Abstract
An embryonic staging system for Molossus rufus (also widely known as Molossus ater) was devised using 17 reference specimens obtained during the postimplantation period of pregnancy from wild-caught, captive-bred females. This was done in part by comparing the embryos to a developmental staging system that had been created for another, relatively unrelated bat, Carollia perspicillata (family Phyllostomidae). Particular attention was paid to the development of species-specific features, such as wing and ear morphology, and these are discussed in light of the adaptive significance of these structures in the adult. M. rufus can be maintained and bred in captivity and is relatively abundant in the wild. This embryonic staging system will facilitate further developmental studies of M. rufus, a model species for one of the largest and most successful chiropteran families, the Molossidae.
Keywords: bat, Chiroptera, Molossus rufus, Molossus ater, embryogenesis, embryonic staging system
Introduction
The bats (order Chiroptera) are one of the most successful mammalian groups. A recent compilation indicates that there are at least 1,116 species of bats, comprising a little more than 20% of all living mammalian species (Simmons, 2005; Wilson and Reeder, 2005), and they are exceedingly abundant mammals in absolute numbers, particularly in tropical regions of the world. Bats also exhibit remarkable diversity with respect to habits, form and function. This can be attributed to the innovations of powered flight and, in the majority of species, echolocation. These have permitted many bats to prosper and evolve in a niche, the night sky, which, among mammals, has been exploited to a limited extent only by those that glide. Bats now perform a variety of ecologically important functions within this niche, including pollination, seed dispersal and predation (particularly of insects).
To fully understand the evolution of bats, knowledge of their ecology, phylogeny and ontogeny is needed. The study of bat ecology and species-specific characteristics allows us to postulate what selective pressures may have encouraged the development and diversification of these adaptations. An analysis of the embryonic and postnatal growth of specialized structures (such as bat wings), and comparisons with the formation of homologous structures in model organisms, provides an anatomical basis to begin understanding the genetic modifications that have played a role in their evolution.
Previous studies have described early oviductal and/or uterine development of the embryo in many bat species (Rasweiler, 1993; Badwaik and Rasweiler, 2000), and the postnatal development of a variety of anatomical features (Pedersen, 1995; Reep and Bhatnagar, 2000; Vater, 2000; Phillips, 2000; Adams and Thibault, 2000; Hermanson, 2000; Elangovan et al., 2007). Relatively few studies have examined development during the post-implantation period, and most of these have not included a precise temporal framework (instead using embryo crown-rump length or weight as indicators of relative age). These have focused upon the brain (Misek, 1989, 1990; Pirlot and Bernier, 1974, 1991; Reep and Bhatnagar, 2000), pituitary gland and vomeronasal organ (Reep and Bhatnagar, 2000), and aspects of limb development and skeletogenesis (Adams, 1992a, b; Wyant and Adams, 2007).
Recently, we used conceptuses collected after timed, captive matings to create a detailed embryo staging system for the short-tailed fruit bat, Carollia perspicillata (family Phyllostomidae) (Cretekos et al., 2005). Subsequently, this system has been used as a framework for detailed descriptions of the embryonic development of a pteropodid bat (Rousettus amplexicaudatus; Giannini et al., 2006) and a vespertilionid bat (Pipistrellus abramus; Tokita, 2006). Staging systems had previously been devised for another pteropodid (Syconycteris australis; Lawrence, 1991) and the vespertilionid Myotis lucifugus (Adams, 1992a).
Here, we add to current knowledge of bat embryonic development through a description of this process in the black mastiff bat, Molossus rufus (family Molossidae), correlated with some major structure-function relationships in the adult. The molossids are generally known as fast flying, acrobatic insectivores, although many (e.g. M. rufus) are also highly adept at non-volant, quadrupedal movement. It seems reasonable to expect that the morphological characteristics that facilitate the distinctive locomotor activities of this group would be reflected in their patterns of embryonic development.
Although the subject of this study has also been widely referred to in previous studies (including the collection of the actual embryos) as Molossus ater, Dolan (1989) made the case that this species was actually first identified and described by Geoffroy Saint-Hilaire (1805a, b) as M. rufus. According to the rules of zoological nomenclature (International Code of Zoological Nomenclature, Fourth Edition, 1999), the latter name should therefore take precedence.
M. rufus is found widely in the neotropics, from northern Mexico to northern Argentina (Dolan, 1989). These animals are relatively large (20–40 grams) compared to most other insectivorous bats (Rasweiler, 1990a), and the adults have wings with a high aspect ratio and wing-loading (Fenton et al., 1998). These long, narrow wings combined with low frequency echolocation calls (20–30 kHz) allow M. rufus to specialize in open-air foraging with fast, sometimes erratic flight patterns, and an apparent preference for the consumption of hard-shelled insect prey, e.g. beetles (Freeman, 1981a, b; Fenton et al., 1998).
The available evidence indicates that the Trinidadian population of M. rufus that provided breeding stock for the present study is reproductively synchronized, and that some of the females may carry two pregnancies per year in quick succession (Rasweiler, 1988; Badwaik et al., 1998). The reproductive biology of M. rufus has also been extensively studied under controlled conditions in captivity (Rasweiler, 1987, 1988, 1990a, b, 1991a, b, 1992, 1993; Badwaik et al., 1998), revealing this species to be an interesting model for studies of the biology of menstruation, trophoblastic growth and differentiation, and placental morphogenesis. During its menstrual cycle, M. rufus also exhibits, within its uterus, the most pronounced example of physiological (nonpathological) angiogenesis thus far observed in any adult mammal (Rasweiler, 1991a). Our description of the postimplantation embryology in M. rufus further advances the utility of this bat species for developmental studies.
Materials and Methods
Source of animals and species identification
Over the course of 23 years, more than 2000 M. rufus were collected on the West Indian island of Trinidad by one of the authors (JJR). These were captured with mist nets or live traps, as they exited from their diurnal roosts to forage at dusk or dawn, or when they returned and attempted to re-enter these roosts. The nets or traps were carefully positioned to catch most of the bats as they dropped from their exit holes and initially began to fly off. Many additional M. rufus were observed within their roosts. The roosts were found under roofs or in the attics of buildings at 48 sites scattered over a wide area of central Trinidad, roughly outlined by the towns and villages of Arima, Valencia, Sangre Grande, Nestor, Mamon, Mundo Nuevo, Tabaquite, Gran Couva, Freeport, Chaguanas, Caroni and Arouca.
On the basis of their larger adult size and pelage coloration (always a lustrous jet black), these bats were readily distinguished from the two smaller species of Molossus that also live on Trinidad - Molossus sinaloae trinitatus and Molossus molossus (Carter et al., 1981). Recently, however, a small number of exceptional M. rufus (about 6 individuals) exhibiting their russet (reddish) color phase were observed by the present authors intermingled with a larger number of black animals in one additional roost. The great preponderance of black individuals in this heavily-sampled population raises a serious question as to whether M. rufus is really a more appropriate species name for these bats than M. ater.
Captive maintenance
Some of the M. rufus (adult females and a smaller number of males in breeding condition) were retained, transported to New York and maintained at Cornell University Medical College with methods described elsewhere in detail (Rasweiler 1987, 1988). The bats were kept in a warm room (29–31°C) with a controlled light cycle (12 hours light: 12 hours dark). Initially, they were housed in sexually-segregated groups in Jewell-type animal cages (45.7 cm deep × 48.3 cm wide × 35.6 cm high). A portion of each cage (15.2 cm wide) on the right side was almost completely enclosed, except for a small (7.6 cm wide × 5.1 cm high) access hole at floor level, to provide a darkened retreat.
Each day, without exception, the bats were fed mealworms (the larvae of Tenebrio molitor) ad libitum in shallow pans. Initially, the mealworms were fattened for 2–3 weeks on a calcium-supplemented medium and then sprayed with a liquid multivitamin supplement (diluted 1:1 with water) immediately prior to being offered to the bats. Later in the studies, the mealworms were sprayed with the diluted vitamin mix, dusted directly with calcium carbonate and then presented to the bats. Water was also continuously provided via plastic chick fountains, consisting of a reservoir bottle and a water trough.
Observations of locomotor activity
M. rufus is specialized for a way of life that is significantly different in a number of respects from those of many other bat species. Observations were made by one of the authors (JJR) on M. rufus resting or moving about in their natural, diurnal roosts in 48 buildings on Trinidad. This was accomplished by peering into attics or subroof crawl spaces through access holes (“manholes”) from the human living quarters with the assistance of a flashlight, or by actually entering the roosts (which generally disturbed the bats). Observations were also frequently made on M. rufus when they left or re-entered their roosts at dusk or dawn, while actively foraging in flight for insects (which occurs while there is substantial daylight), and in captive breeding colonies maintained for about five years at Cornell. In both the field and captivity, bats occasionally fell to the ground or floor, and attempted to escape by crawling or running.
Timing of embryonic development
Following their transport to New York, the bats were initially housed in sexually-segregated groups for 2–7 months. Single males were then placed with groups of females to permit breeding activity.
Histological studies provided evidence that many of the M. rufus became polyestrous when maintained in sexual isolation in captivity. These studies also revealed that the species is a spontaneous ovulator with a functional luteal phase to its cycle. The cycle is terminated by true menstruation (Rasweiler, 1988, 1991a).
Following the introduction of males, vaginal aspirates were obtained from every female each morning and examined for spermatozoa (Rasweiler, 1987). As M. rufus did not exhibit a limited period of estrus, it proved impossible to correlate the first appearance of spermatozoa in aspirates with ovulation and fertilization. Most females exhibited an extended period of sperm-positive aspirates, however, and the time elapsed from its onset was generally correlated with the stage of embryonic development found in pregnant animals. Records of the days on which sperm-positive aspirates were obtained from many of the bats have been published elsewhere (Rasweiler, 1988, 1990b).
Measurements of the uterus and embryo
M. rufus was found to ovulate only from the right ovary and to carry conceptuses only in the right oviduct and uterine horn. Upon dissection, the greatest diameter of the right uterine horn was measured. In the initial studies (Rasweiler, 1990b), embryos at the limb bud stage or later were removed from the uterus, weighed, fixed in Zenker’s fluid for 16–20 hrs, and measured. The embryos were then washed overnight in running tap water and dehydrated through graded alcohols to 70% ethanol for storage. When necessary, mercuric chloride precipitated on the embryos was removed by treatment with Lugol’s solution, followed by a 5% solution of sodium thiosulfate to remove the iodine. In later studies, initial fixation of the embryos was done while they were still in utero. Both embryonic weight and crown-rump length of each embryo were then recorded post-fixation. These measurements have been recorded in Rasweiler (1990b) and Table 1 of the present paper.
Table 1.
Summary of M. rufus embryonic development
| Stage | Reference specimens1 | Day female was killed3 | Uterus diameter (mm) | Crown-rump length (mm) | Mass (gm) | Key features* |
|---|---|---|---|---|---|---|
| 13 | MA25C2 | 40 | 5.0 | 5.0 | 0.03 | Hindlimb buds form; forelimb AER; 3 pharyngeal arches. |
| 14 | MA24C2 | 29 | 8.0 | 7.0 | 0.04 | Forelimb longer than wide; hindlimb AER; nasal pits; cervical flexure; propatagium primordium. |
| MA41 | 38 | 9.5 | 6.4 | 0.04 | ||
| MA66 | 10 | 8.0 | 5.8 | 0.03 | ||
| 15 | MA29 | 40 | 10.0 | 7.2 | 0.09 | Hand and footplate; plagiopatagium primordium; auditory hillocks; premaxillary centers. |
| MA74B2 | 41 | 10.5 | 9.0 | 0.07 | ||
| 16 | MA15 | 44 | 12.0 | 9.7 | 0.13 | Pinnae and antitragii present; vibrissae condensations; uropatagium primordium; fore- and hindlimb digital condensations. |
| MA92B2 | 36 | 10.0 | 10.0 | 0.08 | ||
| 17 | MA53 | 34 | 11.0 | 11.4 | 0.17 | Prominent snout; cervical flexure absent; eyelids; fore- and hindlimb interdigit tissue receding. |
| 18 | MA22 | 62 | 13.0 | 13.5 | 0.33 | Mouth open and thumb free; forelimbs cross at midline; crescent-shaped pinnae; eyes begin to close; thumb and hindlimb claw primordia. |
| MA25 | 66 | 12.0 | 12.9 | 0.26 | ||
| MA44 | 40 | 12.1 | 12.1 | 0.20 | ||
| MA83B2 | 43 | 12.5 | 12.0 | 0.39 | ||
| 20 | MA16 | 50 | 13.5 | 16.7 | 0.70 | Eyes closed; pinnae extend towards facial midline; salient crus helix; knee flexure; mouth obscured by forelimb; digits II–IV juxtaposed/chirpatagium narrow. |
| MA21 | 54 | 14.5 | 18.5 | 0.77 | ||
| 21 | MA102 | 60 | 19.0 | 19.0 | 0.45 | Autopod folded onto zeugopod; mouth open and tongue visible. |
| 22 | MA782 | 70 | 19.0 | 28.0 | 2.80 | Eyes begin to open; teeth formed; antitragii broad and erect. |
Italicized text indicates unique features of M. rufus when compared to other bat species that were staged according to the C. perspicillata staging system (Cretekos et al., 2005; Giannini et al., 2006; Tokita, 2006).
Measurements reported in Rasweiler, 1990b.
Measurements not included in Rasweiler, 1990b; C-R length and weight recorded post-fixation.
Counted from the beginning of a prolonged period of sperm-positive vaginal aspirates.
AER, apical ectodermal ridge.
Results
Characteristics of locomotor activity
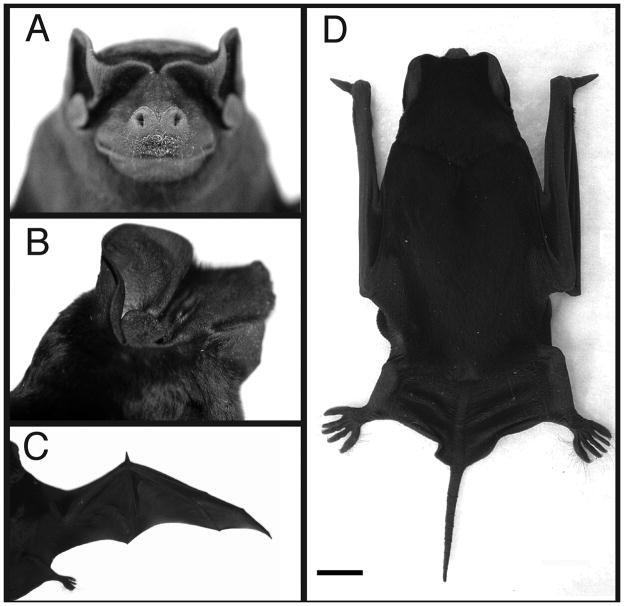
Extensive observations were made on locomotor activity by M. rufus during the course of both field work (48 building roosts) and captive experimentation with the animals. Like other molossid bats, M. rufus possesses long, narrow wings and compact craniofacial features (Fig. 1A–D). These are thought to be adaptations, in part, for their rapid mode of flight (Freeman, 1981a; Vaughan, 1966). When foraging, M. rufus is also capable of sudden, seemingly erratic, changes in direction, which are presumably used in part to catch insects. M. rufus forages twice per night, at dusk and dawn. At both times, the animals are flying outside their roosts when there is significant daylight, and they are therefore at risk of predation by raptors. On one occasion, during the course of field work on Trinidad, M. rufus leaving a roost to forage at dusk used sudden, evasive flight maneuvers to successfully avoid being captured by a pair of bat falcons (Falco rufigularis) (Badwaik and Rasweiler, 2000). Chase et al. (1991) have also observed bat falcons and owls hunting the closely-related M. molossus.
Fig. 1.
Gross morphology of adult M. rufus. A. Frontal view of head. B. Lateral view of head. C. Dorsal view showing extended wing. D. Dorsal view showing wings retracted for quadrupedal movement; scale bar = 1 cm.
Within their natural roosts (or wire cages in captivity), M. rufus were observed to walk or scamper quadrupedally across flat, inclined or vertical surfaces with great agility. This ability may help them to avoid predation by barn owls (Tyto alba), and possibly other predators (e.g. snakes), that also sometimes occupy the same attic roosts (JJR unpubl. observ.; Esberard and Vrcibradic, 2007). M. rufus were never seen to take to flight within their natural roosts (even when disturbed by the entrance of a human observer) and have generally proved to be incapable of flight in captivity (e.g. when escaping during their routine care or handling, and dropping to the floor of a relatively large animal room). As feeding time approaches in the wild, the bats can often be heard crawling across ceiling panels and out into roof overhangs to exit, usually from holes along the edges of the roofs.
Upon exiting from such holes, M. rufus do not immediately take to active flight. Rather, they first drop several meters to pick up lift and momentum before flying off. This was observed many hundreds of times in the course of catching the bats. When individuals occasionally dropped to the ground during collecting activities in the field, they could scamper there but generally could not directly take flight. Rather, they would have to first ascend to an elevated position by crawling and then drop to take flight.
Courtship behavior was also frequently observed during periods when M. rufus were being cared for or worked with in the captive breeding colonies established in New York. This was possible, because the animals were sometimes active in the open portions of their cages during daylight hours. Courtship activity typically involved extensive movement and interactions of the animals on all fours. The animals possess a cutaneous throat or gular gland, which is only active in adult males. Males were sometimes noted crawling about their cages to mark cage surfaces or females with the secretion of their gular glands. Females also sometimes exhibited intense interest in the gular glands of adult males and would approach the males on all fours, in an effort to nuzzle the glands. On occasion, this led the females to crawl under the males, into the normal dorsum-to-venter mating position, and coitus eventually took place (Rasweiler, 1987, 1992). Finally, if two adult breeding males were placed together in a cage, fighting would often erupt. This too was conducted on all fours.
Thus, for numerous reasons, quadrupedal movement is very important to these animals, and they are highly adept at it.
Timing of embryonic stages
It has been well-documented elsewhere that M. rufus does not exhibit a limited period of estrus (Rasweiler, 1987, 1988, 1990b). Instead, most females usually exhibit intermittent brief periods of sperm-positive vaginal aspirates, as well as one or more longer periods of such aspirates. Although clear evidence was obtained that mating activity was not always closely correlated with ovulation, the possibility that spermatozoa may also sometimes be stored for prolonged periods in the female tract of M. rufus remains to be explored.
The time elapsed from the onset of the first prolonged period of sperm-positive aspirates was usually correlated with the stage of embryonic development found in pregnant animals (Table 1), although there were some exceptional cases. This general pattern is best appreciated, however, when data for younger and more advanced conceptuses are also examined (see Rasweiler, 1988, 1990b). Because embryonic development was not closely correlated with the onset of a prolonged period of sperm-positive vaginal aspirates, it was impossible to determine the duration of each embryonic stage.
Stages of embryonic development
One hundred-thirty-eight M. rufus embryos were generated from captive matings. Sixty-five embryos were collated into nine developmental stages beginning at stage 13 and ending with stage 22; we did not have a specimen for stage 19 (Figs. 2, 3). Between two and six embryos were observed for stages 13 to 20. For stages 21 and 22, we had 10 and 29 embryos, respectively. The 17 embryos used as reference specimens are listed in Table 1. Seventy-three embryos were earlier or later in development than these numbered stages (Rasweiler, 1988, 1990b). Some embryos of M. rufus may be slightly more or less mature than those of the reference specimens. In such cases it would be appropriate to label such embryos as “early” or “late” stage embryos (e.g. 13E or 13L).
Fig. 2.
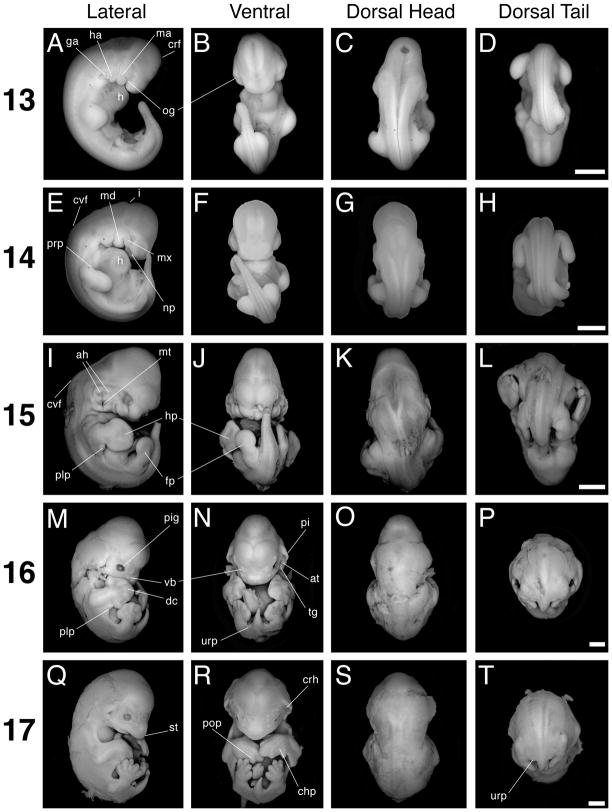
Stages 13 to 17. Scale bar = 1 mm in all panels. The first column (A, E, I, M, Q) shows lateral views with dorsal to the left, the second column (B, F, J, N, R) shows ventral views, the third column (C, G, K, O, S) shows dorsal views of head and trunk, and the fourth column (D, H, L, P, T) shows dorsal views of the trunk and tail. A–D: Stage 13 specimen. E–H: Stage 14 specimen. I–L: Stage 15 specimen. M–P: Stage 16 specimen. Q–T: Stage 17 specimen. at, antitragus; ah, auditory hillock; crh, crus helix; chp, chiropatagium; cvf, cervical flexure; crf, cranial flexure; dc, digit condensation; fp, foot plate; ga, glossopharyngeal arch; h, heart; ha, hyoid arch; hp, hand plate; i, isthmus; ma, mandibular arch; md, mandible; mt, auditory meatus; mx, maxilla; np, nasal pit; og, oral groove; pi, pinna; pig, pigment; plp, plagiopatagium; pop, posterior oriented phalanx; prp, propatagium; st, snout; tg, tragus; urp, uropatagium; vb, developing vibrissa.
Fig. 3.
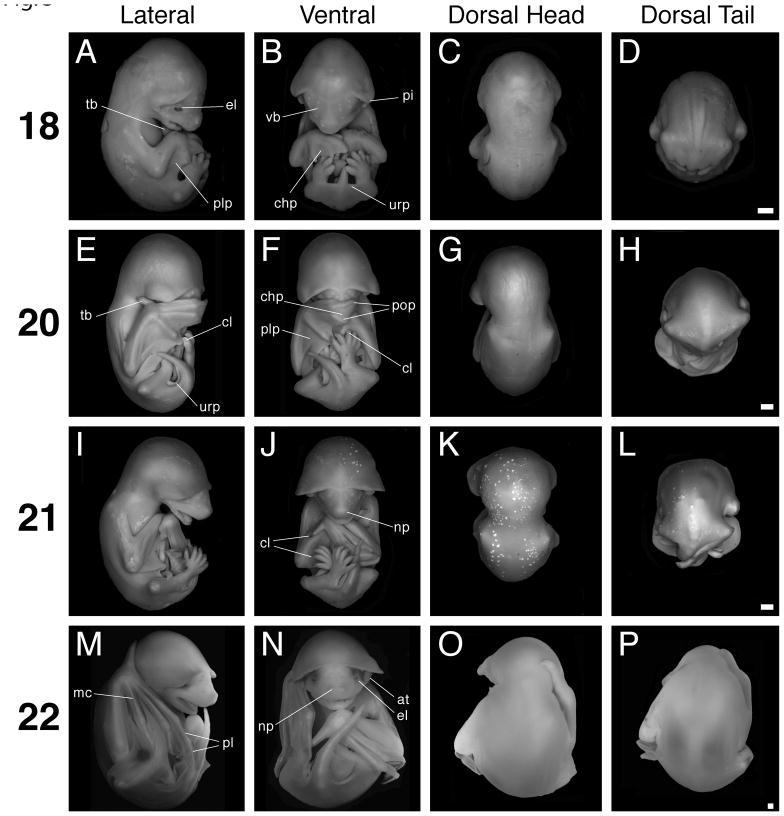
Stages 18 to 22. Scale bar = 1 mm in all panels. The first column (A, E, I, M) shows lateral views with dorsal to the left, the second column (B, F, J, N) shows ventral views, the third column (C, G, K, O) shows dorsal views of head and trunk, and the fourth column (D, H, L, P) shows dorsal views of the trunk and tail. A–D: Stage 18 specimen. E–H: Stage 20 specimen. I–L: Stage 21 specimen. M–P: Stage 22 specimen. at, antitragus; chp, chiropatagium; cl, claw; el, eyelid; mc, metacarpal; np, nasal pit; pi, pinna; pl, phalanx; plp, plagiopatagium; pop, posterior oriented phalanx; tb, thumb; urp, uropatagium; vb, developing vibrissa.
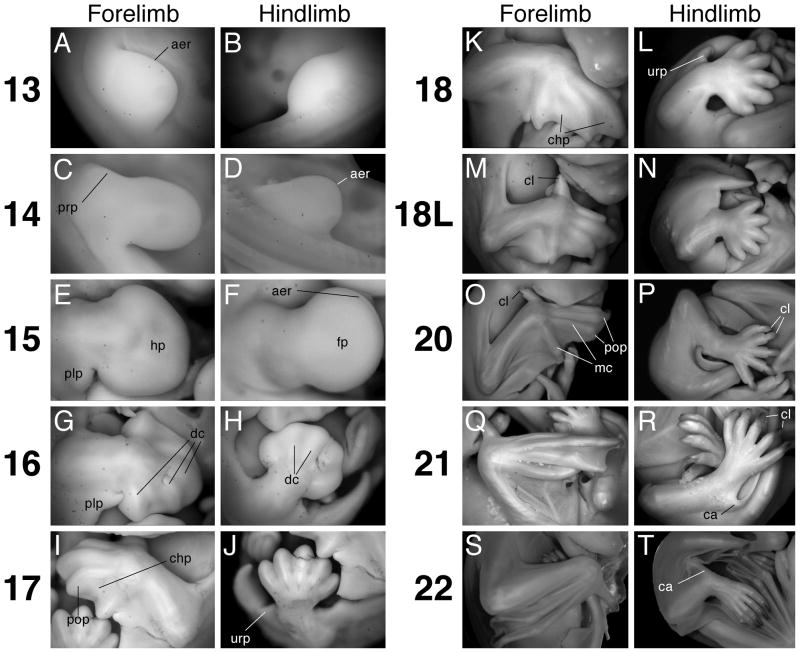
Stage 13
The reference specimen for this stage possesses 33 somite pairs, and late stage 13 embryos are presumed to have up to 36 somite pairs (see description for stage 14 below). Additionally, this stage is hallmarked by the presence of both fore- and hindlimb buds (Figs. 2A–D, 4A, B). The forelimb buds possess an apical ectodermal ridge (AER), which likely becomes more visible by late stage (Fig. 4A). Three pharyngeal arches are distinct and set in relief compared to the rest of the cranium. The oral groove appears as a slit on the anterior border of the first, or mandibular, arch and separates it into an elongated distal mandibular component and a shorter proximal maxillary component (Fig. 5A). In ventral view the oral groove is visible as a horizontal slit. The second, or hyoid, arch is asymmetrically shaped, with a broader proximal and narrower distal component. The third, or glossopharyngeal, arch is tucked underneath the second arch and protrudes towards the anterior end of the embryo (Fig. 5A). Also, the otic vesicle is apparent dorsal to the hyoid arch (Fig. 5A). Just anterior to the mandibular arch the lens vesicle is recognized as a whitish opaque condensation. The heart protrudes ventrally from the main body of the embryo and contacts the future viscerocranium (Fig. 2A). Additionally, the dorsal convexity of the embryo causes the tip of the curling tail to nearly touch the head (Fig. 2A).
Fig. 4.
Limb Morphology. A. Forelimb at stage 13. B. Hindlimb at stage 13. C. Forelimb at stage 14. D. Hindlimb at stage 14. E. Forelimb at stage 15. F. Hindlimb at stage 15. G. Forelimb at stage 16. H. Hindlimb at stage 16. I. Forelimb at stage 17. J. Hindlimb at stage 17. K. Forelimb at stage 18. L. Hindlimb at stage 18. M. Forelimb at stage 18 Late (L). N. Hindlimb at stage 18L. O. Forelimb at stage 20. P. Hindlimb at stage 20. Q. Forelimb at stage 21. R. Hindlimb at stage 21. S. Forelimb at stage 22. T. hindlimb at stage 22. aer, apical ectodermal ridge; ca, calcar; chp, chiropatagium; cl, claw; dc, digit condensation; fp, foot plate; hp, hand plate; mc, metacarpal; plp, plagiopatagium; prp, propatagium; pop, posterior oriented phalanx; urp, uropatagium. All panels show dorsal surface of the right limb (except for panels B, I, J, L, and N) with anterior toward the top and proximal at left (except for panels B, I, J, L, and N); views are not to scale.
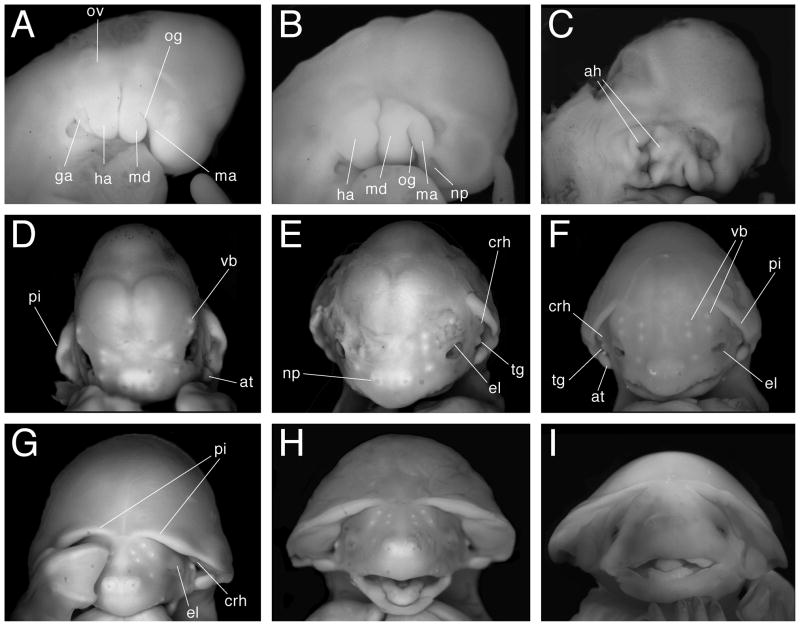
Fig 5.
Craniofacial development. Panels A–C show lateral views of developing head with anterior to the right. Panels D–I show face-on views with anterior to the top. A. Stage 13. B. Stage 14. C. Stage 15. D. Stage 16. E. Stage 17. F. Stage 18. G. Stage 20. H. Stage 21. I. Stage 22. ah, auditory hillock; at, antitragus; crh, crus helix; el, eyelid; ga, glossopharyngeal arch; ha, hyoid arch; ma, maxilla; md, mandible; np, nasal pit; og, oral groove; ov, otic vesicle; pi, pinna; tg, tragus; vb, developing vibrissa. Views are not to scale.
Stage 14
An early stage 14 reference specimen possessed 37 somite pairs. The late stage 14 reference specimen possessed 36 somite pairs; however, its tail was broken, and the embryo probably had an actual total of approximately 40 somite pairs (Fig. 2E–H). Stage 14 M. rufus embryos therefore possess at least between 37–40 somite pairs. Stage 14 is also easily identified by the morphology of the forelimb, which has now elongated so as to be longer than it is wide (Figs. 2E, 4C). Additionally, hindlimb AERs are present at this stage, with the AER becoming more obvious in late stage (Fig. 4D). The cleft between the mandibular and hyoid arches has deepened because of the increased size of the arches. Moreover, the anterior margin of the mandibular arch almost eclipses the eye (Figs. 2E, 5B). The oral groove, too, is more conspicuous, being deepened by the swelling of the proximal and distal portions of the mandibular arch. Only the proximal base of the third pharyngeal arch is visible posterior to the second arch (Fig. 5B). The nasal pits are visible as shallow, broad depressions in the future viscerocranium (Fig. 5B). As opposed to stage 13, the oral groove is not visible in ventral view, because it is obscured by the mandibular component of the first arch (Fig. 2F). The asymmetric shape of the hyoid arch has given way to three apparent segments: proximal, medial, and distal (Fig. 5B). These segments presage the development of the auditory hillocks. By late stage 14 the cleft between arches one and two begins to widen (Fig. 5B). The ventral body cavity, in between the fore- and hindlimbs, has distended so as to give the entire embryo a cuboidal appearance (Fig. 2E). Moreover, the somite pairs are difficult to discern in the anterior half of the embryo because of body wall thickening and somite differentiation (Fig. 2G). Also noticeable is a notch along the dorsal midline of the body opposite the first arch. This is the external expression of the isthmus between the mid- and hindbrain (Fig. 2E). Additionally, the cervical flexure is apparent at this stage (Fig. 2E). The anterior edge of the proximal forelimb displays a region of thickened tissue (Fig. 4C). From this region the propatagium, or the segment of the wing membrane that extends from the shoulder to the wrist, will likely develop.
One of the stage 14 embryos (collected from bat MA66) provides a good example of development not being well-coordinated with the onset of the first prolonged period of sperm-positive aspirates from the mother. In this case, the mother had been killed 10 days after the onset of this period (Table 1); however, sperm-positive aspirates had also been obtained from this female 13 and 14 days prior to that. It seems most likely that conception had actually occurred in association with the earlier period of breeding activity (see Rasweiler 1988 see Rasweiler 1990b for the complete breeding record for this animal).
Stage 15
The most obvious external changes at this stage involve alterations in limb geometry and pharyngeal arch modifications (Figs. 2I, J, 4E, F, 5C). The fore- and hindlimb autopods (distal regions of each limb) are paddle shaped, indicating that the hand plate has developed (Fig. 4E, F). The posterior arc of the forelimb handplate is slightly larger than the anterior arc, reflecting anterior-posterior (A–P) asymmetries in autopod development (Fig. 4E). Between the fore- and hindlimb, the plagiopatagium primordium has developed as a rounded bulge of tissue on the posterior side of the proximal forelimb (Fig. 4E). The plagiopatagium is the wing membrane section that extends from the fifth forelimb digit to the ankle. The premaxillary centers, which are bounded by the maxillary component of the first arch and the facial midline, are discernible as bulges that are larger than the maxillary components (Figs. 2I, J, 5C). The mandibular components of the first pharyngeal arch pair have fused at the midline, forming the precursor of the lower jaw. Additionally, roundish tissue swellings punctuate the posterior-lateral margin of the mandibular component of the first arch; these are aligned with tissue swellings along the anterior-lateral margin of the second arch so that the cleft between the two arches appears discontinuous and deeper (Figs. 2I, 5C). The external auditory canal (or meatus) will eventually form from the deepened and widened cleft, and the conspicuous mounds of tissue lining this depression are the auditory hillocks. The increased bulkiness of this stage is most apparent when viewed from the back, where one can see that the greatest width of the embryo occurs between the forelimb buds (Fig. 2K, L). Lateral body wall thickening all but entirely obscures any view of the somites. The notch demarcating the midbrain-hindbrain boundary is still present, and the cranial flexure is still evident (Fig. 2I); thus the overall contours of the embryo remain the same as stage 14.
Stage 16
The auditory hillocks have fused to form the pinna of each ear, as well as other external ear structures (Figs. 2M–O, 5D). The external ears have changed their orientation relative to other facial features. It is now more instructive to talk of the ears as being aligned with the embryo’s A–P axis rather than the proximal-distal (P–D) axis associated with the pharyngeal arches of earlier stages. Thus, the anterior margins of the ears developed from the proximal margins of the pharyngeal arches, whereas the posterior margins of the ears developed from the arches’ distal margins. Additionally, at this stage the external ear is restricted to the side of the head. Near a pinna’s posterior base one can see the bulbous antitragus (Figs. 2N, 5D). Along and near the ventral face of a pinna’s anterior base two noticeable tissue aggregations developed from the auditory hillocks. We call the ventral-most of these two tissue aggregations the tragus (Fig. 2N). We call the second tissue aggregation, which is dorsal to the tragus and adjoins both the side of the head and the anterior border of the pinna, the crus helix; this structure becomes more prominent in later stages. The maxillary components of the first pharyngeal arch pair and the premaxillary centers are now fused at the midline. The mandibular process does not protrude ventrally as much as the maxilla and thus the embryo possesses an “overbite.” The retina is obviously pigmented (Fig. 5D). In profile view there is a noticeable, though not salient, indentation along the profile of the head so as to suggest to the observer a forehead above the eyes and the beginnings of a snout (Fig. 2M). Five opaque ectodermal condensations are readily visible on either side of the facial midline – these are vibrissae precursors (Figs. 2M, N, 5D). Two condensations appear above the eye, two develop medial to the eye, and one is conspicuous midway between the nasal pits and the eye. These are presumably developing tactile vibrissae as observed on Rhinolophus ferrumequinum (Schneider, 1963). Additionally, when viewed laterally three more condensations are visible – two just ventral to the antitragus, and one midway along the maxilla. Though the cervical flexure is still apparent, it is not as pronounced as in earlier stages. The notch marking the isthmus between the mid and hindbrain is reduced or absent. Both the foot and hand plate exhibit opaque digit condensations (Figs. 2M, N, 4G, H). In the forelimb, future digit III is longer than all other digits and the first and fifth digit condensations extend in the anterior and posterior direction, respectively. Digits I and V on the forelimb autopods extend beyond the interdigit tissue; therefore each hand plate is spade shaped (Fig. 4G). The distal margin of the foot plate appears corrugated. This may be the result of interdigit tissue recession; alternatively, the digit condensations may have grown beyond the interdigit tissue (Fig. 4H). Due to growth along their A–P axes the hand and foot plates contact each other along their posterior and anterior edges, respectively (Fig. 2N). The uropatagium, which will become the segment of the wing membrane that connects the ankle to the tail, is evident as a slight lip of tissue connecting the bases of the hindlimbs to the ventral base of the tail (Fig. 2N, P). The tip of the tail is free of uropatagium. The plagiopatagium is also more distinct as a continuous layer of skin extending from the forelimb wrist to the anterior border of the proximal hindlimb (Fig. 2M).
Stage 17
Maturation of facial and limb structures hallmark this stage. The rounded snout is now a prominent facial feature, and the overbite created by the maxilla is exaggerated by a downward turn (Figs. 2Q, R, 5E). Also, due to the rising of the head relative to the rest of the body, the cervical flexure is absent and the chin no longer rests on the chest (Fig. 2Q). The bulbous regions of the cranial vault are reduced so that above the eyes the head appears rounder; the trend towards a rounder cranium continues for the rest of development (Fig. 5E). This trend is highlighted by the observation that the anterior pinnae bases are closer to the apex of the head relative to stage 16. The antitragus is approximately equal in size to the eye, while the tragus is now prominent and cone-shaped (Fig. 5E). The crus helix, which is anterior and slightly dorsal to the tragus, is larger and more elongated than the tragus. Between stages 17 and 20 this structure fuses with the ventral surface of the pinna (Figs. 2R, 5E). The anterior bases of the pinnae are no longer confined to the sides of the head, but are nearer to the midline (Fig. 5E). The primordial eyelids are conspicuous as thin margins of thickened tissue around each eye (Fig. 5E). The maturing bones of the forearm are evident, as they protrude out of the membranous patagia (Fig. 2Q, R). Particularly obvious is the flexure at the elbow, and less evident is the flexure at the wrist (Figs. 2Q, R, 4I). The distal margins of the autopods now meet at the midline (Fig. 2R). Fore-and hindlimb digits are clearly distinct from the interdigital tissue, and the interdigital tissue of the hindlimb is clearly receding (Fig. 4I, J). Also, the interdigit tissue is noticeably reduced between the first and second forelimb digits, though still present. The distal tip of digit IV on each autopod conspicuously bends towards posterior, foreshadowing the final orientation of this digit’s first phalange in the adult (Figs. 2R, 4I). The orientation of this digit (as well as Digit III, described in stage 20) may aid in this species’ ability for rapid flight and quadrupedal locomotion, as has been suggested for other bat species (Vaughan, 1966; Norberg and Rayner, 1987). The head no longer appears disproportionately larger than the rest of the body; this results in an overall appearance that is developmentally more advanced, but less bulky than stage 16 embryos (Fig. 2Q, R). Additionally, when viewed from the back, the trunk width between the shoulders is not markedly greater than the width of the posterior half of the trunk (Fig. 2S, T).
Stage 18
The thumb conspicuously extends towards the head, and depending on the specimen, may sit inside the open mouth or juxtaposed against the neck (Figs. 3A, 4M). The tissue between the thumb and digit II has completely receded. The forelimb digits are longer, with the distal tips of digit III from each arm meeting or crossing at the midline (Fig. 3B). Often, the overlying forelimb will also cover the distal extremities of the hindlimbs (Figs. 3B, 4K). The degree to which the forelimbs overlap increases from early to late stage 18 (Fig. 3B). The propatagium follows the edges of the stylopod and zeugopod very closely, such that near the wrist and the proximal base of the stylopod it is difficult to discern (Figs. 3A, 4M). Though the first and fifth forelimb digits extend away from the center of the autopod, digits II–IV of the forelimb are juxtaposed (Figs. 3B, 4K, M). The hindlimb digits are free of interdigit tissue (Fig. 4L, N). In late stage 18 specimens, thumb claw primordia are just perceptible as slight constrictions of tissue at the distal tips of the digits (Fig. 4M). The knee-joints are clearly articulated and as perceptible as the elbow flexure (Figs. 3B, D, 4L, N). The entire distal margin of the pinna curves in towards the side of the head, such that the ear “hugs” the head (Figs. 3C, 5F). The antitragus is more lobate in appearance, looking like a miniature limb bud, and is approximately equal in size to the eye. It stands erect and is situated nearly flush against the side of the head (Fig. 5F). The tragus appears triangular. At late stage 18, the anterior bases of the pinnae extend towards the facial midline at least as far as the medial border of the eye (Fig. 5F). The vibrissae condensations are more evident as white, slightly raised dots on the face (Fig. 5F). The eyelids begin to grow over the eye (Figs. 3A, 5F). Additionally, the overbite observed in earlier stages is not as prominent because the mandible has grown out relative to the maxilla (Fig. 3A). Along the interior edges of the proximal region of the mandible one can see swollen bulges of tissue flanking the tongue. These tissue condensations may be the precursors of the teeth. The nasal pits face forward, and the nasal process is clearly set in relief to the rest of the snout (Fig. 5F). In late stage 18 embryos, the indented contour that in profile view distinguished the snout and the forehead is becoming less evident (Fig. 3A). As in earlier stages, the snout is rounded (Fig. 3A). The uropatagium that flanks the tail is straight, meeting the base of the tail at a 90° angle. Moreover, the tail appears rounder at its base and more conical in overall appearance (Figs. 3B, 4N).
Stage 19
No reference standard specimens were collected for this stage.
Stage 20
The pinnae have unfurled so that their ventral surfaces are easily visible again, as in stage 16 and 17 (Figs. 3G, 5G). Moreover, the antitragus now extends laterally away from the side of the head. Thus the antitragus appears as a flat, table-like structure, oriented in the same plane as the mouth (Fig. 5G). The anterior pinnae bases have extended closer to the facial midline (Figs. 3F, 5G). The tragus has reduced in size to a bead-like nodule. Now of more prominence than the tragus is the crus helix (Fig. 5G). Due to the unfurling of the pinnae, their proximity to the midline, and the increasing roundness of the apical cranium, the upper two thirds of the head look like one distinct anatomical structure – a helmet, or mushroom cap (Fig. 5G). The eyes are closed. The mouth is slightly ajar, and the tongue is visible (Fig. 5G). Vibrissae condensations are macroscopically visible. There is no longer any indentation between the forehead and the snout in profile view. This welding of the snout and the forehead results in a head that appears both more robust and teardrop-shaped (Figs. 3E, 5G). One or both forelimb autopods either cover the face or lie tucked underneath the chin (Figs. 3E, F, 4O, 5G). Depending on the specimen, the hindlimb digits may be entirely or almost entirely hidden by the overlying forelimb autopods (Fig. 3F). Well-defined claws are clearly visible on the thumbs (Fig. 4O). Hindlimb claws are also pointed and readily distinguishable (Fig. 4P). The chiropatagium is easily discernable between digits IV and V, but is not easily discernable between digits II, III and IV (Figs. 3E, 4O). The first phalanx of digit III, like digit IV in stage 17, is now conspicuously oriented towards the embryo’s posterior pole (Fig. 3F, 4O). The posterior-facing tips of digits III and IV, coupled with the approximately equal length of the autopod and zeugopod, permit the autopod to fold back onto the zeugopod. It has been suggested that this compact configuration contributes to the molossids’ marked ability for terrestrial locomotion (Vaughan, 1966). Flexure in the knee, ankle, wrist, and elbow joints is readily discernible as the patagia become thinner and increasingly membranous (Figs. 3E, F, H, 4O). The uropatagium is folded under the ventral base of the tail and thus no longer meets the base of the tail at a 90° angle (Fig. 4P).
Stage 21
The forelimbs, particularly the autopods, are in new positions relative to the head. The wrists have moved slightly ventral and are either positioned alongside the open mouth or underneath the chin (Figs. 3I, J, 4Q). The autopods do not fully extend across the midline, but instead are approximately aligned with the embryo’s A–P axis, with the digits pointing towards the posterior pole (Figs. 3J, 4Q). The autopod is partially or fully folded back onto the zeugopod and the chiropatagium is folded in between the digits (Fig. 3I, 4Q), presaging the functional orientation of these limb elements during quadrupedal terrestrial locomotion. The hindlimbs are crossed (Figs. 3I, J, L, 4R). The thumb and hindlimb claws appear keratinized, and pigment is visible on their dorsal side (Fig. 4R). The ventral faces of the pinnae bubble out, as if they had been inflated; this is especially evident near their distal rim (Fig. 5H). Thus the crus helix observed in the last stage is temporarily conflated with the bubbling underside of the pinna. It is not clear whether this observation is an artifact of the fixation process or a real developmental event. The eyes are still closed. The tongue is readily visible in the open mouth (Fig. 5H). The regions of swollen tissue that flanked the tongue in earlier stages have developed into a ridge of tooth primordia (Fig. 5H). The tip of the snout is now more angular than round (Fig. 3I). The calcar, though partially obscured by the crossed hindlimbs and creased uropatagium, is visible for the first time extending away from the ankle into the uropatagium towards the base of the tail (Fig. 4R).
Stage 22
Teeth have developed within the open mouth (Fig. 5I). The upper and lower jaws are mature and broad, appearing as wide as a line drawn from the lateral-most corner of one eye to the other. In this respect the snout appears as it does in the adult (Figs. 1A, B, 5I). The eyes have begun to open again (Figs. 3N, 5I). The nasal processes protrude away from the tip of the snout, making the nose appear as a discrete facial structure (Fig. 5I). The antitragii now stand erect, with their distal tips projecting into the external ear cavity. Additionally, the antitragii bases are broader and flatter than in earlier stages and therefore appear egg-shaped, as they will be in the adult (Figs. 1A, B, 3N, 5I). The bubbling of the ventral face of the pinna observed in the previous stage is now mostly absent except near the pinna’s distal rim. Therefore, the crus helix is again readily discernible (Fig. 5I). The wrists are either juxtaposed against the chin, or lying alongside the neck (Figs. 3M, N, 4S). The thumb is roughly as long as the snout, and if the forelimb is apposed to the neck the thumb may extend dorsally around it – like a collar (Fig. 3M, O, P). The hindlimbs are uncrossed and, like the tail, they extend towards anterior where they contact the chin (Figs. 3M, N, 4T). In this compact configuration, which makes the entire embryo appear spherical, the keel-like calcar is often fully visible (Figs. 3M, N, 4T). In summary, the embryo looks much like an adult except that it lacks fur and the pinnae do not yet stand erect (Figs. 1A, B, 3M, N, 5I).
Discussion
The external morphology described here for M. rufus is the first embryological staging series produced for any species of molossid bat, and is based in part on the staging system developed for Carollia perspicillata (Phyllostomidae) (Cretekos et al., 2005). Perhaps not surprisingly, the embryonic staging system for M. rufus bears many similarities to other bat species (Tokita, 2006; Giannini et al., 2006) that were derived from the C. perspicillata staging system. In relation to other bat species for which staging systems exists, M. rufus embryos display a number of species-specific characters. Several of these unique anatomical characters, identifiable during development, have known or suspected roles in the natural history of the adult bats.
The shape and orientation of the wings of M. rufus embryos are noticeably different from those of some other, reasonably well-studied bat species by stage 18; augmentation of these differences continues into later stages. Whereas digits II–IV remain in close proximity in stage 18 M. rufus forelimbs, the entire autopod, with adjoining chiropatagium, is extended in C. perspicillata and Pipistrellus abramus (Cretekos et al., 2005; Tokita, 2006). The pteropodid, Rousettus amplexicaudatus, also passes through a stage (Stage 22) wherein the digits and chiropatagium are outstretched (Giannini et al., 2006). The extended autopod in the latter species reveals their relatively rounded and broad forelimbs. This is in contrast to the pointed, narrow shape of the M. rufus autopod – a distinctive feature of M. rufus adults. During M. rufus development, the chiropatagium between digits II, III and IV never seems to accumulate between stages 16–22 as it does in the aforementioned species. Interestingly, stage 20 of P. abramus is noted for the folding of accumulated dactilopatagium between the digits (Tokita, 2006). Although M. rufus wings appear corrugated in later stages, this is not the case between digits II and III, suggesting that reduction of dactilopatagium development between these two digits is important for adult bat wing shape. Additionally, the dactilopatagium between digits IV and V never develops to the same distal extent as that observed in C. perspicillata or P. abramus. This provides M. rufus forelimbs with a characteristic notch between digits IV and V and a noticeably narrower wing along the A–P axis. The long, narrow wings of M. rufus observed during embryological development presumably affect the flight and feeding behaviors of the adults. As was suggested for other molossid bats, the narrow, pointed wings of M. rufus may aid in its pursuit of certain types of flying insects. This may also affect foraging site preferences towards unobstructed locales (Vaughan, 1966; Freeman, 1981a).
During stage 17 digit IV is posteriorly oriented. The same is true for digit III at stage 20. This orientation is unique to M. rufus when compared to the forelimb development for other staged species (Lawrence, 1991; Cretekos et al., 2005; Tokita, 2006; Giannini et al., 2006). The orientation of the distal phalanges of digits III and IV are believed to be an adaptation that, in concert with other anatomical peculiarities, provide M. rufus adults, and other bats within the Molossidae, with the marked ability of quadrupedal terrestrial locomotion (Vaughan, 1966; Schutt and Simmons, 2006). Notably, Cheiromeles have evolved plagiopatagium-derived pouches into which the distal phalanges of the forelimb can be inserted during quadrupedal locomotion (Schutt and Simmons, 2006). Though no such pouches exist for M. rufus, it is apparent from the doubled-over forelimbs of stages 21 and 22 that the orientation of digits III and IV may effectively wrap around the elbow. In the adult, such a configuration would prevent the tip of the wing from being fully exposed during quadrupedal movement. In this orientation the chiropatagium is snuggly tucked into the autopod, and the plagiopatagium is partially collapsed onto the zeugopod, presaging the orientation of these limb elements during adult quadrupedal locomotion.
The pinnae (ears) of M. rufus, like the wings, become increasingly indicative of this species in later stages of development. At stage 16, for all species staged similarly to C. perspicillata, the pinnae begin to develop along the side of the head. As early as stage 17 in M. rufus embryos, however, the anterior borders of the pinnae have migrated nearer to the facial midline. This extension of the anterior pinnae borders to the midline continues throughout development, so that the ears are as much a feature of the forehead as they are of the side of the head. The conspicuous positioning of the pinnae is complemented by the development of what we have identified as the crus helix – the keel-like structure that punctuates the anterior margin of a pinna’s ventral face. In the adult this structure appears at least partly responsible for restricting the erect pinna to the side of the head, just above the eye (see Fig. 1A, B). The development of the crus helix was not noted in the staging series reported for the vespertilionids or pteropodids, although it is likely present in some form, as the crus helix is a recognized structure just above the tragii in numerous mammalian pinnae. The prominent and apparently rigid crus helix in M. rufus likely evolved to support the unique position of the pinnae along the front and side of the head. The location and timing of the developing crus helix suggests that its unique features – rigidity and prominence in relation to the tragus and antitragus – originate from a derived proliferative region of the auditory hillocks, which already proliferate to give rise to the external ear structures. The unique positioning of M. rufus’ pinnae along the forehead is not without postulated ecological importance. Vaughan (1966) suggested that the morphology of some molossid bat ears might aid in the bats’ ability for rapid, sustained flight, because the broad ears are drawn in towards the head and are thus parallel to the airstream during flight. It is worth noting that P. abramus is also an insectivorous bat and yet, both during development and in the adults of this species, the pinnae do not migrate to the midline (Tokita, 2006). Thus, although M. rufus’ unique pinnae may aid it during rapid flight, P. abramus apparently required no modification of its pinnae in order to catch prey. Therefore, shape differences in the pinnae are probably also driven by the foraging and navigational needs of each species, which rely heavily upon echolocation (Dumont, 2006).
Previous authors noted that within the family Molossidae, jaw thickness, temporal muscle size, and the size and number of teeth correlate with and may predict well the feeding behavior of particular species (Freeman, 1979). M. rufus crania, for example, with their thick jaws and few, but large teeth correlated well with this bat’s reported habit of preying upon hard-shelled insects, such as beetles (Freeman, 1979 and references therein, 1981a, b; Fenton et al., 1998). The broad, deep jaws of M. rufus are readily apparent by stage 20 (and less so at earlier stages). The craniofacial structure of C. perspicillata, a frugivore, at comparable stages does not exhibit either the broadness of the snout or the welding of the snout to the forehead that gives M. rufus embryo crania their robust, tear-drop shapes (cf., Cretekos et al., 2005). Instead, at stage 20 C. perspicillata embryos the snout is barrel-shaped and does not extend laterally much beyond the medial border of the eyes when viewed ventrally. Although P. abramus is also an insectivore, its snout is largely obscured during stages 18–24, and little mention was made of its fetal snout morphology (Tokita, 2006).
The broad snout of M. rufus also helps to create a relatively large oral cavity that may be used to hold accumulations of insects. Goodwin and Greenhall (1961) reported that these bats have large “cheek pouches”, which become stuffed to capacity during foraging. The animals then supposedly return to their roosts to chew and swallow their food. Although Fenton et al. (1998) found some insect remains in the mouths of M. rufus returning to their roosts after foraging, no evidence of insects in cheek pouches was observed. Also, Murray and Strickler (1975) failed to find functional cheek pouches in two dissected M. rufus. In captivity, however, these bats have often been observed to greatly engorge their mouths with mealworms when being hand-fed and to masticate large masses of worms for extended periods before completing their ingestion (Rasweiler, unpubl. observ.). The ability to temporarily accumulate at least modest amounts of food in their mouths could be of considerable adaptive significance to M. rufus. It may enable the bats to quickly catch large numbers of flying insects during daily periods of peak abundance and to sufficiently chew the insects prior to swallowing. By increasing hunting efficiency, it may also reduce the amount of time that foraging bats must be outside of their roosts and at risk of predation. Studies by Fenton et al. (1998) indicate that M. rufus can forage very efficiently. Presumably any accumulation of food in the bats’ mouths must be balanced against their need to use oral echolocation calls for hunting and navigational purposes. M. rufus and a number of other molossids are generally considered to be oral, rather than nasal, signal emitters (Pedersen, 1993, pers. comm.; Goudy-Trainer and Freeman, 2002).
The present study is important because it provides the first detailed embryonic staging system for a representative of the Molossidae, one of the largest and most successful chiropteran families. This family contains 100 species (Simmons, 2005; Wilson and Reeder, 2005). Some of these are extraordinarily abundant in the wild and therefore of great ecological importance, particularly as predators of insects. For example, summer colonies of Mexican free-tailed bats (Tadarida brasiliensis) occupying some caves in the southwestern United States may be the largest aggregations of mammals in the world. These may have been even much larger in the recent past; however, there is uncertainty about the accuracy of historical population estimates (Betke et al., 2008). Molossid bats also differ significantly in habits, form and function from many other commonly studied bats. Prior to the present study, however, embryonic staging systems had been devised only for representatives of three other families (the Phyllostomidae, Pteropodidae and Vespertilionidae) of this very diverse mammalian order. The present study is also noteworthy because the embryos were obtained from females bred under controlled conditions in captivity.
Having a system available for precisely staging embryos harvested either from captive-bred females or from a reproductively-synchronized wild population (e.g. that existing on the island of Trinidad)(Rasweiler, 1988; Badwaik et al., 1998) opens the door to much more probing studies of significant reproductive, developmental and evolutionary problems. It provides a means of more accurately characterizing synchronized or seasonal breeding patterns in the wild. For example, observations made on embryonic development in C. perspicillata bred under controlled conditions in captivity played a major role in the discovery of a period of developmental delay in the wild population (Rasweiler and Badwaik, 1997).
Finally, the availability of an embryonic staging system can be of great value for studies of evolutionary developmental biology. Staging series can provide a morphological basis for functional characterization of DNA sequences that control development (Cretekos et al., 2001; Baguna and Garcia-Fernandez, 2003). Gene expression patterns obtained throughout the development of similar structures at similar points of developmental maturity in two different species can be used as a starting point for functional genomic studies (Cretekos et al., 2001; Chen et al., 2005; Sears et al., 2006; Weatherbee et al., 2006; Cretekos et al., 2007). Recently, comparative mammalian embryology emboldened the decision to test the functional relevance of a putatively important genetic sequence during limb development by moving it from one species, the bat, C. perspicillata, to the mouse, Mus musculus (Cretekos et al., 2008).
Acknowledgments
National Institutes of Health (NIH) HD17739 and RR05396 to JJR and National Science Foundation IBN 0220458 and the Ben F. Love Endowment to RRB. CJC was supported by NIH training grants CA09299 and HD07325. MJN was supported by NIH training grant HD07325. DH was supported by the Society for Integrative and Comparative Research Grants-in-Aid-of-Research Award and by the National Research Foundation of South Africa Prestigious Masters Scholarship
We thank the Department of Zoology, University of the West Indies, Trinidad, and particularly Mr. P. Deoraj and Professor J.S. Kenny, for generous assistance with the field aspect of these studies. We are also grateful to the Wildlife Section, Forestry Division, Ministry of Agriculture, Land and Marine Resources of the Republic of Trinidad and Tobago for providing collecting and export permits.
Literature Cited
- Adams RA. Stages of development and sequence of bone formation in the little brown bat, Myotis lucifugus. J Mammal. 1992a;73:160–167. [Google Scholar]
- Adams RA. Comparative skeletogenesis of the forearm of the little brown bat (Myotis lucifugus) and the Norway rat (Rattus norvegicus) J Morphol. 1992b;214:251–260. doi: 10.1002/jmor.1052140302. [DOI] [PubMed] [Google Scholar]
- Adams RA, Thibault KM. Ontogeny and evolution of the hindlimb and calcar: assessing phylogenetic trends. In: Adams RA, Pedersen SC, editors. Ontogeny, functional ecology, and evolution of bats. Cambridge: Cambridge University Press; 2000. pp. 317–332. [Google Scholar]
- Badwaik NK, Rasweiler JJ, IV, Muradali F. Co-expression of cytokeratins and vimentin by highly invasive trophoblast in the white-winged vampire bat, Diaemus youngi, and the black mastiff bat, Molossus ater, with observations on intermediate filament proteins in the decidua and intraplacental trophoblast. J Reprod Fert. 1998;114:307–325. doi: 10.1530/jrf.0.1140307. [DOI] [PubMed] [Google Scholar]
- Badwaik NK, Rasweiler JJ., IV . Pregnancy. In: Crichton EG, Krutzsch PH, editors. Reproductive biology of bats. London: Academic Press; 2000. pp. 221–294. [Google Scholar]
- Baguna J, Garcia-Fernandez J. Evo-devo: the long and winding road. Int J Dev Biol. 2003;47:705–713. [PubMed] [Google Scholar]
- Betke M, Hirsh DE, Makris NC, McCracken GF, Procopio M, Hristov NI, Tang S, Bagchi A, Reichard JD, Horn JW, Crampton S, Cleveland CJ, Kunz TH. Thermal imaging reveals significantly smaller Brazilian free-tailed bat colonies than previously estimated. J Mammal. 2008;89:18–24. [Google Scholar]
- Carter CH, Genoways JJ, Loregnard RS, Baker RJ. Occasional Papers of the Museum. 72. Texas Tech University; 1981. Observations on bats from Trinidad, with a checklist of species occurring on the island; p. 27. [Google Scholar]
- Chase J, Small MY, Weiss EA, Sharma D, Sharma S. Crepuscular activity for Molossus molossus. J Mammal. 1991;72:414–418. [Google Scholar]
- Chen C, Cretekos CJ, Rasweiler JJ, IV, Behringer RR. Hoxd13 expression in the developing limbs of the short-tailed fruit bat, Carollia perspicillata. Evol Dev. 2005;7:130–141. doi: 10.1111/j.1525-142X.2005.05015.x. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Rasweiler JJ, IV, Behringer RR. Comparative studies on limb development in mice and bats: a functional genetic approach towards a molecular understanding of diversity in organ formation. Reprod Fertil Dev. 2001;13:691–695. doi: 10.1071/rd01115. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Weatherbee SD, Chen C-H, Badwaik NK, Niswander L, Behringer RR, Rasweiler JJ., IV Embryonic staging system for the short-tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive-bred animals. Dev Dyn. 2005;233:721–738. doi: 10.1002/dvdy.20400. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Deng JM, Green ED, Rasweiler JJ, IV, Behringer RR NISC Comparative Sequencing Program. Isolation, genomic structure and developmental expression of Fgf8 in the short-tailed fruit bat, Carollia perspicillata. Int J Dev Biol. 2007;51:333–338. doi: 10.1387/ijdb.062257cc. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Wang Y, Green ED, Martin JF, Rasweiler JJ, IV, Behringer RR NISC Comparative Sequencing Program. Regulatory divergence modifies limb length between mammals. Genes Dev. 2008;22:141–151. doi: 10.1101/gad.1620408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan PG. Special Publications. Vol. 29. The Museum, Texas Tech University; 1989. Systematics of Middle American mastiff bats of the genus Molossus; p. 71. [Google Scholar]
- Dumont ER. The correlated evolution of craniofacial morphology and feeding behavior in New World fruit bats. In: Zubaid A, McCracken GF, Kunz TH, editors. Functional and evolutionary ecology of bats. New York: Oxford University Press; 2006. pp. 160–177. [Google Scholar]
- Elangovan V, Raghuram H, Yuvana Satya Priya E, Marimuthu G. Wing morphology and flight development in the short-nosed fruit bat Cynopterus sphinx. Zool. 2007;110:189–196. doi: 10.1016/j.zool.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Esberard CEL, Vrcibradic D. Snakes preying on bats: new records from Brazil and a review of recorded cases in the Neotropical Region. Rev Brasiliera Zool. 2007;24:848–853. [Google Scholar]
- Fenton MB, Rautenbach IL, Rydell J, Arita HT, Ortega J, Bouchard S, Hovorka MD, Lim B, Odgren E, Portfors CV, Scully WW, Syme DM, Vonhof MJ. Emergence, echolocation, diet and foraging behavior of Molossus ater (Chiroptera: Molossidae) Biotropica. 1998;30:314–320. [Google Scholar]
- Freeman PW. Specialized insectivory: beetle-eating and moth-eating molossid bats. J Mammal. 1979;60:467–479. [Google Scholar]
- Freeman PW. A multivariate study of the family Molossidae (Mammalia: Chiroptera): morphology, ecology, evolution. Fieldiana Zool New Series. 1981a;7:1–173. [Google Scholar]
- Freeman PW. Correspondence of food habits and morphology in insectivorous bats. J Mammal. 1981b;62:166–173. [Google Scholar]
- Geoffroy Saint-Hilaire E. Note sur une petite famille de chauve-souris d’Amérique, désignee sous le nom générique de Molossus. Bulletin des Sciences, par la Société Philomatique de Paris. 1805a;3:278–279. [Google Scholar]
- Geoffroy Saint-Hilaire E. Mémoire sur quelques chauve-souris d’Amérique formant une petite famille sous le nom Molossus. Annales Museum National D’Histoire Naturelle (Paris) 1805b;6:150–156. [Google Scholar]
- Giannini N, Goswami A, Sanchez-Villaga MR. Development of integumentary structures in Rousettus amplexicaudatus (Mammalia: Chiroptera: Pteropodidae) during late-embryonic and fetal stages. J Mammal. 2006;87:993–1001. [Google Scholar]
- Goodwin GG, Greenhall AM. A review of the bats of Trinidad and Tobago. Bull Am Mus Nat Hist. 1961;122:187–301. [Google Scholar]
- Goudy-Trainor A, Freeman PW. Call parameters and facial features in bats: a surprising failure of form following function. Acta Chiropt. 2002;4:1–16. [Google Scholar]
- Hermanson JW. A comparative perspective of the ontogeny of flight muscles in bats. In: Adams RA, Pedersen SC, editors. Ontogeny, functional ecology, and evolution of bats. Cambridge: Cambridge University Press; 2000. pp. 333–361. [Google Scholar]
- Lawrence MA. Biological observations on a collection of New Guinea Syconycteris australis (Chiroptera, Pteropodidae) in the American Museum of Natural History. Am Mus Novit. 1991;(3024):27. [Google Scholar]
- Misek I. Prenatal development of the neocortex of the large mouse-eared bat (Myotis myotis, Microchiroptera) Folia Zool. 1989;38:339–347. [Google Scholar]
- Misek I. Morphogenesis of the mammalian neocortex. II. Prenatal development of the neocortex in selected mammals. Acta Scientiarum Naturalium Academiae Scientiarum Bohemoslovacae Brno. 1990;24:1–40. [Google Scholar]
- Murray PF, Strickler T. Notes on the structure and function of cheek pouches within the Chiroptera. J Mammal. 1975;56:673–676. [Google Scholar]
- Norberg UM, Rayner JMV. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. P Roy Soc B-Biol Sci. 1987;316:335–427. [Google Scholar]
- Pedersen SC. Cephalometric correlates of echolocation in the Chiroptera. J Morphol. 1993;218:85–98. doi: 10.1002/jmor.1052180107. [DOI] [PubMed] [Google Scholar]
- Pedersen SC. Cephalometric correlates of echolocation in the Chiroptera: II. Fetal development. J Morphol. 1995;225:107–123. doi: 10.1002/jmor.1052250109. [DOI] [PubMed] [Google Scholar]
- Phillips CJ. A theoretical consideration of dental morphology, ontogeny and evolution in bats. In: Adams RA, Pedersen SC, editors. Ontogeny, functional ecology, and evolution of bats. Cambridge: Cambridge University Press; 2000. pp. 247–274. [Google Scholar]
- Pirlot P, Bernier R. Embryonic brain-growth in a fruit bat. Anat Embryol. 1974;146:193–208. doi: 10.1007/BF00315595. [DOI] [PubMed] [Google Scholar]
- Pirlot P, Bernier R. Brain growth and differentiation in two fetal bats: Qualitative and quantitative aspects. Am J Anat. 1991;190:167–181. doi: 10.1002/aja.1001900206. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV Prolonged receptivity to the male and the fate of the spermatozoa in the female black mastiff bat, Molossus ater. J Reprod Fertil. 1987;79:643–654. doi: 10.1530/jrf.0.0790643. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV Ovarian function in the captive black mastiff bat, Molossus ater. J Reprod Fert. 1988;82:97–111. doi: 10.1530/jrf.0.0820097. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV The black mastiff bat (Molossus ater): A novel mammalian model for studies of ovarian, uterine, and placental biology. J Exp Zool Supplement. 1990a;4:210–212. doi: 10.1002/jez.1402560446. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV Implantation, development of the fetal membranes, and placentation in the captive black mastiff bat (Molossus ater) Am J Anat. 1990b;187:109–136. doi: 10.1002/aja.1001870202. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater. Am J Anat. 1991a;191:1–22. doi: 10.1002/aja.1001910102. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV Development of the discoidal, hemochorial placenta in the black mastiff bat (Molossus ater): evidence for a role of maternal endothelial cells in the control of trophoblastic growth. Am J Anat. 1991b;191:185–207. doi: 10.1002/aja.1001910205. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ., IV . Reproductive biology of the black mastiff bat, Molossus ater. In: Hamlett WC, editor. Reproductive biology of South American vertebrates. New York: Springer-Verlag; 1992. pp. 262–282. [Google Scholar]
- Rasweiler JJ., IV Pregnancy in Chiroptera. J Exp Zool. 1993;266:495–513. doi: 10.1002/jez.1402660603. [DOI] [PubMed] [Google Scholar]
- Rasweiler JJ, IV, Badwaik NK. Delayed development in the short-tailed fruit bat, Carollia perspicillata. J Reprod Fert. 1997;109:7–20. doi: 10.1530/jrf.0.1090007. [DOI] [PubMed] [Google Scholar]
- Reep RL, Bhatnagar KP. Brain ontogeny and ecomorphology in bats. In: Adams RA, Pedersen SC, editors. Ontogeny, functional ecology, and evolution of bats. Cambridge: Cambridge University Press; 2000. pp. 93–136. [Google Scholar]
- Schneider H. Die Sinushaare der grossen Hufeisennase Rhinolophus ferrum equinum (Schreber, 1774) Z Saugetierk. 1963;28 (6):343–349. [Google Scholar]
- Schutt WA, Simmons NB. Quadrupedal bats: form, function, and evolution. In: Zubaid A, McCracken GF, Kunz TH, editors. Functional and evolutionary ecology of bats. New York: Oxford University Press; 2006. pp. 145–159. [Google Scholar]
- Sears KE, Behringer RR, Rasweiler JJ, IV, Niswander LA. Development of bat flight: morphologic and molecular evolution of bat wing digits. Proc Natl Acad Sci USA. 2006;103:6581–6586. doi: 10.1073/pnas.0509716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons NB. Order Chiroptera. In: Wilson DE, Reeder DA, editors. Mammalian species of the world. A taxonomic and geographic reference. 3. Vol. 1. Baltimore: John Hopkins University Press; 2005. pp. 312–529. [Google Scholar]
- Tokita M. Normal embryonic development of the Japanese pipistrelle, Pipistrellus abramus. Zoology. 2006;109:137–147. doi: 10.1016/j.zool.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Vater M. Evolutionary plasticity and ontogeny of the bat cochlea. In: Adams RA, Pedersen SC, editors. Ontogeny, functional ecology, and evolution of bats. Cambridge: Cambridge University Press; 2000. pp. 137–173. [Google Scholar]
- Vaughan TA. Morphology and flight characteristics of molossid bats. J Mammal. 1966;47:249–260. [Google Scholar]
- Weatherbee SD, Behringer RR, Rasweiler JJ, IV, Niswander LA. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc Natl Acad Sci USA. 2006;103:15103–15107. doi: 10.1073/pnas.0604934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Reeder DM. Introduction. In: Wilson DE, Reeder DA, editors. Mammalian species of the world. A taxonomic and geographic reference. 3. Vol. 1. Baltimore: John Hopkins University Press; 2005. pp. xxv–xxix. [Google Scholar]
- Wyant KA, Adams RA. Prenatal growth and development in the Angolan free-tailed bat, Mops condylurus (Chiroptera: Molossidae) J Mammal. 2007;88:1248–1251. [Google Scholar]