Abstract
Vitamin D regulates calcium homeostasis in the body and may play a major role in regulating immune responses to tuberculosis (TB). Pilot studies suggest that vitamin D supplementation may improve outcomes in pulmonary TB (PTB), but clinical evidence using vitamin D in TB treatment is limited. We present a case of vitamin D deficiency in a woman with refractory drug-susceptible PTB. Antituberculous therapy and the correction of vitamin D deficiency resulted in clinical and microbiologic improvement at month 13 of her treatment. The basis for vitamin D/TB interactions and a brief literature review are discussed. Data from controlled trials are needed to evaluate the efficacy of vitamin D as adjunctive TB therapy.
Keywords: immunity, treatment, tuberculosis, vitamin D
Tuberculosis (TB) is a common and deadly infectious disease caused by the bacterium Mycobacterium tuberculosis. The World Health Organization (WHO) estimates that there were 9.2 million new cases of tuberculosis worldwide in 2006 resulting in 1.7 million deaths, making TB the second leading cause of death due to an infectious disease.1 The emergence of drug resistance, worsening malnutrition in the settings of political instability and civil unrest, and the detrimental effects of HIV coinfection have challenged TB eradication programs worldwide.1
Vitamin D regulates calcium homeostasis for optimal skeletal health. Vitamin D is obtained from only a few dietary sources and is primarily synthesized in the skin after exposure to ultraviolet B (UVB) radiation from the sun.2 Although vitamin D deficiency is common in human populations worldwide,3 patients with dark skin pigmentation are at increased risk due to their higher absorption of UVB by melanin, leading to the limited cutaneous production of vitamin D.4 Non-classical functions of vitamin D, including its immunomodulatory properties, are currently under investigation. Recently, Liu et al5 demonstrated that vitamin D upregulated the production of cathelicidin, a potent antimicrobial peptide, in TB-infected macrophages. Vitamin D deficient sera from African-American subjects were not able to upregulate cathelicidin production and restrict growth of M tuberculosis in absence of exogenous vitamin D supplementation.5 Thus, vitamin D deficiency may contribute to decreased clearance of TB infection.
We present a case of vitamin D deficiency in an African-American patient with refractory TB safely and rapidly corrected with oral ergocalciferol over a period of 8 weeks. Improvement in vitamin D status was accompanied by clinical and microbiologic improvements in the patient’s TB outcome.
Case Description
A 63-year-old African-American female presented with a two-week history of productive cough, right-sided chest pain, and progressive shortness of breath, also accompanied by a two-month history of subjective fevers, night sweats, and weight loss. The past medical history was negative except for cosmetic surgery. The patient was taking no outpatient medications and reported no drug allergies. The patient was a retired hospital laundry worker, smoked two packs of cigarettes per day, consumed three to four alcoholic drinks daily, and denied any recreational drug use. TB risk factors included a close friend treated for smear positive PTB eight years prior to her current presentation. Family history was noncontributory.
On physical examination, the patient’s body temperature was 99°F, heart rate was 72 beats per minute, blood pressure was 138/86, and body weight was 101 pounds. The neck was supple without meningismus or cervical lymphadenopathy. The cardiovascular examination was unremarkable, and the pulmonary examination was significant for crackles in the right upper lung field. Abdominal examination revealed no hepatosplenomegaly. The remainder of the physical examination was unremarkable.
Laboratory evaluation revealed a total leukocyte count of 16,500 cells per μL with 87% segmented neutrophils, 6% lymphocytes, 7% monocytes without observed band forms, eosinophils, or basophils. Hematocrit was 33.3% with hemoglobin concentration at 10.7 g/dL. The patient’s platelet and coagulation indices were within the normal range. The plasma transaminase and bilirubin levels were normal and the albumin concentration was 3.3 g/dL. The patient’s electrolyte panel was normal with a creatinine concentration of 0.6 mg/dL and serum calcium level of 9.0 mg/dL. Viral hepatitis and human immunodeficiency virus (HIV) serologies were negative. Sputum microscopy revealed multiple acid fast bacilli, and sputum culture grew M tuberculosis sensitive to isoniazid, rifampin, and ethambutol.
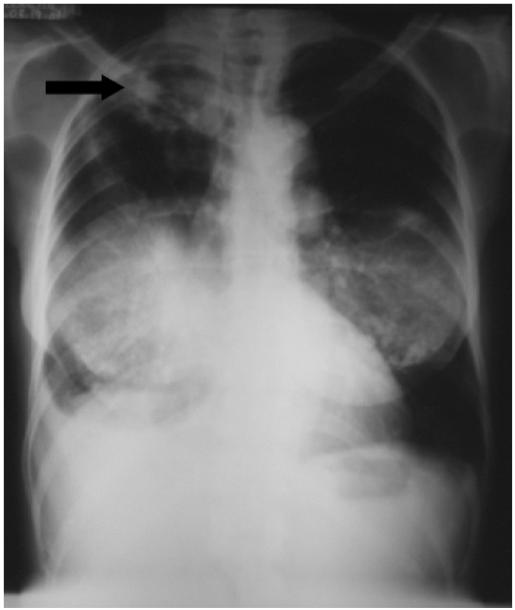
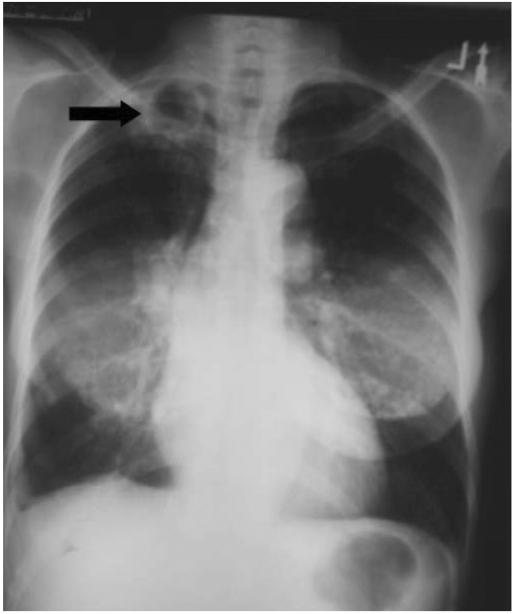
Chest radiography (Fig. 1) and sputum microscopy results were deemed consistent with PTB, and the patient was started on directly observed TB therapy (DOT) with isoniazid, rifampin, pyrazinamide, and ethambutol. The patient demonstrated excellent compliance with DOT, and responded with clearance of sputum cultures after 1 month of therapy; however, she experienced progressive weight loss and subsequent treatment failure with positive sputum cultures for M tuberculosis at 4 months after the initiation of anti-TB therapy. Although resistance was ruled out, the patient’s TB regimen was changed to isoniazid, rifampin, levofloxacin, ethambutol, and amikacin to improve antimicrobial tissue penetration. The patient failed this regimen despite documented compliance with medication administration, and severe vitamin D deficiency was diagnosed at month 10 of total therapy with a markedly low serum 25-hydroxyvitamin D 25(OH)D concentration of 7 ng/mL. The patient was treated with 50,000 units of ergocalciferol orally three times weekly for eight weeks while continuing isoniazid, rifampin, ethambutol, and levofloxacin; a repeat serum 25(OH)D and serum calcium levels were 81 ng/mL and 9.4 mg/dL, respectively, at 10 weeks after initiation of vitamin D therapy. No adverse events were observed, and the patient demonstrated significant radiographic improvement (Fig. 2) with negative sputum cultures at month 13 of total therapy.
Discussion
Contemporary observational studies suggest that, on average, patients with TB, especially those with darker skin pigmentation,6 have lower serum levels of vitamin D than healthy controls despite matching on key demographic parameters such as sex, age, ethnicity, diet, and geographical location.7 The presence of vitamin D deficiency may also correlate with increased disease severity in patients with PTB8 and may be a major contributing factor to the increased burden of TB-related disease observed in patient populations with darker skin pigmentation, such as African Americans.9 Taken together, these studies provide evidence to support screening patients with TB for evidence of concomitant vitamin D deficiency.
While vitamin D deficiency is known to have detrimental effects on skeletal health, additional functions of vitamin D are currently being delineated, and these may have a significant impact on future management of infectious diseases such as TB. Vitamin D appears to modulate the innate immune response through increased expression of cathelicidin (LL-37), an antimicrobial peptide with potent activity against M tuberculosis. Recent translational studies in healthy TB contacts found that whole blood from vitamin D–supplemented contacts restricted growth of bacillus-calmette-guerin (BCG) (an M tuberculosis surrogate) more effectively than blood from the unsupplemented control group.10
In addition, a randomized, placebo controlled, pilot clinical trial of vitamin D supplementation as adjunctive therapy to conventional anti-TB drug therapy in 67 PTB patients in Indonesia demonstrated significantly higher sputum conversion rates at earlier time points in the vitamin D group (n = 34) compared to placebo (n = 33).11 These pre-clinical and early clinical data suggest a potential therapeutic benefit of vitamin D in TB, but more studies are needed to further evaluate the role of vitamin D as potential adjunctive therapy.
Although the consequences of uncorrected vitamin D deficiency have been well characterized,3 no universally accepted vitamin D repletion regimen has been adopted.3,12 A recent study suggests that more than 1000 IU of vitamin D daily is necessary to restore vitamin D status.13 Weekly high dose regimens have been proposed as a safe and effective method to rapidly correct vitamin D.14-16 As patients with TB are likely to have extensive derangements in vitamin D metabolism,6 our clinical experience supports the need to diagnose potential vitamin D deficiency in TB-infected patients and consider a more aggressive repletion strategy in TB patients with vitamin D deficiency.
Conclusion
Recent literature suggests that vitamin D deficiency may be an important contributor to pathogenesis of TB-related disease worldwide. We report a case of vitamin D deficiency in an African-American patient with recurrent treatment failure of drug-susceptible PTB. Our clinical experience suggests that rapid correction of vitamin D deficiency in TB patients can be achieved with an oral regimen of high dose vitamin D. Our case supports the hypothesis that vitamin D treatment may improve clinical outcomes in patients with TB; however, larger randomized clinical studies are necessary to further verify the role of vitamin D in TB therapy.
Key Points.
Tuberculosis (TB) patients have a high prevalence of vitamin D deficiency.
Recent studies suggest a link between vitamin D status and the immune response to Mycobacterium tuberculosis and other pathogens.
Correcting vitamin D deficiency is safe and inexpensive; further investigations are needed to evaluate the role of vitamin D supplementation in enhancing treatment outcomes in TB patients.
Fig. 1.
Chest roentgogram demonstrating right upper lobe cavitary lesion consistent with diagnosis of pulmonary tuberculosis.
Fig. 2.
Repeat chest roentgenogram demonstrated diminished cavity size at month 13 of total TB therapy. Fifty thousand units of oral vitamin D (ergocalciferol) was given three times weekly in combination with TB therapy during months 10 and 11 of total TB treatment.
Acknowledgments
The authors thank Roberto Pacifici, MD, principal investigator of NIH grant T32DK007298, for his generous support of work related to this publication.
Supported by NIH grants T32DK007298 (to A. V. Y.), K24 RR023356 (to T. R. Z.), and K23 AR054334 (to V. T.).
References
- 1.Global Tuberculosis Control: surveillance, planning, financing. WHO Report 2008. World Health Organization; Geneva: [Accessed November 19, 2008]. Available at: http://www.who.int/tb/publications/global_report/2008/summary/en/index.html. [Google Scholar]
- 2.Tangpricha V. Vitamin D deficiency in the Southern United States. South Med J. 2007;100:384–385. doi: 10.1097/01.smj.0000209229.90862.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesize vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Sita-Lumsden A, Lapthorn G, Swaminathan R, et al. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007;62:1003–1007. doi: 10.1136/thx.2006.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 8.Grange JM, Davies PD, Brown RC, et al. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985;66:187–191. doi: 10.1016/0041-3879(85)90035-2. [DOI] [PubMed] [Google Scholar]
- 9.Stead WW, Senner JW, Reddick WT, et al. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 10.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 11.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 12.Johnson MA, Kimlin MG. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr Rev. 2006;64:410–421. doi: 10.1111/j.1753-4887.2006.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JS, Kantorovich V, Wu C, et al. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab. 1999;84:2729–2730. doi: 10.1210/jcem.84.8.5899. [DOI] [PubMed] [Google Scholar]
- 15.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 16.Przybelski R, Agrawal S, Krueger D, et al. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19:1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]