experienced therapeutic-induced remission, but not sera from untreated patients or healthy donors
To further verify whether MPD5 antigen epitope is immunogenic in vivo, an MPD5 peptide was synthesized according to the amino acid sequence of the second antigen epitope of MPD5, from aa 30 to aa 47 (Figs. 1D and E). MPD5 peptide-specific IgG antibody responses, shown as the OD405 ratio (OD405 MPD5 peptide/OD405 coating control) (Fig. 3), as we previously reported(18), were negligible in the serum samples of all 23 healthy donors [the mean + 3SD = 1.38]. Thus, in Fig. 3, an OD405 ratio higher than 1.38 is considered positive for anti-MPD5 IgG antibody responses. As summarized in Fig. 3, MPD5 epitope-specific IgG antibody responses were detected in serum samples from 5 out of 20 (25%) PV patients receiving IFN-α therapy, and in 7 out of 34 serum samples (21%) from PV patients receiving other therapies (Fig. 3). Of note, a good correlation existed between increased MPD5 expression in granulocytes, as detected by quantitative PCR, and the specific antibody responses detected by ELISA in PV patients receiving IFN-α and other therapies. These results suggest that the antigenic epitope in MPD5 protein, which is naturally translated, was immunogenic in the PV patients receiving therapy. It is noteworthy that serum results obtained with the MPD5 ELISA corresponded well with those achieved through the phage plaque assay (not shown).
DISCUSSION
To the best of our knowledge, the identification of novel unconventional antigen MPD5 is a part of the first such study in PV and other BCR-ABL negative MPDs(3,19,20). Prior to the current report, whether unconventional cryptic antigens (21)can elicit humoral immune responses has not been well defined. Our recent results on T cell antigen epitope of CML66(22) indicate that integrated humoral and T cell immune responses to SEREX antigens are truly tumor-specific. Of note, the results of our analysis employing the methods(10) suggest that protein sequences of MPD5 have a high potential to encode T cell antigen epitopes. Several new systems contribute to protein antigen presentation and co-stimulation, and shape the T cell responses including Qa-1(23), and ICOS-B7RP-1(24). The question of whether these new systems participate in elicitation of T cell responses to unconventional protein antigens, such as MPD5, remains unknown.
We demonstrated that upregulation of untolerized antigen structure via alternative splicing is a novel mechanism in generating the immunogenicity of tumor antigens(8,18). The current study on MPD5 suggests that overexpression of “intron antigens” in tumor cells is one of the mechanisms involved in eliciting immune responses. In addition, overexpressed antigens must have untolerized antigen epitopes partially by splicing(25). Furthermore, overexpressed antigens must access the antigen presentation pathway and immune system by the following mechanisms(3): (1) releasing from damaged tumor cells followed by cross-presentation; (2) translocating across the intracellular membranes and entering exosome for the MHC class II antigen presentation pathway. Notwithstanding, the correlation between antigen-specific IgG immune responses and remission in PV patients(1) indicates that immune responses mediated by unconventional antigen(s) may contribute to MPD remission(5,6,8).
Acknowledgments
We are grateful for the assistance of Drs. C.J. Wu at Harvard University, M. Talpaz at the University of Michigan, and Candice Price and Alec Merber at New York-Presbyterian Hospital.
This work was supported by grants from the NIH (AI054514, X-FY), the Leukemia & Lymphoma Society, the Myeloproliferative Disorders Foundation (X-FY), and the Cancer Research & Treatment Fund (RTS).
References
- 1.Lengfelder E, Berger U, Hehlmann R. Interferon alpha in the treatment of polycythemia vera. Ann Hematol. 2000;79:103. doi: 10.1007/s002770050563. [DOI] [PubMed] [Google Scholar]
- 2.Liu E, Jelinek J, Pastore YD, Guan Y, Prchal JF, Prchal JT. Discrimination of polycythemias and thrombocytoses by novel, simple, accurate clonality assays and comparison with PRV-1 expression and BFU-E response to erythropoietin. Blood. 2003;101:3294. doi: 10.1182/blood-2002-07-2287. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Z, Liu E, Yan Y, Silver RT, Yang F, Chen IH, Chen Y, Verstovsek S, Wang H, Prchal J, Yang XF. An unconventional antigen translated by a novel internal ribosome entry site elicits antitumor humoral immune reactions. J Immunol. 2006;177:4907. doi: 10.4049/jimmunol.177.7.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CJ, Yang XF, Mclaughlin S, Neuberg D, Canning C, Stein B, Alyea EP, Soiffer RJ, Dranoff G, Ritz J. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang XF, Wu CJ, Mclaughlin S, Chillemi A, Wang KS, Canning C, Alyea EP, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2001;98:7492. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang XF, Wu CJ, Chen L, Alyea EP, Canning C, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CJ, Biernacki M, Kutok JL, Rogers S, Chen L, Yang XF, Soiffer RJ, Ritz J. Graft-versus-leukemia target antigens in chronic myelogenous leukemia are expressed on myeloid progenitor cells. Clin Cancer Res. 2005;11:4504. doi: 10.1158/1078-0432.CCR-05-0036. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Yang XF. New concepts in tumor antigens: their significance in future immunotherapies for tumors. Cell Mol Immunol. 2005;2:331. [PubMed] [Google Scholar]
- 9.Yang XF, Weber GF, Cantor H. A novel Bcl-x isoform connected to the T cell receptor regulates apoptosis in T cells. Immunity. 1997;7:629. doi: 10.1016/s1074-7613(00)80384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng B, Yang F, Huston DP, Yan Y, Yang Y, Xiong Z, Peterson LE, Wang H, Yang XF. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J Allergy Clin Immunol. 2004;114:1463. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JK, Siddiqui AA, Krishnaswamy GA, Dykes R, Berk SL, Magee M, Joyner W, Cummins J. Oral use of interferon-alpha stimulates ISG-15 transcription and production by human buccal epithelial cells. J Interferon Cytokine Res. 1999;19:923. doi: 10.1089/107999099313460. [DOI] [PubMed] [Google Scholar]
- 12.Rosner B. Multisample Inference. In: Rosner B, editor. Fundamentals of Biostatistics. 5. Duxbury Thomas Learning; Australia, Canada, Mexico, Singapore, Spain, United Kingdom, United States: 2000. p. 511. [Google Scholar]
- 13.Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem. 2003;278:52454. doi: 10.1074/jbc.M308080200. [DOI] [PubMed] [Google Scholar]
- 14.Romeo G, Fiorucci G, Chiantore MV, Percario ZA, Vannucchi S, Affabris E. IRF-1 as a negative regulator of cell proliferation. J Interferon Cytokine Res. 2002;22:39. doi: 10.1089/107999002753452647. [DOI] [PubMed] [Google Scholar]
- 15.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak M. Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7:563. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 17.Furuno M, Kasukawa T, Saito R, Adachi J, Suzuki H, Baldarelli R, Hayashizaki Y, Okazaki Y. CDS annotation in full-length cDNA sequence. Genome Res. 2003;13:1478. doi: 10.1101/gr.1060303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Phan L, Yang F, Talpaz M, Yang Y, Xiong Z, Ng B, Timchenko NA, Wu CJ, Ritz J, Wang H, Yang XF. A novel mechanism of alternative promoter and splicing regulates the epitope generation of tumor antigen CML66-L. J Immunol. 2004;172:651. doi: 10.4049/jimmunol.172.1.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y. SEREX review. Cancer Immunity. 2004 http://www.cancerimmunity.org/SEREX/
- 20.Yang X, Mirkovic D, Zhang S, Qe Zhang Y, Yan Z, Xiong F, Yang IH, Chen L, Li Wang H. Processing sites are different in the generation of HLA-A2.1-restricted T cell reactive tumor antigen epitopes and viral epitopes. Intl J Immunopathol Pharmacol. 2006;19(4) doi: 10.1177/039463200601900415. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shastri N, Schwab S, Serwold T. Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules. Annu Rev Immunol. 2002;20:463. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, Chen Y, Yang F, Chen IH, Xiong Z, Wang J, Lachman LB, Wang H, Yang X-F. HLA-A2.1-restricted T cells are reacted to SEREX-defined tumor antigen CML66L and suppressed by CD4+CD25+ regulatory T cells. Intl J Immunopathol Pharmacol. 2006 Sept 29; doi: 10.1177/039463200702000109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Werneck MB, Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006;212:51. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Nurieva RI. Regulation of immune and autoimmune responses by ICOS-B7h interaction. Clin Immunol. 2005;115:19. doi: 10.1016/j.clim.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, I, Chen H, Xiong Z, Yan Y, Wang H, Yang XF. Model of stimulation-responsive splicing and strategies in identification of immunogenic isoforms of tumor antigens and autoantigens. Clin Immunol. 2006;121:121. doi: 10.1016/j.clim.2006.06.007. [DOI] [PubMed] [Google Scholar]