Abstract
Purpose of review
The mitogen-activated protein (MAP) kinases are intracellular signaling proteins which play a central role in controlling the activity of pathways that regulate production and activity of multiple mediators of joint tissue destruction. The therapeutic potential of MAP kinase inhibition in osteoarthritis was reviewed.
Recent findings
Results from basic research studies support the role of MAP kinases as central mediators that regulate expression of pro-inflammatory cytokines and metalloproteinases but also as potential pain mediators as well. Cell culture and animal model studies suggest that inhibition of MAP kinases might slow progression of osteoarthritis but trials of MAP kinase inhibitors in humans with osteoarthritis have not yet been reported. Safety concerns of the currently available inhibitors have limited their initial use to trials in conditions considered more severe than OA.
Summary
MAP kinase inhibition has the potential to slow disease progression in osteoarthritis and also might reduce pain; however, safety concerns have limited the use of general MAP kinase inhibitors in humans. Further understanding of the function of specific isoforms of the MAP kinases as well as upstream and downstream effectors may lead to the development of more specific inhibitors with less toxicity that could eventually be used as structure-modifying drugs for OA.
Keywords: Osteoarthritis therapy, articular cartilage, MAP kinases
Introduction
Progression of osteoarthritis (OA) is characterized by destruction of the articular cartilage and other soft tissues in the joint, such as ligaments and, in the knee, the menisci. This tissue destruction is accompanied by remodeling and hypertrophy of neighboring bone and varying degrees of synovial inflammation [1*]. The destruction, remodeling, and inflammation of joint tissues leads to joint failure manifested as joint pain and loss of function. New treatments for OA, sometimes called structure-modifying OA drugs (SMOADs), are needed that can slow or halt the structural changes in joint tissue with the anticipation that this will result in significant pain reduction and improvement in function.
The structural changes in an OA joint are thought to be due to an imbalance in degradative (catabolic) and synthetic (anabolic) activity resulting in excessive production of matrix degrading enzymes and insufficient matrix repair. Contributing factors to the imbalance in homeostasis include the direct effects of mechanical loading on joint tissues, autocrine and paracrine signaling initiated by cytokines and the damaged matrix itself (matrix fragments), and an alteration in the phenotype of the chondrocytes which are the cells in the articular cartilage responsible for maintenance of the cartilage matrix [1*-3**]. Inhibiting the cell signaling pathways that regulate catabolic and anabolic activity would theoretically restore homeostasis in the joint and perhaps promote repair.
Central to the matrix damage that occurs in OA is the increased production, by chondrocytes, of matrix degrading enzymes including the matrix metalloproteinases (MMPs). Inhibition of the synthesis and/or activity of MMPs has been a focus in developing structural-modifying treatments for OA. However broad-spectrum MMP inhibitors have not been shown to slow progression of OA and appear to have dose limiting toxicity due to musculoskeletal side effects [4]. Inhibitors with greater specificity for MMPs thought to be central to OA, such as MMP-13 and aggrecanase-2, are being developed which might have greater efficacy and less toxicity. As an alternative to directly inhibiting MMP activity, the signaling pathways involved in MMP regulation are also being explored as therapeutic targets. MMP production is regulated by several signal transduction pathways, including those of the mitogen-activated protein (MAP) kinases. Because the MAP kinases are also central regulators of additional cell signaling pathways that control cell proliferation, survival, matrix synthesis, and production of pain mediators, they have been considered to be potential therapeutic targets for several diseases including osteoarthritis.
MAP kinase signaling
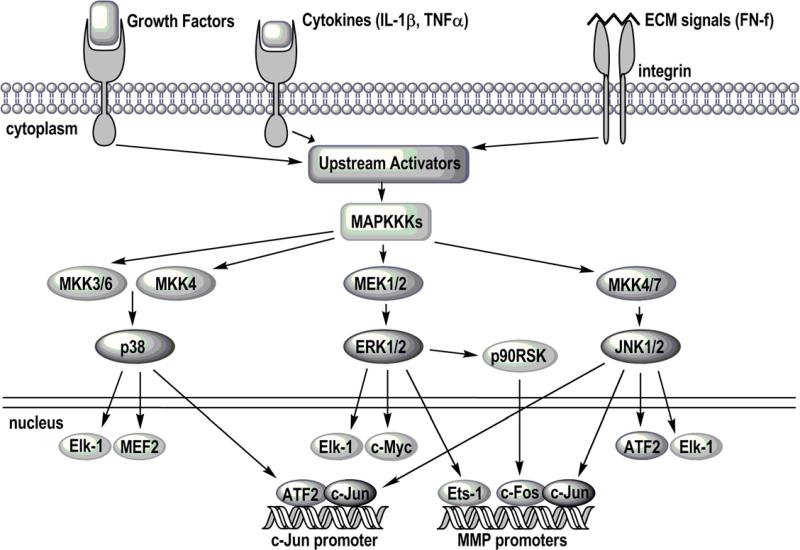
The MAP kinases are Ser/Thr kinases divided into 4 subfamilies that include extracellular regulated kinase (two isoforms - ERK 1 and 2), c-Jun NH2-terminal kinase, also called stress activated protein kinases or SAPK (three isoforms - JNK 1,2, and 3), p38 (four isoforms - α,β,γ,δ), and ERK5 [5]. Less is known about ERK5 and it has not been studied in relation to arthritis so this review will focus on the others. The MAP kinases are activated by a diverse range of stimuli including cytokines, growth factors, and matrix proteins that bind to various receptor tyrosine kinases, G-protein coupled receptors, cytokine receptors, and integrins. Signals generated from these cell surface receptors initiate a cascade of signaling events that lead downstream to the activation of one or more of the MAP kinases (Figure 1). The involvement of multiple signaling proteins within a complex signaling network allows for regulation at various steps in the pathway and integration of signals from a diversity of stimuli.
Figure 1. MAP kinase signaling pathways.
A diverse array of receptors, including growth factor, cytokine, and integrin receptors, activate signaling pathways which lead to the activation of the MAP kinase pathways. MAP kinase kinase kinases (MAPKKKs) activate MAP kinase kinases (MKKs; MAP and ERK kinase or MEK), which in turn activate the MAP kinases (p38, ERK1/2, and JNK). The MAP kinases activate other kinases, such as p90RSK, and transcription factors that regulate gene transcription including Elk-1, MEF2, ATF2, c-Myc, Ets-1, and c-Jun. In this way, all three MAP kinases are involved in the expression of matrix metalloproteinases (MMPs) and other mediators involved in the development of OA.
The MAP kinases are activated by phosphorylation of specific Thr and Tyr residues by MAP kinase kinases (MKKs). ERK1 and ERK2, which are both widely expressed in multiple cell types including various joint tissues and are most commonly found together, are activated by MKK1 and MKK2 respectively, which are also referred to as MAP and ERK kinases or MEKs 1 and 2 [5]. Classic inhibitors used in many in vitro studies of ERK1/2 are PD98059 and U0126 which inhibit the activity of MEK1/2. p38 is also widely expressed although most tissues do not express all 4 isoforms. MKK3 and MKK6 are the most common activators of p38 and p38 is inhibited directly by several compounds including SB203580 which inhibits p38α and p38β, but not p38δ or p38γ [6]. JNK1 and JNK2 are widely expressed in tissues, while JNK3 expression is limited to mainly brain, testes, and heart tissue [7]. JNK is most often activated by MKK4 and MKK7 and can be inhibited by compounds such as SP600125 which inhibits all 3 isoforms [8]. Recent work in synoviocytes from patients with rheumatoid arthritis has shown that IL-1 activation of JNK by MKK4 and MKK7 requires the upstream activity of the TGF-β activated kinase 1 (TAK1) [9].
Once activated, the MAP kinases in turn, activate other protein kinases and several transcriptional regulatory proteins [5]. The latter include Elk-1, Ets, c-Myc, c-Jun, ATF-2, p53 and MEF2. Probably best characterized is ERK activation of Elk-1 and c-Myc, p38 activation of ATF-2 and MEF2, and JNK activation of c-Jun. These transcription factors regulate the expression of a host of genes relevant to OA including genes involved in the inflammatory response, regulation of cell proliferation, and production of matrix degrading enzymes such as MMPs. JNK may be particularly important because of its unique ability to activate c-jun, a key AP-1 component [7]. Besides promoting MMP expression, AP-1 can regulate the expression of pro-inflammatory cytokines such as TNFα and IL-1. These cytokines can then act in an autocrine/paracrine manner to maintain JNK activation and activate JNK in additional cells, further increasing cytokine and MMP production. In this way, JNK integrates many signals generated by different stimuli to amplify the normal levels of MMP production, making it a potential target for treating OA.
MAP kinase function with relevance to osteoarthritis
MAP kinases have been shown to be activated in OA cartilage and there is evidence, at least for ERK, that they can play a key role in the cartilage destruction seen in OA. Levels of activated (phosphorylated) JNK in human OA cartilage appear to be greater than the levels present in normal cartilage [10, 11]. Phosphorylation of p38 was also higher in human OA compared to normal tissue while phosphorylated ERK was found in both [11]. In a dog model of surgically-induced OA, ERK1/2, JNK, and p38 were all activated to a greater degree in OA compared to normal tissue [12]. However, results of immunohistochemistry experiments examining MAP kinase phosphorylation in situ must be interpreted with caution because of problems with antibody specificity, the transient nature of phosphorylation, and the possibility that alterations in phosphorylation may occur during tissue processing.
In the dog OA model, a compound (PD-0200347) that serves as a ligand of voltage gated Ca++ channels reduced the levels of phosphorylated ERK, but not p38 or JNK [12]. This compound had been previously noted to reduce the development of OA lesions in the dog model in association with a reduction in MMP production [13] suggesting that inhibition of ERK activation was sufficient to reduce OA lesions in this model. In support of this, a study from the same group using a rabbit model of OA demonstrated that a MEK 1/2 inhibitor, which blocks ERK activation, also reduced the severity of the OA lesions [14]. Likewise, avocado-soybean unsaponifiables (ASU), which are used to treat OA in Europe, were reported to inhibit IL-1 induced ERK but not p38 or JNK in chondrocytes in vitro [15]. Whether JNK or p38 specific inhibitors would give similar results to ERK inhibition has not been determined.
Recent studies have continued to support a key role for MAP kinases in the regulation of MMP production by chondrocytes. In addition to cytokine regulation of MMP expression [11], MAP kinases are required for induction of MMPs by matrix fragments generated during the development of arthritis including fibronectin fragments [16] and collagen fragments [17*, 18*]. MAP kinases, at least p38, also play a role in MMP expression induced when type II collagen binds to the discoidin domain receptor. Inhibition of any one of the 3 major MAP kinases (ERK, p38, and JNK) was able to inhibit fibronectin fragment induced MMP-13 expression [16] while only p38 inhibition, and not JNK, blocked MMP-13 expression mediated by the discoidin domain receptor [17*]. Similar to fibronectin fragments, stimulation of MMP-13 by bFGF required activity of ERK, p38, and JNK [19, 20].
Cytokine stimulation of MAP kinase activity may also contribute to the development of OA through down regulation of peroxisome proliferators-activated receptor gamma (PPARγ). Activation of PPARγ has been shown to have chondroprotective effects in OA associated with reduced activation of ERK1/2 and p38 [12]. But OA cells appear to express less PPARγ than normal chondrocytes, possibly due to the downregulation of PPARγ by IL-1 [21]. In this study, inhibition of either p38 or JNK, but not ERK, was found to prevent IL-1 induced down regulation of PPARγ in chondrocytes.
MAP kinases are also involved in the regulation of chondrocyte anabolic activity. IGF-I stimulation of proteoglycan synthesis requires activity of the PI-3 kinase, while there is some evidence that ERK may be a negative regulator of proteoglycan synthesis [22]. A recent study found that chondrocytes express receptors for sphingosine-1-phosphate, a metabolite of ceramide, and that sphingosine-1-phosphate stimulation of ERK and p38 was associated with inhibition of aggrecan expression [23]. Treatment with the MAP kinase inhibitors PD98059 and SB203580, to inhibit ERK and p38α respectively, blocked sphingosine-1-phosphate induction of PGE2, but the effect of these inhibitors on aggrecan expression was not determined. LPS activation of toll-like receptor 4 can inhibit chondrocyte proteoglycan synthesis and under these conditions inhibition of p38, but not ERK, partially restored it [24]. In contrast to the inhibition of proteoglycan synthesis by p38 and ERK, a JNK-dependent increase in proteoglycan synthesis was demonstrated in chondrocyte cell lines following cyclical mechanical stimulation [25].
The role of the MAP kinases in OA tissues other than the articular cartilage has received little attention. A recent study found evidence for p38 activation in ruptured menisci [26]. Another recent report found that MMP-1 production by OA synovial fibroblasts stimulated with adiponectin required p38 activity [27]. Other stimuli that may contribute to the development of OA in older adults include basic calcium phosphate crystals that are commonly found in OA joints. These crystals can stimulate MMP production by chondrocytes or by synovial fibroblasts. Recent work in fibroblasts demonstrated that activation of ERK was required for basic calcium phosphate crystals to stimulate c-fos which is a regulator of MMP expression in these cells [28]. Another recent study has shown a role for both ERK and p38 in the calcium phosphate crystal induced MMP-13 production by OA synovial fibroblasts [29]. In addition, p38 has been shown to mediate increased chondrocyte MMP-3 expression in response to monosodium urate crystals [30].
Besides a role in regulating processes relevant to joint tissue destruction, mounting evidence suggests that MAP kinases may also be important in mediating pain signals. In an animal model of inflammatory joint pain, rats were injected with Freund's adjuvant in the knee joint to induce inflammation and this was found to induce activation of ERK in dorsal root ganglions [31*]. In this study, intrathecal injection of the MEK inhibitor UO126 was found to reduce pain behavior elicited by passive motion of the inflamed joint, suggesting a role for ERK signaling in joint pain. Studies outside of the joint have also suggested that ERK may have a role in pain signaling [32, 33]. In addition to ERK, there is evidence that p38 may also play a role in neuropathic pain and a clinical trial of a p38 inhibitor for the treatment of neuropathic pain is listed on www.clinicaltrials.gov.
Inhibition of MAP kinases: potential benefit versus risks
Despite initial animal model studies suggesting that MEK/ERK inhibition would be a potential therapeutic target in OA [14], inhibitors of the ERK pathway have received little attention in additional animal model studies or in clinical trials in humans. This is most likely because of the potential toxicity of systemic ERK inhibition given the knowledge that the ERK MAP kinase pathway is involved in a multitude of growth factor signaling pathways that regulate cell proliferation and tissue homeostasis. JNK and p38 are most commonly activated by inflammatory and stress-induced signals and so inhibition of these MAP kinases is thought to have a better risk-benefit ratio than ERK inhibition.
A number of JNK inhibitors, both direct and indirect, have been developed and tested as potential therapeutics for several diseases, including rheumatoid arthritis (RA), which like OA results in joint destruction despite its different etiology. In rat adjuvant arthritis, a model for inflammatory arthritis, subcutaneous administration of the JNK inhibitor SP600125 significantly reduced joint damage (cartilage and bone erosion) in comparison to the vehicle control, while only a modest decrease in paw swelling/inflammation was observed [8]. SP600125 treatment also markedly decreased JNK kinase activity, AP-1 activation, and MMP-13 mRNA levels in the synovium [8]. Treatment of TNFα-stimulated synoviocytes with R406, a novel inhibitor of spleen tyrosine kinase (Syk), markedly suppressed JNK activation, AP-1 DNA binding, and MMP expression, while having minor effects on p38 and ERK [34]. Celecoxib, one of the commercially available selective COX-2 inhibitors, was also shown to inhibit IL-1β-stimulated JNK and NFκB activation (but not p38 or ERK), resulting in a significant suppression of MMP production in normal, OA, and RA articular chondrocytes [35].
Although JNK inhibitors have not been tested in animal models of OA, there is evidence indicating JNK inhibitors may effectively prevent further cartilage degradation in OA. Diverse stimuli found to be present in OA synovial fluid and cartilage, including TNFα, IL-1, fibronectin fragments, and bFGF all require JNK activation for stimulation of chondrocyte MMP production, suggesting it is a key mediator of MMP production in articular cartilage [20, 36-38]. In the rat adjuvant arthritis study by Han et al [8], the finding that cartilage erosion was reduced to a greater degree than joint inflammation suggests that JNK inhibition might have effects on the cartilage independent of the synovium. Although the synovial inflammatory response is likely the primary initiator of joint damage in RA, it is believed that OA synovial inflammation is secondary to the initial cartilage damage. Thus, inhibition of JNK may be more effective in the treatment of OA in comparison to its use as an RA therapeutic.
Enthusiasm for the use of JNK inhibitors in the treatment of OA has been dampened by concerns about toxicity. The JNK inhibitors recently examined in clinical trials listed on www.clinicaltrials.gov for early Parkinson's disease and myeloid leukemia were found to be ineffective at the doses tolerated leading to the studies’ termination. Possible problems with inhibiting JNK may be due to the important role it plays in other cellular functions such as cell proliferation, cell differentiation, the inflammatory response, and apoptosis. JNK is required for both T-cell function and the regulation of non-transcription factors like Bcl-2 family members that are involved in programmed cell death [7]. Until new and improved inhibitors are developed, the side effects and toxicity typically observed with the currently available JNK inhibitors may outweigh the benefits of using them for the treatment of OA.
p38 inhibition has been suggested to be potentially beneficial as therapeutic strategy in inflammatory disease processes such as sepsis, Crohn's disease, and rheumatoid arthritis [6]. This is mainly due to the ability of p38 to control inflammatory cytokine expression. Several different p38 inhibitors have been tested in animal models of rheumatoid arthritis [39-41]. In each of these studies, p38 inhibition was shown to reduce disease severity and maintain joint integrity with a reduction in the loss of cartilage and bone.
Several p38 inhibitors have advanced into clinical trials in human subjects for treatment of rheumatoid arthritis, but only a few have made it as far as phase II. The problem with these compounds has been similar to that seen with the JNK inhibitors with a poor safety profile, including adverse effects in the central nervous system and liver [42]. Due to these safety issues, new strategies for p38 inhibition are under consideration. One strategy is to investigate molecules downstream of p38 that may be more selective in their biological activities [42]. In fact, a recent study using an animal model of rheumatoid arthritis (collagen-induced arthritis) has shown that MAPKAP kinase 2 (MK2), a molecule downstream of p38, is important in disease progression [43]. It remains to be seen if this molecule or other molecules downstream of p38 could be a therapeutic target in arthritis. A second potential strategy is to investigate the role of p38 isoforms other than p38α [6]. Most studies to date have focused almost exclusively on p38α and inhibitors that target this particular isoform. Not as much is known about the role of the other isoforms in the context of arthritis, although a recent report has been published demonstrating the activation of both p38α and p38γ within synovial tissue of rheumatoid arthritis subjects, suggesting that both of these molecules may be important in the disease process [44].
Conclusion
Accumulating evidence supports a central regulatory role of the MAP kinases in mediating inflammatory and matrix degrading processes that contribute to joint tissue destruction in osteoarthritis. Because MAP kinases also appear to be involved in pain signaling pathways, the potential exists for MAP kinase inhibitors to reduce both pain and structural progression in OA. However, due to the toxicity of the currently available MAP kinase inhibitors, risk-benefit considerations have precluded their use in clinical trials in humans with OA. New strategies are emerging such as the development of non-ATP competitive and isoform specific inhibitors that may have greater specificity with reduced toxicity [42, 45]. An alternative is to inhibit proteins downstream of the MAP kinases that mediate the effects of MAP kinase activation in OA. Further work is needed to better identify the details of the MAP kinase pathways involved in OA, including tissues other than articular cartilage.
Acknowledgments
The research of the authors was supported by NIH grants AR49003, AG16697, and GM063485.
References and recommended reading
- * 1.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [This is a very well written, comprehensive, and up-to-date review of the basic biology of osteoarthritis.] [DOI] [PubMed] [Google Scholar]
- 2.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- ** 3.Aigner T, Fundel K, Saas J, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–44. doi: 10.1002/art.22174. [This study utilized gene microarrays to compare the differential expression of genes in chondrocytes from 78 samples of normal and OA cartilage. Importantly, samples from tissue with histologic evidence of early disease were compared to normal tissue and late stage disease. The analysis of such a large number of samples from different disease stages provides a unique data base. Many of the genes found to be differentially regulated are targets of MAP kinase signaling. Of interest, dual-specificity phosphatase 1, which can inhibit MAP kinase signaling by inactivating MAP kinases, was down-regulated in OA tissue.] [DOI] [PubMed] [Google Scholar]
- 4.Krzeski P, Buckland-Wright C, Balint G, et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther. 2007;9:R109. doi: 10.1186/ar2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avruch J. MAP kinase pathways: the first twenty years. Biochim Biophys Acta. 2007;1773:1150–60. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–26. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 7.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–8. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Z, Boyle DL, Chang L, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammaker DR, Boyle DL, Inoue T, Firestein GS. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther. 2007;9:R57. doi: 10.1186/ar2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy R, Rediske J, Koehne C, et al. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9:294–9. doi: 10.1053/joca.2000.0388. [DOI] [PubMed] [Google Scholar]
- 11.Fan Z, Soder S, Oehler S, et al. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171:938–46. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boileau C, Martel-Pelletier J, Brunet J, et al. PD-0200347, an alpha2delta ligand of the voltage gated calcium channel, inhibits in vivo activation of the Erk1/2 pathway in osteoarthritic chondrocytes: a PKCalpha dependent effect. Ann Rheum Dis. 2006;65:573–80. doi: 10.1136/ard.2005.041855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boileau C, Martel-Pelletier J, Brunet J, et al. Oral treatment with PD-0200347, an alpha2delta ligand, reduces the development of experimental osteoarthritis by inhibiting metalloproteinases and inducible nitric oxide synthase gene expression and synthesis in cartilage chondrocytes. Arthritis Rheum. 2005;52:488–500. doi: 10.1002/art.20809. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier JP, Fernandes JC, Brunet J, et al. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003;48:1582–93. doi: 10.1002/art.11014. [DOI] [PubMed] [Google Scholar]
- 15.Gabay O, Gosset M, Levy A, et al. Stress-induced signaling pathways in hyalin chondrocytes: inhibition by Avocado-Soybean Unsaponifiables (ASU). Osteoarthritis Cartilage. 2008;16:373–84. doi: 10.1016/j.joca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–85. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Xu L, Peng H, Glasson S, et al. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–73. doi: 10.1002/art.22761. [The discovery that chondrocytes express a receptor for native type II collagen that, when activated, stimulates production of MMP-13 through a MAP kinase pathway was somewhat surprising. The finding suggests an additional mechanism for the upregulation of MMPs during the development of OA related to the early loss of non-type II collagen matrix proteins that would allow type II collagen to come into contact with the discoidin domain receptor 2.] [DOI] [PubMed] [Google Scholar]
- *18.Ruettger A, Schueler S, Mollenhauer JA, Wiederanders B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2008;283:1043–51. doi: 10.1074/jbc.M704915200. [This study suggests that in addition to native type II collagen (see reference #17), collagen peptides derived from collagen cleavage can also feedback and regulate expression of matrix degrading enzymes in a MAP kinase dependent manner.] [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Manner PA, Horner A, et al. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–73. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Im H-J, Muddasani P, Natarajan V, et al. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–21. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afif H, Benderdour M, Mfuna-Endam L, et al. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res Ther. 2007;9:R31. doi: 10.1186/ar2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389:723–9. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuko K, Murata M, Nakamura H, et al. Sphingosine-1-phosphate attenuates proteoglycan aggrecan expression via production of prostaglandin E2 from human articular chondrocytes. BMC Musculoskelet Disord. 2007;8:29. doi: 10.1186/1471-2474-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobacz K, Sunk IG, Hofstaetter JG, et al. Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 2007;56:1880–93. doi: 10.1002/art.22637. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Millward-Sadler SJ, Lin H, et al. Evidence for JNK-dependent up-regulation of proteoglycan synthesis and for activation of JNK1 following cyclical mechanical stimulation in a human chondrocyte culture model. Osteoarthritis Cartilage. 2007;15:884–93. doi: 10.1016/j.joca.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Papachristou DJ, Papadakou E, Basdra EK, et al. Involvement of the p38 MAPK-NF-kappaB Signal Transduction Pathway and COX-2 in the Pathobiology of Meniscus Degeneration in Humans. Mol Med. 2008;14:160–6. doi: 10.2119/2007-00138.Papachristou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehling A, Schaffler A, Herfarth H, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–78. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 28.Major ML, Cheung HS, Misra RP. Basic calcium phosphate crystals activate c-fos expression through a Ras/ERK dependent signaling mechanism. Biochem Biophys Res Commun. 2007;355:654–60. doi: 10.1016/j.bbrc.2007.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy ES, Morgan MP, Doherty GA, et al. Mechanism of basic calcium phosphate crystal-stimulated matrix metalloproteinase-13 expression in osteoarthritic synovial fibroblasts: inhibition by prostaglandin E2. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.079582. in press. [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Liote F, Rose DM, et al. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal-induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 2004;50:247–58. doi: 10.1002/art.11486. [DOI] [PubMed] [Google Scholar]
- *31.Seino D, Tokunaga A, Tachibana T, et al. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–203. doi: 10.1016/j.pain.2006.02.032. [The mechanisms controlling joint pain in arthritis have been poorly understood. This study provides eveidence for a unique role for MAP kinases as not only mediators of an inflammatory response but also of pain that accompanies inflammation.] [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- 33.Carrasquillo Y, Gereau RWt. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27:1543–51. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha HS, Boyle DL, Inoue T, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther. 2006;317:571–8. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsumi R, Ito H, Hiramitsu T, et al. Celecoxib inhibits production of MMP and NO via down-regulation of NF-kappaB and JNK in a PGE2 independent manner in human articular chondrocytes. Rheumatol Int. 2007 doi: 10.1007/s00296-007-0511-6. [DOI] [PubMed] [Google Scholar]
- 36.Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–11. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Liacini A, Sylvester J, Li WQ, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208–17. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 38.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–76. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 39.Badger AM, Griswold DE, Kapadia R, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175–83. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa M, Myoui A, Tomita T, et al. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum. 2003;48:2670–81. doi: 10.1002/art.11227. [DOI] [PubMed] [Google Scholar]
- 41.Medicherla S, Ma JY, Mangadu R, et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318:132–41. doi: 10.1124/jpet.105.098020. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci. 2007;28:286–95. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Hegen M, Gaestel M, Nickerson-Nutter CL, et al. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177:1913–7. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- 44.Korb A, Tohidast-Akrad M, Cetin E, et al. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54:2745–56. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- 45.Bogoyevitch MA. Therapeutic promise of JNK ATP-noncompetitive inhibitors. Trends Mol Med. 2005;11:232–9. doi: 10.1016/j.molmed.2005.03.005. [DOI] [PubMed] [Google Scholar]