Abstract
A growing body of data points to intraclonal heterogeneity and hierarchy of growth potential, but also plasticity of cellular differentiation within human tumors. Recent studies have also identified surprising overlap between pathways that regulate pluripotency in embryonal stem (ES) cells and oncogenesis. While there is a long history of targeting embryonal tissues towards cancer vaccines, recent identification of critical stemness pathways in ES cells, as well as putative cancer stem cells (CSCs) provides novel opportunities for antigen-specific targeted therapy. Here we discuss recent insights into the capacity of the immune system to target these pathways. Immunologic targeting of pathways associated with stemness has implications for both immune regulation of tumor growth as well as regenerative therapies with embryonal stem cells.
Cancer cells share several properties with stem cells including the capacity for long term persistence and self renewal[1,2] Pathways that regulate the biology of stem cells have striking overlap with critical checkpoints that regulate the growth of cancer cells[3-5]. In this review, we discuss recent insights into the capacity of the immune system to target these pathways and argue that such pathways are potentially important targets for the capacity of the immune system to control cancer. The ability to harness the properties of these immune responses also has implications for the emerging field of regenerative medicine targeting embryonal stem (ES) cells.
Pluripotency, Stem Cells and Cancer
A major insight in developmental biology has been the recent demonstration that a limited set of genes are sufficient to induce pluripotency in adult differentiated cells[6,7] ••. However these studies also indicate that induction of pluripotency is intricately linked to cancer. Indeed formation of tumors is used as one of the criteria for evaluating the induction of stemness itself and tumorigenicity of stem cells in regenerative medicine is directly proportional to their pluripotency (Figure 1). Interestingly, genes such as p53 which regulate oncogene-mediated induction of cancer also regulate the formation of such induced pluripotency stem (iPS) cells[3,4,8,9]. The presence of embryonal stem cell like gene expression programs is detected in several human cancers and correlate with adverse outcome[10-12] ••. Expression of these genes also correlates with subtypes of cancers typically associated with aggressive clinical course, such as those with undifferentiated histology[11]. At least a proportion of these programs may be directly activated by oncogenes such as Myc, implicated in several human tumors[13]. Recently, more direct evidence linking pluripotency genes to cancers has also emerged. For example, aberrant expression of OCT4 is sufficient to induce tumors in mice[14]. SOX2 was recently identified as a common target of genomic amplification and a lineage survival oncogene in patients with lung cancer[15]. Myc is already a well recognized oncogene. Together these data suggest that inappropriate expression of stem cell programs may be a hallmark of both murine and human tumors, and perhaps its Achilles heel.
Figure 1.
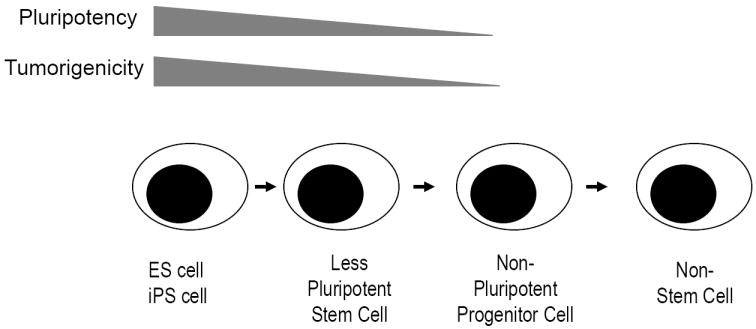
Relationship between pluripotency and tumorigenicity in regenerative medicine. The capacity of stem cells to induce tumors in the host is directly proportional to their pluripotency.
Pioneering studies in leukemia, later extended to solid tumors have suggested the presence of intraclonal hierarchy with subpopulation of tumor cells enriched for clonogenic growth, termed cancer stem cells (CSCs)[16,17] ••. These cells have typically been defined on the basis of their ability to seed tumors in animal hosts, to self renew and to spawn differentiated progeny. Several groups have documented the enrichment of ES associated genes in CSCs, suggesting that these cells may utilize similar programs for self renewal[2]. Part of the controversy regarding CSCs results from differences in frequencies of these cells depending on the specific model used[18,19]. Indeed, recent studies suggest that the CSC like properties may be a function of the cell type of origin, stromal microenvironment, accumulated somatic mutations and the stage of malignant progression. The CSC model therefore needs to be interpreted in light of evidence regarding the plasticity of the differentiation status of tumor cells. Thus, interactions of tumor cells with their microenvironment can lead to altered differentiation, termed epithelial-mesenchymal transition (EMT) in the case of solid tumors[20]. Such dedifferentiation can also be seen experimentally in hematopoietic tumors with a differentiated cell phenotype, such as myeloma, particularly in the context of signals from the microenvironment [21]. Experimental data suggest a strong overlap between EMT and stem cell phenotype in cancer[20]. The phenotypic plasticity of tumor cells also suggests that a dynamic equilibrium may exist between CSCs and non-CSCs, depending on signals from the microenvironment[22] •. We suggest that a critical target from the perspective of tumor immunity may not be a particular cell type (which may be a moving target), but the property of stemness itself.
Immune targeting of stemness- pros / cons
Sequencing of the cancer genomes has illustrated the plethora of mutations that exist in each tumor, some of which drive the oncogenic process[23]. These may, in principle, represent attractive targets for tumor immunity[24]. Other classes of targets include non-mutated differentially expressed antigens, as well as proteins (such as cancer-testis antigens) aberrantly expressed as a result of epigenetic alterations in tumor cells. While a case can be made for each of these classes of antigens, which, if any of these antigens might serve as a true tumor rejection antigens in the clinic remains unknown[25]. We argue that one size fits all may not apply for immune therapy of cancer and that optimal targets for tumor immunity may depend on the underlying genetic lesions within tumors, and the biology of the resulting tumors. In this regard, tumor types most dependent on CSCs for their growth kinetics may be the best suited for approaches targeting stem cell genes. The concept that CSCs and non-CSCs may exist in a dynamic equilibrium also argues for a need to target CSCs, or genes associated with stemness. One prediction from this concept is that unless CSCs are effectively targeted, tumor immunity might paradoxically lead to enrichment of less differentiated cells, such as those with EMT. Such an observation has indeed been made in some experimental murine models[22]. However whether this happens clinically remains to be shown. Targeting only the more differentiated or transit amplifying compartment may also set up a vicious cycle of homeostatic regeneration, analogous to chronic wounds. Such a process has been implicated in the setting of autoimmune myopathies, but may also have a parallel in cancer immunity[26].
Immune targeting of stem cell genes also carries potential risks. The most obvious risk relates to pathways shared with normal adult stem cells. In this setting, autoimmunity would carry substantial risk of toxicity to normal stem cells. Immune tolerance to pathways shared between CSCs and adult stem cells also represent a potentially formidable challenge. We suggest that the group of genes most attractive as immune targets in this setting are genes expressed in or shared between cancer (or CSCs), and embryonal stem cells (ESCs), but not adult stem cells or their progeny (Figure 2). T cells against such targets may also be less susceptible to tolerance mechanisms to prevent autoreactivity to normal tissues or stem cells. In this regard, it is of interest that at least some of the ESC associated genes expressed in putative CSCs appear to be dispensable for the function of adult stem cells. One example is the pluripotency gene OCT4, which has been shown to be oncogenic in vivo, but is dispensable for the function of adult stem cells[14,27]. However at this time, the capacity of the human immune system to target such genes is not well understood.
Figure 2.
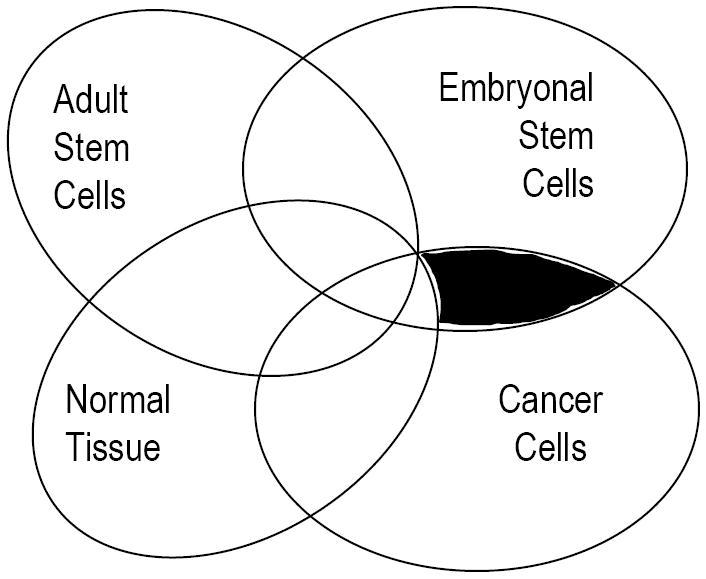
Embryonal stem cell antigens as targets of cancer immunity. Genes restricted to ES cells and cancer, but not expressed by normal adult stem cells or their differentiated progeny (shaded area) may be potential targets for cancer vaccines. Some of these genes can regulate stemness in both cancer and ES cells, and are the most attractive targets.
Immune responses to ES associated genes in cancer
Attempts to vaccinate against cancer using embryonic material has a long history of over 100 years in cancer immunology (reviewed recently by Brewer et al)[28]. Much of these early attempts preceded any biologic understanding of the properties of stem cells. Interestingly, even in these studies, the protective effects of vaccination were limited predominantly to early stage, but not later stage embryos, which led to much confusion. Nonetheless, investigations in this field led to the discovery of several oncofetal antigens, such as carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and prostate specific antigen (PSA), which continue to have clinical utility to date and serve as important targets for cancer therapy, including vaccines. It is however only recently that we have a much better appreciation of the stemness programs that regulate different stages of development. For example, recent studies have shown that the a limited set of core transcriptional factors, namely SOX2, OCT3/4 and Nanog regulate the stemness and pluripotency of ES cells[29]. These insights provide novel opportunities to explore immune recognition of these antigens or pathways.
Evidence supporting the ability of the human immune system to mediate T cell responses against ES associated stemness genes came initially from antigen discovery approaches applied to cohorts of patients with clinical cancer or premalignant states. Multiple myeloma is a plasma cell tumor preceded by common premalignant state, monoclonal gammopathy of undetermined significance (MGUS). Analysis of host response against a panel of tumor antigens suggested that targets of host response in MGUS differed from those in myeloma. Interestingly, the top gene differentially targeted by the immune system in MGUS was the pluripotency gene, SOX2[30] ••. The presence of naturally occurring T cell responses against SOX2 in MGUS patients was predictive of an indolent course and markedly reduced likelihood of progression to clinical myeloma requiring chemotherapy. Expression of SOX2 correlated with the putative clonogenic compartment in MGUS, and SOX2 specific T cells inhibited the clonogenic growth of MGUS cells in culture. Antibodies against SOX2 have also been observed in patients with lung cancer, wherein they correlate with improved outcome, although cellular immunity to this antigen in patients with lung cancer has not yet been examined[31] ••. Antibodies to SOX2 are also detected in patients with meningioma, a benign tumor with an indolent course in most patients[32]. Other investigators have recently made similar observations when comparing targets of immune response in preneoplastic to malignant lesions. For example, immunity to another developmental antigen OFD1 was detected in MGUS, but not in myeloma[33]. OFD1 is also implicated in morphogenesis, although its role in carcinogenesis is not presently clear. Together these studies suggest the possibility that the nature of specific targets of spontaneous immunity may be predictive of clinical outcome in patents with cancer or more importantly, in otherwise healthy individuals or those with preneoplastic states.
Two other genes relevant to the biology of ES cells and targets of anti-tumor immune response are the tumor suppressor gene p53 and telomerase reverse transcriptase (TERT). However in contrast to the pluripotency genes discussed above, these genes are also expressed in and are important for the function of nonmalignant cells, as well as adult stem cells. Preclinical studies described anti-tumor efficacy of immunity against p53[34,35]. Both humoral and cellular responses against p53 can be detected in patients with cancer, and early phase studies to harness these responses are ongoing[34,35]. Similarly, both naturally occurring and vaccine induced T cell responses against hTERT can be elicited in patients with cancer[36]. However, whether T cells against these antigens can mediate the rejection of human tumors remains to be established. As the expression of these genes is not restricted to tumor cells, potential toxicity in terms of reactivity to normal tissues is also a potential concern. Another class of genes, cancer-testis antigens represent genes expressed predominantly in germ cells and a subpopulation of tumor cells, but not in normal tissues[37]. The expression of these genes is linked to the altered methylation status of the cancer genome and often correlates with adverse outcome of tumors. These genes were among the first defined human tumor antigens and have been extensively studied, particularly as the lack of expression in normal tissues makes them attractive targets for vaccines. It has been argued that the subpopulation of tumor cells expressing cancer-testis antigens may be enriched in CSCs [38], however the functional significance of C/T antigens in cancer is still largely unknown. The emerging data discussed above suggests that the immune system surprisingly lacks tolerance to antigens expressed on ES cells. It is of interest to ask whether there is a much broader repertoire of ES associated genes to which the human immune system can potentially respond. It would be important to better understand the properties of this immune response, and the mechanistic basis for the apparent lack of immune tolerance to this set of genes. The capacity of the immune system to target stem cell associated genes is particularly relevant for two emerging clinical areas, targeting putative CSCs; and ES / iPS derived regenerative medicine, as discussed below.
Immune targeting of cancer stem cells
CSCs have been shown to be intrinsically resistant to traditional chemotherapies and implicated in disease recurrence[1]. This has prompted exploration of alternate approaches. Recent data suggest that immune based approaches may be particularly attractive towards targeting CSCs. One strategy is to target CSCs via monoclonal antibodies targeting antigens differentially overexpressed on these cells. Two recent examples of such an approach are antibodies targeting CD123 and CD47, which have been shown to eradicate leukemia stem cells in preclinical models[39,40] ••. It is likely that in the coming years, several other targets overexpressed on putative CSCs will be found, which may provide novel opportunities for targeting these cells.
Another approach involves harnessing cellular immune responses against these cells. Such an approach was tested in the context of minor histocompatibility antigen reactive T cells against leukemia stem cells[41,42]. T cells against a Y chromosome encoded antigen, DDX3Y, were identified in the context of a patient in clinical remission following sex mismatched allogeneic stem cell transplant, and shown to target leukemia stem cells[43]. Anti-tumor T cells in MGUS, particularly those reactive against ES antigen SOX2 can inhibit the clonogenic growth of tumor cells. Indeed, the expression of SOX2 was shown to be enriched in CD138- subpopulation of tumor cells, thought to be enriched in the clonogenic potential in MGUS[30]. Other investigators have also shown the capacity of T cells to inhibit the clonogenic growth of MGUS cells in culture[44]. Data regarding targeting CSCs via anti-tumor T cells has also emerged from solid tumors[45,46]. T cell immunity against SOX2 and SOX6 has been explored in the context of glioma stem cells[47,48]. Dendritic cell (DC) mediated targeting of neurospheres known to be enriched in CSCs led to greater anti-tumor immunity in mice compared to targeting bulk tumor cells[49]. In another preclinical 9L glioma CSC model, DCs loaded with glioma CSCs, but not daughter cells or conventionally cultured 9L cells prolonged survival in animals bearing 9L CSC tumors. CSCs in human glioma are thought to be enriched in CD133+ subpopulation[50]. Brown et al demonstrated the capacity of cytomegalovirus (CMV) pp65 specific T cells to kill pp65 expressing glioma CSCs, supporting the capacity of T cells to target these cells[51]. Even injection of bulk ES or iPS cells has been explored and shown to induce protective immunity in a colon cancer model[52]. Clearly, better understanding of antigenic targets on CSCs in different tumors is needed to further explore immune targeting of CSCs in the clinic. However, the emerging data do point to the feasibility of immune based targeting of CSCs and suggest that exploring new strategies to harness immunity to these cells or pathways are worthwhile.
Stem cell tumorigenicity and safety of regenerative medicine
Recent discovery of induction of pluripotency by a core set of factors has led to the promise of regenerative medicine using such cells[7]. However such induced pluripotent stem cells are predicted to possess tumorigenic potential equal to or greater than ES cells[53]. The nature of tumors associated with ES or iPS therapy is not restricted to teratomas but includes diverse tumor types[54,55]. Indeed, all four of the core IPS factors are now strongly implicated in cancer, as discussed earlier. The tumorigenic potential of ES or iPS cells seems to be related to their differentiation status[56]. Therefore, one approach being taken to reduce risk of tumors with stem cell based therapy is not to inject the stem cells themselves, but their differentiated progeny. However this approach may reduce the very promise of stem cell therapy and still carries considerable risk, as differentiation is a dynamic process, not an on-off switch. Recent findings that the immune system has the capacity to target ES pluripotency genes [30]suggest the possibility that harnessing such an immune response may allow reduction of tumorigenicity of iPS based regenerative therapy.
Conclusions
Recent insights in stem cell biology have major implications for understanding the development of cancer, as well as harnessing immune response against cancer. In this review, we have tried to argue that pathways or genes that regulate stemness in embryonal or cancer cells may be critical targets for cancer therapy and that these may be targeted via the immune system. The capacity of the immune system to target these genes also has implications for preventing tumors during stem cell based therapies.
Acknowledgments
MVD is supported by funds from the National Institutes of Health, Dana Foundation and Leukemia and Lymphoma Society.
Abbreviations
- ES
embryonal stem cells
- iPS
Induced pluripotency stem cells
- CSC
cancer stem cells
- EMT
epithelial-mesenchymal transition
- MGUS
monoclonal gammopathy of undetermined significance
- TERT
telomerase reverse transcriptase
- DC
dendritic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 3.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Armstrong SA. Cancer: inappropriate expression of stem cell programs? Cell Stem Cell. 2008;2:297–299. doi: 10.1016/j.stem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019.. •• Pioneering study showing the induction of pluripotency in adult cells by a limited set of core factors.
- 7.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glinsky GV. “Stemness” genomics law governs clinical behavior of human cancer: implications for decision making in disease management. J Clin Oncol. 2008;26:2846–2853. doi: 10.1200/JCO.2008.17.0266. [DOI] [PubMed] [Google Scholar]
- 12.Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383:157–162. doi: 10.1016/j.bbrc.2009.02.156.. •• References 10-12 show the strong correlation between the expression of stemness genes and clinical outcome in diverse human cancers.
- 13.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 15.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100.. ••Pioneering studies demonstrating the intraclonal hierarchy in human tumors.
- 18.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 19.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343.. • An interestring study showing immune mediated regulation of dynamic behavior of CSCs versus non-CSCs.
- 23.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 24.Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 25.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 26.Suber TL, Casciola-Rosen L, Rosen A. Mechanisms of disease: autoantigens as clues to the pathogenesis of myositis. Nat Clin Pract Rheumatol. 2008;4:201–209. doi: 10.1038/ncprheum0760. [DOI] [PubMed] [Google Scholar]
- 27.Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer BG, Mitchell RA, Harandi A, Eaton JW. Embryonic vaccines against cancer: an early history. Exp Mol Pathol. 2009;86:192–197. doi: 10.1016/j.yexmp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387.. •• Demonstration of the capacity of human immune system to mediate T cell responses against a core pluripotency gene. Spontaneous immunity correlated strongly with favorable clinical outcome.
- 31.Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci U S A. 2000;97:4198–4203. doi: 10.1073/pnas.97.8.4198.. •• An important early paper identifying embryonic neural proteins as highly immunogenic tumor antigens inducing antibody responses in humans.
- 32.Comtesse N, Zippel A, Walle S, Monz D, Backes C, Fischer U, Mayer J, Ludwig N, Hildebrandt A, Keller A, et al. Complex humoral immune response against a benign tumor: frequent antibody response against specific antigens as diagnostic targets. Proc Natl Acad Sci U S A. 2005;102:9601–9606. doi: 10.1073/pnas.0500404102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blotta S, Tassone P, Prabhala RH, Tagliaferri P, Cervi D, Amin S, Jakubikova J, Tai YT, Podar K, Mitsiades CS, et al. Identification of novel antigens with induced immune response in monoclonal gammopathy of undetermined significance. Blood. 2009;114:3276–3284. doi: 10.1182/blood-2009-04-219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauwen MM, Zwaveling S, de Quartel L, Ferreira Mota SC, Grashorn JA, Melief CJ, van der Burg SH, Offringa R. Self-tolerance does not restrict the CD4+ T-helper response against the p53 tumor antigen. Cancer Res. 2008;68:893–900. doi: 10.1158/0008-5472.CAN-07-3166. [DOI] [PubMed] [Google Scholar]
- 35.Offringa R, Vierboom MP, van der Burg SH, Erdile L, Melief CJ. p53: a potential target antigen for immunotherapy of cancer. Ann N Y Acad Sci. 2000;910:223–233. doi: 10.1111/j.1749-6632.2000.tb06711.x. discussion 233-226. [DOI] [PubMed] [Google Scholar]
- 36.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 37.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 38.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 39.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018.. •• References 39 and 40 demonstrate the feasibility of antibody mediated targeting of putative cancer stem cells
- 41.Faber LM, van der Hoeven J, Goulmy E, Hooftman-den Otter AL, van Luxemburg Heijs SA, Willemze R, Falkenburg JH. Recognition of clonogenic leukemic cells, remission bone marrow and HLA-identical donor bone marrow by CD8+ or CD4+ minor histocompatibility antigen-specific cytotoxic T lymphocytes. J Clin Invest. 1995;96:877–883. doi: 10.1172/JCI118134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci U S A. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosinski KV, Fujii N, Mito JK, Koo KK, Xuereb SM, Sala-Torra O, Gibbs JS, Radich JP, Akatsuka Y, Van den Eynde BJ, et al. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood. 2008;111:4817–4826. doi: 10.1182/blood-2007-06-096313.. •• References 41-43 demonstrate the capacity of human T cells to target leukemia stem cells.
- 44.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, Bonyhadi M, Levitsky H, Whartenby K, Borrello I. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 2005;65:2026–2034. doi: 10.1158/0008-5472.CAN-04-3337. [DOI] [PubMed] [Google Scholar]
- 45.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato N, Hirohashi Y, Tsukahara T, Kikuchi T, Sahara H, Kamiguchi K, Ichimiya S, Tamura Y, Torigoe T. Molecular pathological approaches to human tumor immunology. Pathol Int. 2009;59:205–217. doi: 10.1111/j.1440-1827.2009.02353.x. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M, et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007;96:1293–1301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueda R, Ohkusu-Tsukada K, Fusaki N, Soeda A, Kawase T, Kawakami Y, Toda M. Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int J Cancer. 2009 doi: 10.1002/ijc.24851. [DOI] [PubMed] [Google Scholar]
- 49.Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66:10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 50.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown CE, Starr R, Martinez C, Aguilar B, D’Apuzzo M, Todorov I, Shih CC, Badie B, Hudecek M, Riddell SR, et al. Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res. 2009;69:8886–8893. doi: 10.1158/0008-5472.CAN-09-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Zeng H, Xu RH, Liu B, Li Z. Vaccination with Human Pluripotent Stem Cells Generates a Broad Spectrum of Immunological and Clinical Response against Colon Cancer. Stem Cells. 2009 doi: 10.1002/stem.234. [DOI] [PubMed] [Google Scholar]
- 53.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 55.Shih CC, Forman SJ, Chu P, Slovak M. Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev. 2007;16:893–902. doi: 10.1089/scd.2007.0070. [DOI] [PubMed] [Google Scholar]
- 56.Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]


