Abstract
The cytochrome P4501 (CYP1) gene family comprises four subfamilies in fish; CYP1A, CYP1B, CYP1C, and CYP1D. Only two CYP1 genes, CYP1A1 and CYP1A3, are so far known in rainbow trout (Oncorhynchus mykiss). The present study aimed to identify other CYP1 subfamily genes in rainbow trout, to establish methods for quantitative mRNA expression analysis of these genes, and to determine their basal and induced mRNA expression in gills and liver. Another goal was to examine their mRNA expression in environmentally exposed fish. We cloned four new transcripts, denoted rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3. Levels of these and the previously known rbCYP1A transcripts were determined by real-time PCR in unexposed fish, fish exposed to the potent aryl hydrocarbon receptor (AhR) agonist 3,3’,4,4’,5-pentachlorobiphenyl (PCB126), and fish caged in various waters in the Uppsala region (Sweden). The mRNA expression patterns observed in unexposed rainbow trout (basal levels) were markedly similar to those reported for orthologous genes in other species. All six transcripts were induced by PCB126 in gills and liver, suggesting all genes to be AhR regulated. The caged fish showed clear rbCYP1 induction in gills at all monitoring sites (up to 70-fold the basal level), whereas the liver responses were weak; induction (up to 5-fold) was recorded only at the Uppsala municipal sewage treatment plant outlet. Gill filament EROD activity was induced at all caging sites. Most interestingly, the rbCYP1 gene response patterns in gills differed among cagingsites and among subfamilies. The EROD induction seemed to only reflect induction of rbCYP1A transcription. Response patterns of multiple CYP1 genes in gills and liver could provide an improved monitoring strategy. Such patterns could be used to characterize complex mixtures of AhR agonists and antagonists in aquatic environments.
Keywords: cytochrome P450 1A 1B and 1C (CYP1A CYP1B CYP1C) genes; EROD induction; 3,3'4,4',5-pentachlorobiphenyl (PCB126); rainbow trout; biomonitoring
1. Introduction
The cytochrome P450 1 (CYP1)-catalyzed ethoxyresorufin O-deethylase (EROD) activity is a classical biomarker for exposure to dioxin-like and other aryl hydrocarbon receptor (AhR) agonists in mammals, birds, and fish. EROD activity is traditionally assayed in liver microsomes, although the major EROD-catalyzing CYP1 form (CYP1A) (Lewis, 2004), is expressed in a variety of other tissues including the kidney and the fish gill (Ortiz-Delgado et al., 2008; Sarasquete and Segner, 2000; Smolowitz et al., 1991; Smolowitz et al., 1992) The selection of liver microsomes is favored by the high cellular homogeneity and larger size of the liver compared to kidney and gill.
We previously developed a gill filament-based EROD assay which is used to measure both CYP1 induction and inhibition in fish (Beijer et al., 2010; Jönsson et al., 2002). Gill filaments appear more sensitive than liver when comparing EROD or CYP1A protein induction in response to inducing compounds in the ambient water (Abrahamson et al., 2007; Jönsson et al., 2006; Mdegela et al., 2006). Moreover, the relative sensitivity of EROD activity in gills versus liver seems to be higher for readily metabolized AhR agonists (e.g., benzo(a)pyrene and indigo) than for a persistent inducer (3,3’,4,4’,5-pentachlorobiphenyl, PCB126), presumably because of a lower hepatic bioavailability resulting from first-pass metabolism in extrahepatic tissues (Jönsson et al., 2006). Thus, gill-liver EROD induction ratios might be useful in determining whether the inducing contaminants are persistent or readily metabolized (Abrahamson et al., 2007; Jönsson et al., 2006).
CYP1A inducibility is generally very high compared to that of non-AhR-regulated CYP forms (Buhler and Wang, 1998). Three CYP1 subfamilies other than CYP1A are known in fish, i.e. CYP1B (Di Bello et al., 2007; Leaver and George, 2000), CYP1C (Godard et al., 2005; Itakura et al., 2005; Jönsson et al., 2007a; Wang et al., 2006) and CYP1D (Goldstone et al., 2009; Goldstone and Stegeman, 2008). In zebrafish (Danio rerio) exposure to PCB126 or 2,3,7,8-tetrachlorodibenzo-p-dioxin, induces CYP1A, CYP1B, and CYP1C, but not CYP1D genes and this induction is regulated primarily by AhR2 (Goldstone et al., 2009; Jönsson et al., 2007a; Jönsson et al., 2007b). In rainbow trout (Oncorhynchus mykiss) two CYP1A genes, CYP1A1 and CYP1A3, have been identified so far (Berndtson and Chen, 1994; Råbergh et al., 2000).
The first objective of the present study was therefore to identify genes in the other CYP1 subfamilies in rainbow trout, and to establish methods for quantitative mRNA expression analysis (real-time PCR) of these genes. The second objective was to determine the basal and induced mRNA expression of these genes in gill filaments and liver using unexposed fish, fish exposed experimentally to PCB126, and fish exposed in the field by caging at four freshwater sites in the Uppsala region. For comparative purposes, gill EROD activity was measured in each exposure group. The results revealed four new PCB126 inducible genes belonging to the CYP1B and CYP1C subfamilies. We also demonstrate that the CYP1A, CYP1B and CYP1C subfamily genes were differently induced at the different caging sites.
2. Material and methods
2.1. Fish husbandry
Juvenile rainbow trout (obtained from Näs fiskodling AB, By Kyrkby, Sweden) were kept in the aquarium facility at the Evolutionary Biology Centre, Uppsala University. The fish were held in tanks continuously supplied with aerated tap water (12 °C) and at a maximal density corresponding to 12.5 g biomass L−1. The fish were fed pellets (Dan-ex 1352) from Dana Feed A/S (Horsens, Denmark), at daily rations corresponding to 1% of their body weight. The experiments were approved by the local ethical committee for research on animals.
2.2. Cloning
Rainbow trout CYP1 transcripts were cloned by the initial use of primers designed to four partial salmon (Salmo salar) CYP1B- and CYP1C–like sequences. The salmon sequences were assembled from ESTs obtained by BLAST searches in Gene Bank with the zebrafish CYP1B1, CYP1C1, and CYP1C2 sequences.
Total RNA was extracted from different rainbow trout tissues (heart, gill, and liver) using RNA STAT 60 (Tel. Test Inc. Friendswood, TX, USA), and mRNA was isolated using the MicroPoly(A)Purist™ Kit (Ambion Inc., Austin, TX, USA). For cDNA synthesis the Omniscript Reverse Transcriptase Kit was used with random hexamer primers (both from Qiagen, Hilden, Germany). Amplification of cDNA was performed using the Advantage® 2 Polymerase PCR Kit (Clontech Laboratories Inc., Mountain View, CA, USA) with salmon and rainbow trout CYP1 gene-specific primers synthesized by Sigma-Aldrich (St. Louis, MO, USA) and Eurofins MWG GmbH, (Ebersberg, Germany). For determination of the mRNA 3’ and 5’ends the SMART™ RACE cDNA Amplification Kit (Clontech Laboratories Inc) was used. The PCR products were sequenced (Uppsala Genome Center, Uppsala, Sweden) and assembled, resulting in the full coding sequences of four different genes. These nucleotide sequences and their deduced amino acid sequences were aligned using ClustalW and compared with homologous sequences in zebrafish and human by sequence identity analysis in BioEdit (Hall, 1999).
2.3. Phylogenetic analysis
Additional sequences for phylogenetic investigations, including salmon (Salmo salar) EST sequences for part of CYP1B1, and all of CYP1C2 and CYP1C3, were obtained from GenBank. Accession numbers for the sequences used in the investigation are in the Supplemental information. Phylogenetic relationships were investigated using maximum likelihood (RAxML-7.0.4)(Stamatakis, 2006a) and Bayesian techniques (MrBayes v 3.1.2; (Ronquist and Huelsenbeck, 2003)). MrBayes estimates posterior probabilities using Metropolis-Hastings coupled Monte Carlo Markov chains (MC3). We performed MC3 estimates with uninformative prior probabilities using the WAG model of amino acid substitution (Whelan and Goldman, 2001) and prior uniform gamma distributions approximated with four categories (WAG+I+r). Four incrementally heated, randomly seeded Markov chains were run for 3×106 generations, and topologies were sampled every 100th generation. Burnin value was set to 5×105 generations. The WAG substitution model using the categories approximation (PROTMIXWAG)(Stamatakis, 2006b) was used for RAxML analyses, and 100 randomly seeded bootstrap replicates were performed.
2.4. Exposure
Laboratory exposure via ambient water was performed in disposable, transparent polyethylene bags as previously described (Jönsson et al., 2002). The bags were placed in boxes (45×30×15 cm), filled with Uppsala tap water (15–20 L), and supplied with aeration via air stones. A trough with tap water running around the boxes was used to maintain the exposure temperature constant.
Neither using this exposure system nor using acetone as a carrier (20 ppm) have any significant effect on gill filament EROD activity (Jönsson et al., 2002). The effect of solvent on mRNA expression of CYP1 gene was examined in gills of rainbow trout exposed (24 h) to DMSO or acetone (20 ppm of each) as described above (n=6). Trout kept for 24 h in a polyethylene bag filled with tap water without addition of chemicals and trout sampled directly from the facility tank represented bag controls and tank controls (n=6). The trout body weight was 12±3 g (mean ± standard deviation of the mean; SD).
Basal and induced mRNA expression of CYP1 gene were determined using rainbow trout sampled directly from the facility tank (controls) and trout exposed (24 h) to a nominal concentration of 10 nM PCB126 (Larodan Fine chemicals, Malmö, Sweden; 20 ppm acetone) at 15±1 °C (body weight: 22±3 g; n=6). The trout were not fed during the experiment.
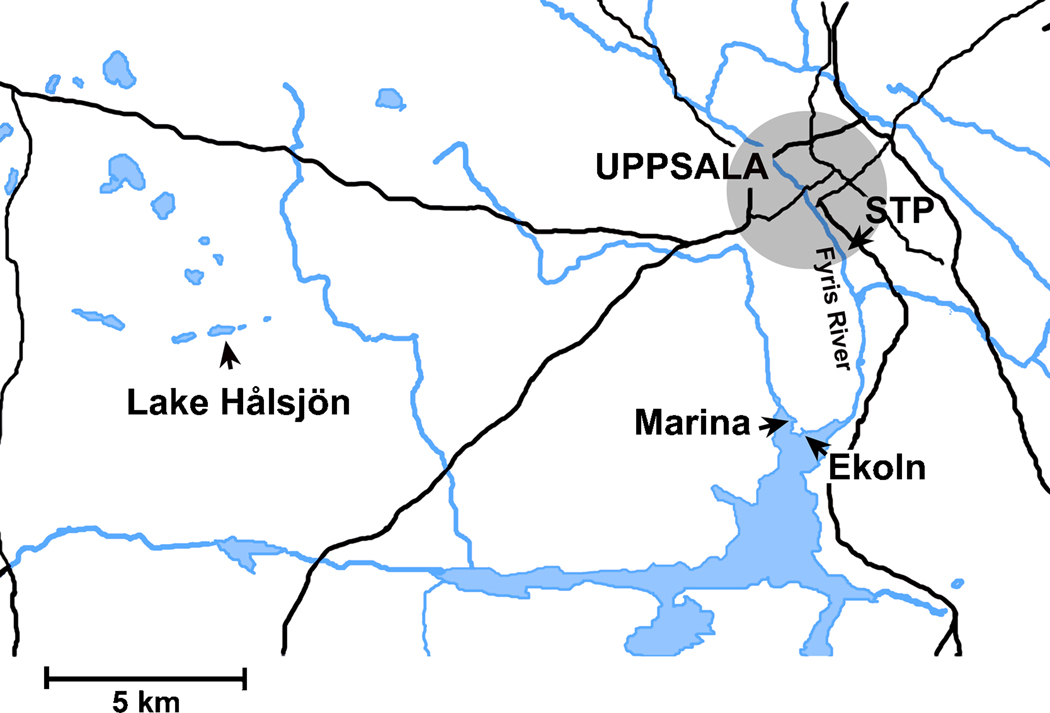
CYP1 mRNA expression was also determined in field-exposed trout. Groups of 12 trout (body weight: 29±5 g) were caged at four different freshwater sites in the Uppsala region (Fig. 1). One cage was placed in Lake Hålsjön, a small lake situated about 22 km west of the Uppsala town center and not directly influenced by larger roads or other pollution point sources. The lake is supplied with water from wells and smaller streams and its catchment area is composed of forests and uncultivated land. One cage was placed among the small boats in a marina in Lake Ekoln (the northernmost part of Lake Mälaren) and one cage was placed at a bridge on the exposed side of a headland nearby the marina (about 200 m to the south). The last cage was placed in the Fyris River at the Uppsala city sewage treatment plant (STP), approximately 5 m downstream from the outlet. Following two days of caging, the trout were transported back to the laboratory for sampling and analysis.
Figure 1.
Map of the Uppsala region showing the locations for the four caging sites used in the environmental exposure experiment. Roads are shown with black and rivers and lakes with blue (grey).
2.5. Sampling
All fish were killed by decapitation. Pieces of gill filaments and liver were dissected, frozen in liquid nitrogen, and stored at −80 °C until real-time PCR analysis. For the gill EROD assay two gill arches were excised and placed in ice-cold HEPES-Cortland (HC) buffer (Jönsson et al., 2002).
2.6. Gill EROD assay
Gill filament EROD activity was determined as previously described (Jönsson et al., 2002). For each fish duplicate groups of 10 filament tip pieces (2 mm long) were placed in wells of a 12-well plate containing HC buffer. The buffer was replaced with “reaction buffer” (0.5 ml) consisting of 7-ethoxyresorufin (1 µM), dicumarol (10 µM; both from Sigma-Aldrich), and DMSO (0.2%; v/v) in HC buffer. After preincubation (10 min) with continuous shaking the reaction was initiated by replacing the buffer with fresh reaction buffer (0.7 ml). After 30 and 50 min of incubation (at 15 °C), 0.2-ml aliquots were transferred from each well to a white Fluoronunc 96-well plate (VWR International, Arlington Heights, IL, USA). Each plate included resorufin standards (Sigma-Aldrich) in reaction buffer (0–250 nM). The fluorescence was determined in a multi-well plate reader (Victor 3; PerkinElmer, Boston, MA, USA) at 544 nm (excitation) and 590 nm (emission). EROD activity is expressed as pmol resorufin filament tip−1 min−1.
2.7. Real-time PCR
Total RNA was isolated with RNA stat-60 (Tel-Test Inc.) and subsequently treated with 3 M LiCl (Sigma-Aldrich) in TRIS buffer. The RNA quantity and quality were determined spectrophotometrically (NanoDrop Technologies, Wilmington, DE, USA). The RNA was reverse-transcribed using the iScript™ cDNA Synthesis Kit (includes both random hexamer and oligo dT primers) from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
Gene-specific real-time PCR primers for the rainbow trout CYP1A1, CYP1A3, CYP1B1, CYP1C1, CYP1C2, CYP1C3, and EF1α transcripts (Table 1) were synthesized by Sigma-Aldrich. To confirm the specificity of the PCR primers the PCR-products were cloned into pGEM-T-Easy vectors (Promega, Mannheim, Germany) and transformed into Escherichia coli Top10-cells (Invitrogen, Paisley, UK). Plasmids were extracted using Qiagens plasmid spin mini prep kit. The sequence reactions were run with M13-primers at the Uppsala Genome Center and analyzed in BLAST.
Table 1.
Sequences (5’-3’) of the gene-specific real-time PCR primers used in the experiments.
| Transcript | Forward primer | Reverse primer | GenBank Acc. No. |
|---|---|---|---|
| rbCYP1A1 | GGAAACTAGATGAGAACGCCAACA | GTACACAACAGCCCATGACAG | AAB69383.1 |
| rbCYP1A3 | GAAACTAGATGAGAACGCCAACG | CTGATGGTGTCAAAACCTGCC | AAD45966.1 |
| rbCYP1B1 | CATTCTGATACTTGTGAGGTTTCC | CAACTGAGACTGGTCTTCCAT | GU325707 |
| rbCYP1C1 | GCAGCACAGAGAAACCTTCAAC | GTCCTTTCCGTGCTCAATCACA | GU325708 |
| rbCYP1C2 | GAGCACAGGGAGACATTTGAC | GGTATCACTGTCCGCCTTG | GU325709 |
| rbCYP1C3 | CATGAGTGATGCCATCATTAACGC | AGGTCTGTGACTGTTCCTTCAACAA | GU325710 |
| rbEF1α | GCAGGTACTACGTCACCATCAT | CACAATCAGCCTGAGATGTACC | CF752904 |
Real-time PCR was carried out using the Rotor-Gene 6000 real-time DNA amplification system (Qiagen, Hilden, Germany). The 20-µl PCR reaction mixtures consisted of iQ SYBR Green Supermix (Bio-Rad Laboratories), forward and reverse primers (5 pmol of each) and cDNA (derived from 0.05 µg RNA). In each sample, the genes were analyzed in duplicate with the following protocol: 95 °C (10 min) followed by 40 cycles of 95 °C (15 s) and 62 °C (60 s). To ensure that a single product was amplified, melt curve analysis was performed on the PCR products at the end of each run.
Relative CYP1 mRNA expression was calculated for each reaction by the EΔΔCt method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008). Distinct PCR efficiency values (E) in gill and liver were determined for each primer pair using the LinRegPCR program (Ruijter et al., 2009). Elongation factor 1-α (EF1α) was selected as the reference gene as it is among the most stable genes for tissue comparison and treatment studies (Jørgensen et al., 2006; McCurley and Callard, 2008). Outlier data were excluded based on the Grubbs test (Grubbs, 1969). Data were log-transformed when the variance differed between groups. In the figures data representing five or six biological replicates are shown as mean + SD. The statistical analyses were performed using Prism 5 by GraphPad Software Inc. (San Diego, CA, USA).
3. Results
3.1. Cloning and sequence analysis of rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3
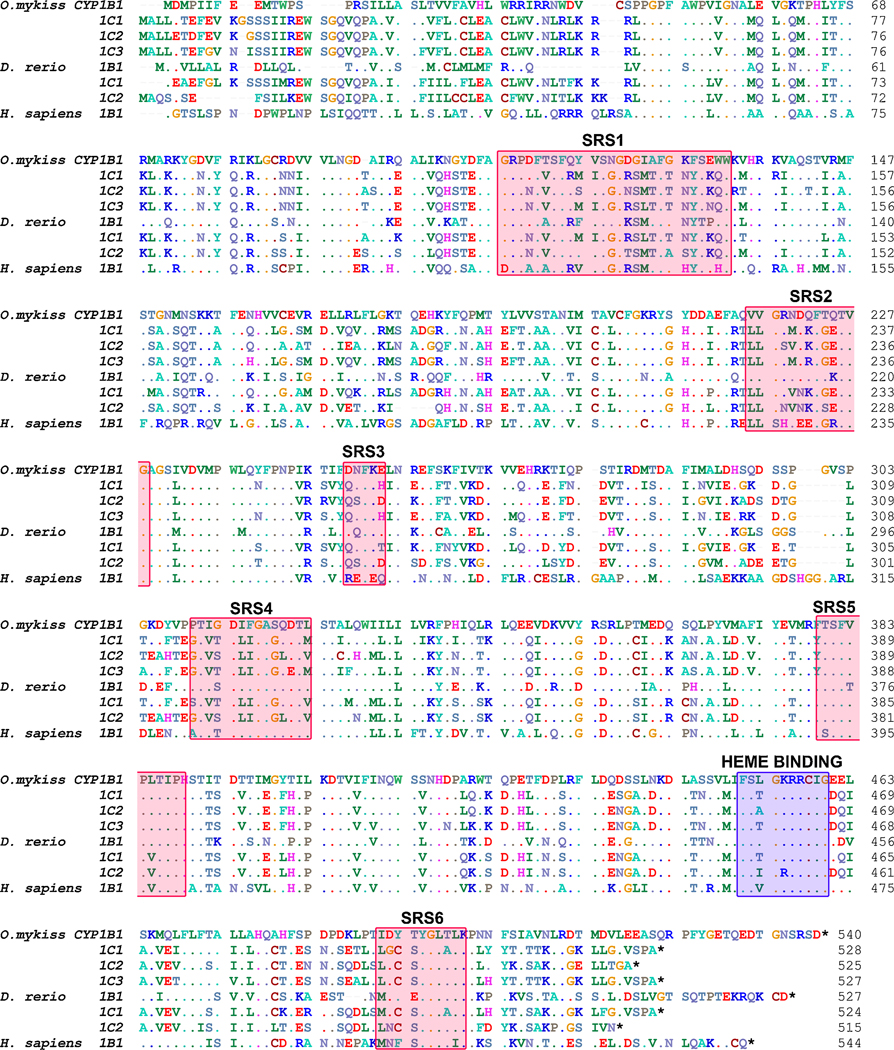
Four complete rainbow trout CYP1 transcript sequences were cloned, and are denoted rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3 (Accession numbers GU325707-GU325710; Figure 2). The results of the sequence identity analysis are shown in Table 2. The deduced amino acid sequence of the cloned rbCYP1B1 was only 47–48% overall identical and 56–57% identical in the substrate recognition site (SRS) in comparison to the three rbCYP1Cs. Higher identities were observed following pair-wise comparisons among the three rbCYP1Cs. The highest amino acid identity scores were obtained for rbCYP1C1 and rbCYP1C3, which showed 93 and 89% identity overall and in the SRS regions, respectively. RbCYP1B1 and zebrafish (zf) CYP1B1 showed a higher similarity in the SRS regions than in the overall amino acid sequence (79 and 64% identical, respectively). When compared with the zebrafish CYP1C overall amino acid sequence and SRS regions, the rbCYP1C1 and rbCYP1C3 were most similar to zfCYP1C1 (82 and 85% identical and 82 and 86% identical), and rbCYP1C2 was most similar to zfCYP1C2 (79 and 86% identical).
Figure 2.
Alignment of the deduced amino acid sequences of the cloned CYP1B1, CYP1C1, CYP1C2, and CYP1C3 transcripts in rainbow trout (Oncorhynchus mykiss) with orthologous sequences in zebrafish (Danio rerio) and human (Homo sapiens) made using ClustalW (Hall, 1999). The location for the heme binding site (blue) and the substrate recognition sites (SRS1–6) (red) (Lewis et al., 2003) of the proposed enzymes are indicated by shading. Accession numbers are available in the supplemental information.
Table 2.
Percentage of sequence identity between pairs of nucleotide (Nucl), or deduced amino acid (AA) sequences), or between substrate recognition site (SRS) regions, as determined after optimal pair-wise alignment with ClustalW in BioEdit (Hall, 1999). Comparisons were made between the cloned rainbow trout CYP1B1 CYP1C1 CYP1C2, and CYP1C3 (coding sequence), and between the cloned sequences, and orthologous sequences in zebrafish and human. Accession numbers are available in the supplemental information. Bold underlined numbers indicate the highest identities observed.
| Rainbow trout | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP1B1 | CYP1C1 | CYP1C2 | CYP1C3 | ||||||||||
| Nucl | AA | SRS | Nucl | AA | SRS | Nucl | AA | SRS | Nucl | AA | SRS | ||
| Rainbow trout | CYP1C1 | 56 | 47 | 56 | |||||||||
| CYP1C2 | 55 | 48 | 57 | 85 | 84 | 82 | |||||||
| CYP1C3 | 56 | 48 | 57 | 94 | 93 | 89 | 83 | 82 | 82 | ||||
| Zebrafish | CYP1B1 | 66 | 64 | 79 | 54 | 48 | 58 | 56 | 50 | 58 | 55 | 49 | 56 |
| CYP1C1 | 55 | 48 | 58 | 75 | 82 | 85 | 73 | 79 | 81 | 75 | 82 | 86 | |
| CYP1C2 | 55 | 47 | 56 | 70 | 73 | 74 | 72 | 79 | 86 | 70 | 72 | 73 | |
| Human | CYP1B1 | 57 | 50 | 58 | 55 | 48 | 57 | 59 | 47 | 55 | 55 | 47 | 55 |
3.2. Phylogenetic analysis
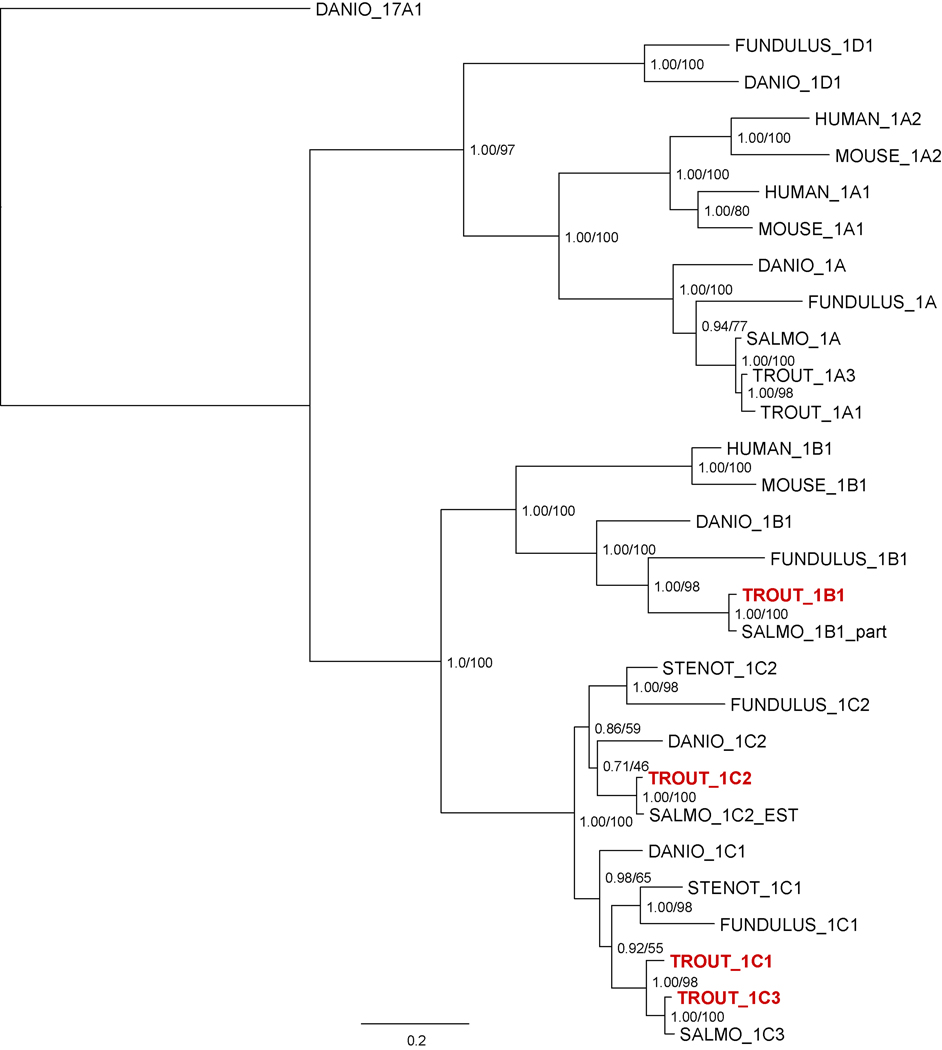
To examine how the rainbow trout CYP1B and CYP1C genes are related to other vertebrate CYP1s we performed phylogenetic analyses of selected vertebrate CYP1 amino acid sequences, including the complete complement of the zebrafish and killifish (Fundulus heteroclitus) CYP1 family (Fig. 3). Both Bayesian and maximum likelihood phylogenetic methods support the phylogeny presented in Figure 3. The newly cloned rbCYP1B1, rbCYP1C2, and rbCYP1C3 cluster with the respective salmon CYP sequences assembled from Genbank. Fish CYP1As form a separate clade from the mammalian CYP1A1 and CYP1A2 sequences, as has previously been described (Goldstone and Stegeman, 2006; Goldstone et al., 2007). RbCYP1A1 and rbCYP1A3 sequences have been previously cloned (Berndtson and Chen, 1994), although the original rbCYP1A1 sequence appears to be an artifactual chimeric hybrid of rbCYP1A1 and rbCYP1A3 (David Nelson, personal communication). Fish CYP1B1 sequences are also distinct from the mammalian CYP1B1 sequences (Goldstone et al., 2007), and the relative relationship of the four species represented is the same between the CYP1As and the CYP1Bs. This is not the case for the CYP1Cs: zebrafish CYP1C2 is clustered with the salmonid CYP1C2, but scup and killifish CYP1C1 are closer to the salmonid CYP1C1 and CYP1C3. However, both bootstrap support and posterior probabilities for the placement of the zebrafish CYP1C2 are low, and alternative topologies place the zebrafish CYP1C2 outside the ((scup, killifish),(trout, salmon)) clade (data not shown).
Figure 3.
Molecular phylogeny of rbCYP1A1, rbCYP1A3, rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3 deduced amino acid sequences with selected other CYP1 sequences including assembled or renamed salmon sequences derived from GenBank (Salmo_1B1_part, Salmo_1C2_EST, Salmo_1C3). Both Bayesian and maximum likelihood techniques recover the topology presented here. Uncertainties in the topology are due to the alterative positioning of the zebrafish CYP1C1 sequence. Clade support values presented at each node represent the Bayesian posterior probability calculated after 3e6 generations and the maximum likelihood bootstrap support calculated from 100 replicates. Accession numbers are available in the supplemental information.
3.3. Basal rbCYP1 mRNA expression patterns in gills and liver
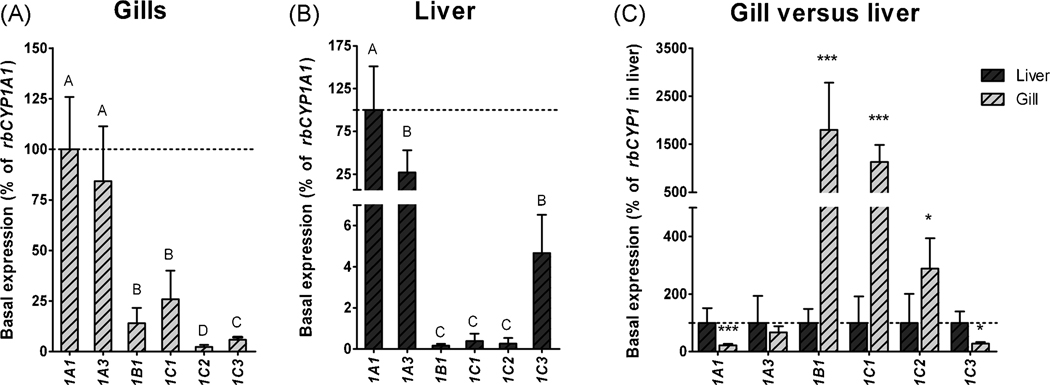
The relative abundance of transcript in unexposed fish (i.e., the basal level of mRNA expression) was examined for the cloned rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3, as well as for rbCYP1A1 and rbCYP1A3 in gills and liver. In each tissue rbCYP1 mRNA expression was calculated as a percentage of the mean value for CYP1A1 mRNA expression (Fig. 4). In gills the mRNA expression of rbCYP1A3 was not significantly different from that of rbCYP1A1 (84% of the rbCYP1A1 level; Fig 3A). However, the rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3 genes all showed a lower mRNA expression than rbCYP1A1 (14, 26, 2 and, 6% respectively; Fig 3A). In the liver, the rbCYP1A3 and rbCYP1C3 transcript levels were 27 and 4% of that of rbCYP1A1, whereas the levels of rbCYP1B1, rbCYP1C1, and rbCYP1C2 were only small fractions (0.2–0.4%) of the rbCYP1A1 level (Fig 3B)
Figure 4.
Relative levels of rbCYP1A1 rbCYP1A3 rbCYP1B1 rbCYP1C1 rbCYP1C2, and rbCYP1C3 mRNA expression in gills (A), in liver (B), and in gills relative to liver (C) in unexposed rainbow trout (basal expression). Basal mRNA expression was calculated as a percentage of rbCYP1A1 mRNA expression in gills or liver (% of mean rbCYP1A1), or for each of the rbCYP1s in liver (% of mean rbCYP1 in liver). EF1α was used as reference gene. The dotted lines indicate 100%. Statistically significant differences between levels of rbCYP1 transcripts within a tissue were examined by one-way ANOVA followed by Tukey’s post hoc test and are shown by different letters. Statistically significant differences between transcript levels in gills and liver were determined by t-test with the Welch correction and are shown by stars (*** = p<0.001 and * = p<0.05), and n=5–6.
Basal mRNA expression of the rbCYP1 genes was compared in gills and liver after calculations using PCR efficiency values for each primer pair determined in gill and liver separately and rbEF1α as reference gene. The rbEF1α Ct values in unexposed fish were similar in gills and liver (the median Ct values were 14.6 and 14.5). The basal levels of rbCYP1B1, rbCYP1C1, and rbCYP1C2 mRNAs were higher in the gill than in the liver (16-, 11- and 3-fold the liver level) whereas the levels of rbCYP1A1 and rbCYP1C3 mRNAs were lower in the gill than in the liver (22 and 27% of the liver level; Fig. 4C). There was no significant difference between the basal rbCYP1A3 mRNA levels in gills and liver.
3.4. Induction patterns in gills and liver of PCB126-exposed rainbow trout
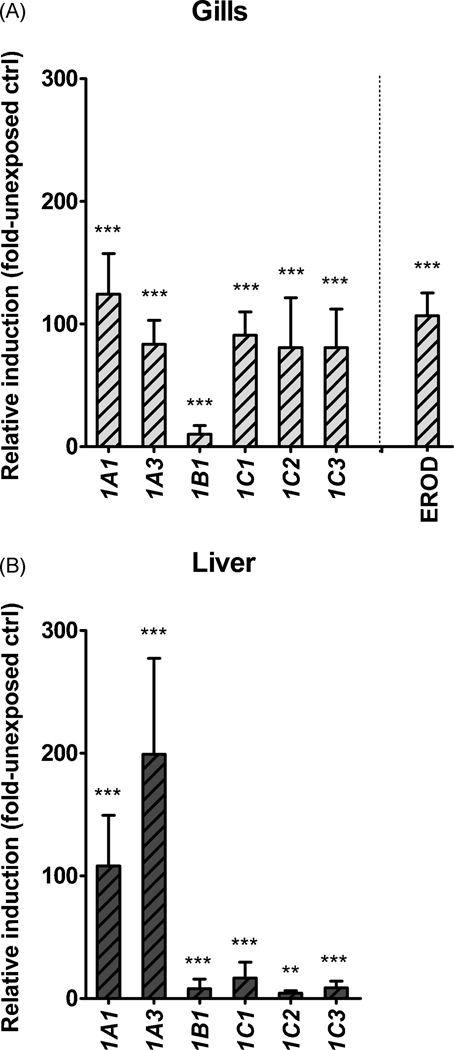
The capability for transcriptional induction of the cloned genes (rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3) and of the two rbCYP1As was examined in gills and liver of trout exposed to waterborne PCB126 (10 nM) for 24 hours. In addition, gill filament EROD activity was analyzed in these fish. Neither the exposure system nor the solvents (DMSO or acetone) had any substantial effect on CYP1 mRNA expression. Therefore we used unexposed fish from the facility tank as controls. PCB126 induced all six CYP1 genes transcriptionally both in gills and liver. In gills rbCYP1A1, rbCYP1A3, rbCYP1C1, rbCYP1C2, and rbCYP1C3 were transcriptionally strongly induced (124-, 83-, 91-, 81-, and 81-fold versus the unexposed control, respectively), whereas the transcriptional induction of rbCYP1B1 was weaker (10-fold versus the unexposed control; Fig. 5A). Gill filament EROD activity displayed a 107-fold induction versus the unexposed control. In the liver the rbCYP1A genes were transcriptionally induced to a considerably higher degree than the rbCYP1B1 and rbCYP1C genes; i.e., rbCYP1A1, rbCYP1A3 rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3 were induced 110-, 200-, 8-, 17-, 4-, and 7fold over the unexposed control, respectively (Fig 4B).
Figure 5.
Relative transcriptional induction of rbCYP1A1 rbCYP1A3 rbCYP1B1, rbCYP1C1 rbCYP1C2, and rbCYP1C3 in gills (A) and liver (B) of PCB126-exposed rainbow trout (n=5–6). Calculations were made using EF1α as the reference gene and the mean values of the different rbCYP1s in unexposed controls as calibrators. Statistically significant differences compared with the unexposed control were examined by one-way ANOVA followed by Bonferroni's post hoc test for selected pairs and are shown by stars (*** = p<0.001 and ** = p<0.01).
3.5. Induction patterns in gills and liver of environmentally exposed rainbow trout
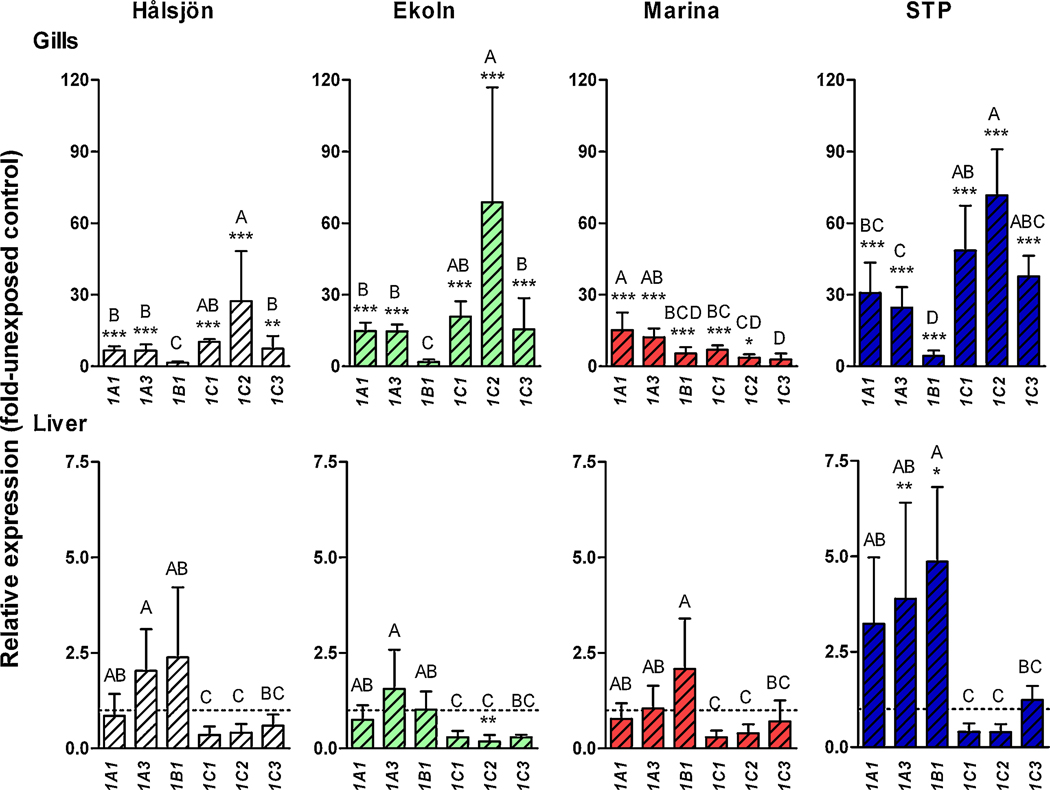
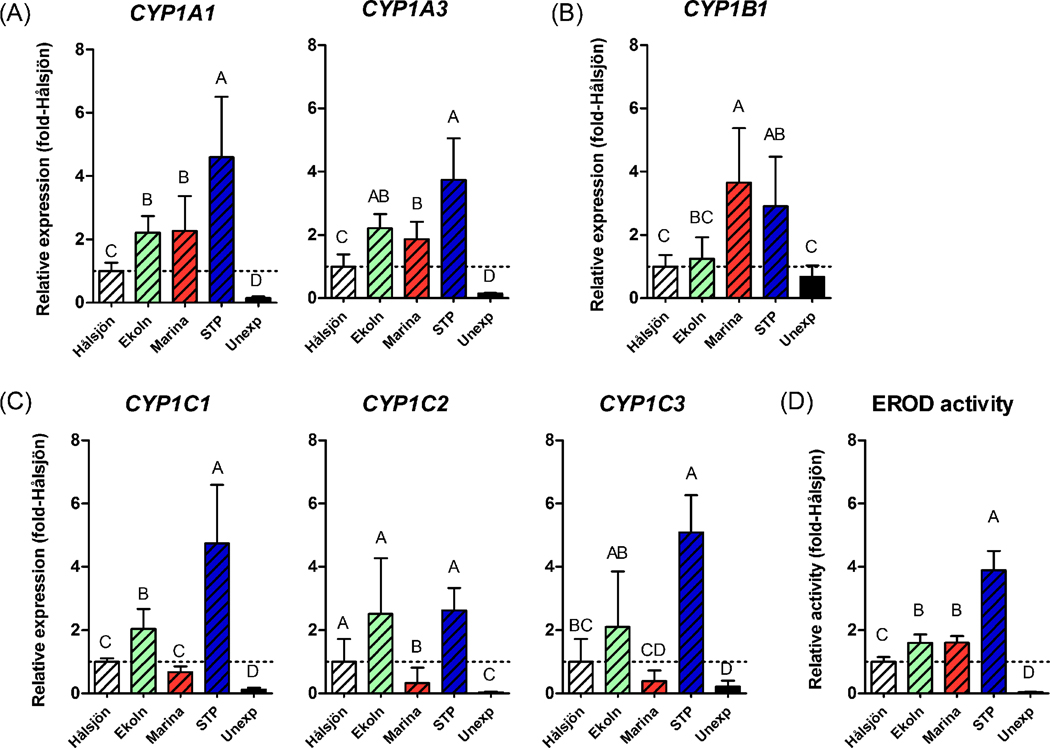
In order to examine the response to environmental exposure we determined rbCYP1 mRNA expression in rainbow trout held in cages for two days at four sites in the Uppsala region: Lake Hålsjön (selected as a reference site), a marina in Lake Ekoln, a site outside this marina, and the Uppsala STP outlet in Fyris River (Fig. 1). For simplicity these sites are denoted “Hålsjön”, “Marina”, “Ekoln”, and “STP” from here on in the text, and figures. Figure 6 shows the rbCYP1 mRNA expression in gills and liver at each caging site (calculated with the level in unexposed fish as a calibrator). Figure 7 shows the mRNA expression of the rbCYP1A, rbCYP1B, and rbCYP1C subfamily genes and for EROD activity in gills of caged and unexposed trout (calculated with the level at Hålsjön as a calibrator).
Figure 6.
Relative mRNA expression patterns of rbCYP1A1 rbCYP1A3 rbCYP1B1 rbCYP1C1 rbCYP1C2, and rbCYP1C3 in gills (A) and liver (B) of environmentally exposed rainbow trout. Groups of 12 fish were exposed by two days of caging at four freshwater sites in the Uppsala region. Calculations were made using EF1α as the reference gene and the mean values of the different rbCYP1s in unexposed controls as calibrators. Statistically significant differences among the rbCYP1 genes at a caging site were examined by one-way ANOVA followed by Tukey’s post hoc test and are shown by different letters (p<0.05). Statistically significant differences compared with the unexposed control were examined by one-way ANOVA followed by Bonferroni's post hoc test for selected pairs and are shown by stars (*** = p<0.001, ** = p<0.01, and * = p<0.05) and n=5–6.
Figure 7.
mRNA expression patterns for rbCYP1A (A), rbCYP1B (B), rbCYP1C (C) subfamily genes and EROD activity (D) in gills of environmentally exposed rainbow trout (n=5–6). Fish were exposed by two days of caging at four freshwater sites in the Uppsala region. Calculations were made using EF1α as the reference gene and the mean values of the different rbCYP1s at the reference site (Hålsjön) as calibrators. Statistically significant differences in rbCYP1 mRNA expression among trout caged at different sites and unexposed controls were examined by one-way ANOVA followed by Tukey’s post hoc test and are shown by different letters (p<0.05).
3.5.1. Response in gills
The rbCYP1A1, rbCYP1A3, rbCYP1C1, and rbCYP1C2 genes and EROD activity were significantly induced in gills of trout from all caging sites, while rbCYP1C3 was induced at all sites except the Marina (Fig. 6). RbCYP1B1 was induced only at the Marina and at the STP, and the level of induction was low at both sites (4- and 5-fold the unexposed control). Considering all gill data together, caging at the STP caused the strongest rbCYP1 induction among the different sites. Most strongly induced at the STP were the rbCYP1Cs (51-, 72-, and 38-fold transcriptional induction for rbCYP1C1, rbCYP1C2, and rbCYP1C3, respectively). RbCYP1C2 was strongly induced also in gills of fish caged at Ekoln (69-fold the unexposed control). Notably, as recorded after repeated measurements, rbCYP1C2 mRNA expression showed a comparatively high individual variation at Ekoln and at Hålsjön (Fig. 6). Gill filament EROD activity in trout caged at Hålsjön, Ekoln, the Marina, and the STP was induced 23-, 37-, 37-, and 90-fold relative to the unexposed control, respectively.
3.5.2. Response in liver
The rbCYP1 gene response of the caged fish was considerably weaker in the liver than in the gills (Fig. 6). Induction in liver was observed only at the STP and only for rbCYP1A3 and rbCYP1B1, which were induced transcriptionally 4- and 5-fold relative to the unexposed control. It is notable that exposure at several caging sites tended to suppress rbCYP1C mRNA expression in liver, although only the rbCYP1C2 level at Ekoln was significantly below the basal level (30% of the unexposed control; Fig. 6).
3.5.3. Response patterns in gills
When comparing the responses in gills at different caging sites, Ekoln and Hålsjön exhibited similar rbCYP1 mRNA expression patterns; rbCYP1B1 was not induced and rbCYP1C2 was strongly induced, whereas the levels of rbCYP1A1, rbCYP1A3, rbCYP1C1 and rbCYP1C3 mRNAs were in-between those of rbCYP1B1 and rbCYP1C2 and showed no significant difference among each other (Fig. 6). The response pattern at the STP was somewhat similar to those at Ekoln and Hålsjön; rbCYP1B1 showed the weakest induction and rbCYP1C2 the strongest induction (Fig. 6). However, although the expression patterns were similar, the magnitude of the response differed, i.e., for most genes the induction was lowest at Hålsjön and highest at the STP. The Marina showed a different pattern than the other sites: rbCYP1B1 mRNA expression was higher at the Marina than at Ekoln and Hålsjön (clearly induced), whereas the rbCYP1C mRNA levels tended to be lower at the Marina than at the other sites. RbCYP1C3 was not significantly induced at the Marina (Fig. 6).
Generally, rbCYP1 genes within the same subfamily responded in a similar way, although the mRNA expression patterns differed between rbCYP1A, rbCYP1B, and rbCYP1C subfamilies (Fig. 7A–7C). The patterns of the two rbCYP1A genes, the rbCYP1A1 pattern in particular, were very similar to the EROD activity pattern (Fig. 7A and 7D. The rbCYP1B1 pattern was characterized by low levels of transcriptional induction (no induction at Hålsjön and Ekoln, and low induction at the Marina and STP (Fig. 7B). Furthermore, in contrast to the other genes rbCYP1B1 induction was not higher at the STP than at the Marina.
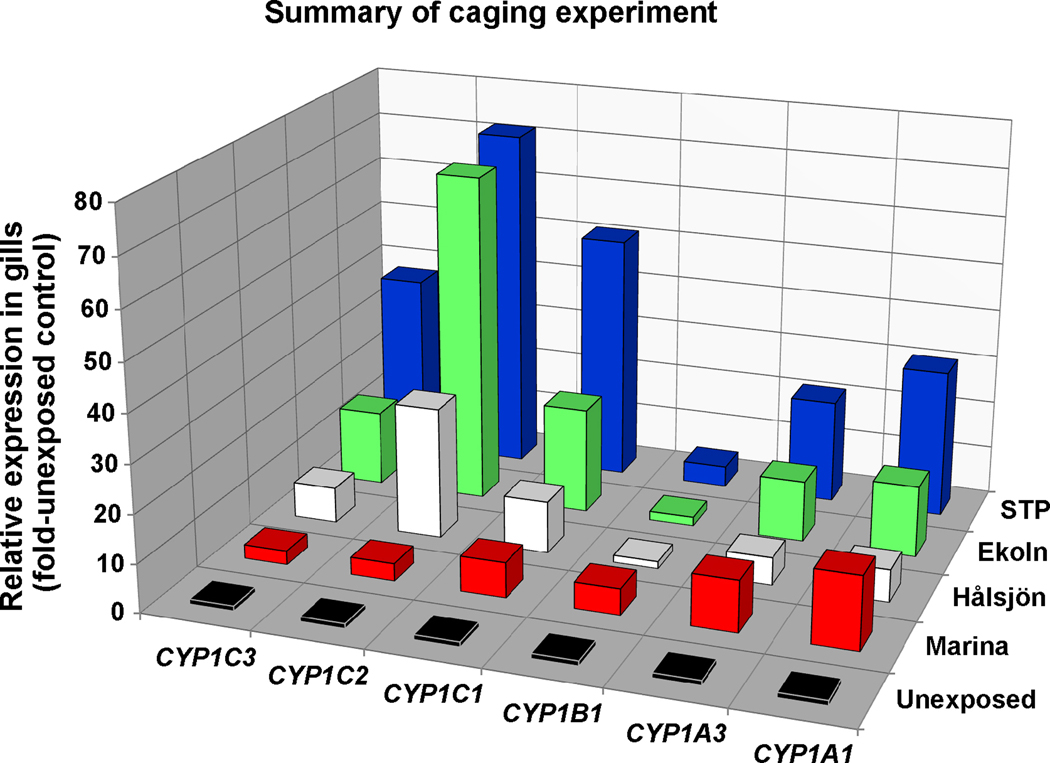
A summary of all rbCYP1 mRNA expression results in gills of environmentally exposed trout is given in Figure 8.
Figure 8.
Summary of all results on CYP1 mRNA expression in gills of rainbow trout caged in the Uppsala region (n=5–6). The bars represent mean values of relative expression data (fold-unexposed control).
4. Discussion
4.1. New rainbow trout CYP1 transcripts
We cloned one CYP1B and three CYP1C transcripts in rainbow trout, and confirmed their subfamily membership by phylogenetic and sequence analyses. These new transcripts were denoted rbCYP1B1, rbCYP1C1, rbCYP1C2, and rbCYP1C3 (Fig. 2). Basal mRNA expression patterns of the full series of rbCYP1 genes, including the previously known rbCYP1A1 and rbCYP1A3, were very similar to those observed in other fish species (Jönsson et al., 2007a; Zanette et al., 2009). All six genes were transcriptionally induced by PCB126 both in gills and liver (Fig. 5), supporting the idea that not only the rbCYP1As but also the cloned rbCYP1B and rbCYP1C genes are transcriptionally regulated by the AhR. Furthermore, five of the six genes were transcriptionally induced in gills in trout caged at various freshwater sites in the Uppsala region (Fig. 6). Induction in liver was found only for rbCYP1A3 and rbCYP1B1, and only at the Uppsala STP (Fig. 6). Moreover, the response patterns of the six rbCYP1 genes in gills were specific to both caging site and to subfamily (Fig. 6–8). Future studies will examine whether mRNA expression patterns of multiple CYP1 genes in gills could be used to characterize complex exposures to AhR agonists in polluted waters.
4.2. Sequence analysis and phylogeny of the rainbow trout CYP1 genes
The tetraploid origin of salmonid fish is reflected by increased DNA content, larger chromosome numbers, and a higher occurrence of duplicated gene loci relative to most other fish species (Bailey et al., 1978; Gharbi et al., 2006; Ohno et al., 1968). Accordingly, two and four AhR2 genes are present in rainbow trout and salmon, respectively (Abnet et al., 1999; Hansson et al., 2004). Rainbow trout have two CYP1A genes, whereas fish generally have only one (Berndtson and Chen, 1994; Morrison et al., 1995). It is therefore notable that we found only one rainbow trout CYP1B gene, while two CYP1B genes are present in the tetraploid carp (Cyprinus carpio) (El-kady et al., 2004a, 2004b). The tetraploidization event occurred earlier in salmonids than in carp (25–100 million years ago in salmonids versus 12 million years ago in carp) (Allendorf and Thorgaard, 1984; David et al., 2003). Consequently, a second trout CYP1B gene could possibly have been lost during evolution. We did not search for two CYP1B genes, however, and therefore the occurrence of a duplicated CYP1B gene in rainbow trout cannot be ruled out.
Interestingly, we found three rainbow trout CYP1C transcripts, rather than two, as found in other fish (Godard et al., 2005). The origin of the third CYP1C in rainbow trout is not clear. In the five fish genomes presently available, the CYP1C genes have tandemly duplicated and are located immediately adjacent to one another on the chromosome. The rbCYP1C3 gene could thus result from either another tandem duplication (presumably of rbCYP1C1), or from the salmonid tetraploidization. RbCYP1C1 and rbCYP1C3 were 94% similar in the deduced amino acid sequence, suggesting that they are very closely related (Table 2). It is presently unknown whether a fourth rainbow trout CYP1C gene exists or once existed. Rainbow trout and zebrafish CYP1C2 were 79% identical in the SRS sequences, but more different when the full AA sequences were compared. The functional implications of these findings are not known.
4.3. Basal CYP1 gene expression patterns
The basal CYP1 mRNA expression pattern in rainbow trout gills (Fig. 4) was similar to those previously described in gills of zebrafish, the rank order of expression being CYP1A > CYP1C1 > CYP1B1 > CYP1C2)(Jönsson et al., 2007a), and killifish (CYP1A > CYP1B1 > CYP1C1 > CYP1C2 (Zanette et al., 2009). In liver, all three species exhibited similar mRNA expression patterns, the CYP1As showing a considerably higher constitutive expression than the CYP1B and CYP1C genes. These strikingly similar patterns for basal mRNA expression suggest conserved physiological functions among CYP1A, CYP1B and CYP1C genes in fish. The mRNA of the “extra” rainbow trout CYP1C gene, rbCYP1C3, was expressed at least 10 times higher than either rbCYP1C1 or rbCYP1C2 in liver (Fig 3B), with unknown functional consequences.
Mammals have CYP1A and CYP1B genes but no CYP1C genes (Godard et al., 2005; Goldstone et al., 2007). Similar to fish, CYP1B1 in mouse and human liver shows a much lower (or undetectable) basal expression than the CYP1A genes (CYP1A1 and CYP1A2)(Choudhary et al., 2005). Consequently, the low basal CYP1B1 mRNA level in adult liver seems to be evolutionary stable among fish and from fish to mammals. It is therefore notable that increased levels of CYP1B1 protein have been observed in human cancers in breast, colon, lung, skin, brain, testis, etc. compared with healthy tissues (Murray et al., 1997). In rainbow trout, the induced CYP1B1 mRNA level was low both in gills and liver (Figs. 4–7).
4.4. CYP1 mRNA expression in PCB126-exposed fish – responses in gills versus liver
Rainbow trout, zebrafish, and killifish differed more in PCB126-induced mRNA expression patterns than in basal mRNA expression patterns of CYP1 genes. The strong rbCYP1A and rbCYP1C induction and the weaker rbCYP1B1 induction by PCB126 in rainbow trout gills (Fig 4) did not match the previously observed PCB126 response pattern in zebrafish gills, where CYP1A, CYP1B1, and CYP1C1 showed 50-, 40-, and 7-fold transcriptional induction and CYP1C2 no induction at all (Jönsson et al., 2007a). The induction pattern in killifish gills was somewhat similar to that in rainbow trout. Following intraperitoneal injection of PCB126 in killifish, CYP1A and CYP1C1 were strongly induced transcriptionally (70- and 100-fold), whereas CYP1B1 and CYP1C2 were moderately induced (appoximately15-fold) (Zanette et al., 2009).
The relative CYP1A induction in liver by PCB126 was considerably higher in rainbow trout (110- and 200-fold; Fig. 5) than in zebrafish and killifish (about 15-fold) (Jönsson et al., 2007a; Zanette et al., 2009). Notably, however, PCB126 exposure resulted in almost identical transcriptional induction levels for hepatic CYP1B1, CYP1C1, and CYP1C2 in rainbow trout (8-, 17-, and 4-fold) and zebrafish (10-, 17- and 4-fold)(Jönsson et al., 2007a). The PCB126 induction pattern of these genes in killifish liver was similar to those in trout and zebrafish liver, although the magnitudes of induction were considerably higher in killifish (roughly 200-, 400-, and 50-fold for CYP1B1, CYP1C1, and CYP1C2) (Zanette et al., 2009). Since PCB126 was administered via intraperitoneal injection to killifish and via water to trout and zebrafish, the differences in induction in liver could be due to different biavailabilities to the liver. However, it could also be influenced by differences in the control (basal) level, as calculations of relative mRNA expression depend on the control. The relative levels of CYP1B1, CYP1C1, and CYP1C2 mRNAs were lower in killifish liver controls (0.001–0.1% of CYP1A mRNA expression) than in zebrafish and rainbow trout liver controls (0.1–1% of CYP1A1 mRNA expression; Fig. 4). Control levels of CYP1 mRNA expression and EROD activity could vary depending the fish feed, which can contain AhR agonists (Easton et al., 2002; Maule et al., 2007).
The clear transcriptional induction by PCB126 of all six rainbow trout CYP1 genes implies that these genes are regulated by the AhR. All of the CYP1A, CYP1B, and CYP1C genes known in zebrafish and killifish are induced by AhR agonists (Jönsson et al., 2007a; Zanette et al., 2009), and in zebrafish embryos their induction by PCB126 is “knocked down” by a morpholino targeting AhR2 (Jönsson et al., 2007b). The CYP1D1 gene in zebrafish and killifish is not inducible by AhR agonists. No CYP1D gene was found in rainbow trout.
4.5. CYP1 mRNA expression in environmentally exposed fish – responses in gills versus liver
Gill filament EROD activity is a sensitive biomarker for AHR agonists in ambient water (Jönsson et al., 2002). In a previous study performed in the Uppsala-Stockholm region, EROD activity was induced in gills of fish from all caging sites, whereas induction in the liver and kidney was less frequently observed (Abrahamson et al., 2007). In line with these findings, all caged fish showed strong transcriptional induction of most rbCYP1 genes in gills in the present study. The liver responses were weaker and significant induction was observed only for rbCYP1A3 and rbCYP1B1 and only at the STP. The induction of EROD activity and rbCYP1 mRNA expression observed in gills of fish caged in the Uppsala region presumably reflects a ubiquitous presence of AHR agonists in this urban area. Several studies report increasing trends for the levels of polycyclic aromatic hydrocarbons (PAHs) in reference and urban areas (Hanson et al., 2009; Van Metre and Mahler, 2005). The weak CYP1 gene response in liver versus gills in the caged fish supports the contention that the inducers were readily metabolized compounds, such as PAHs. Some PAHs are both inducers and substrates for CYP1A and CYP1B enzymes (Shimada and Fujii-Kuriyama, 2004). In trout exposed to waterborne benzo[a]pyrene (BaP), EROD induction in the liver required higher concentrations than induction in the gills (Jönsson et al., 2006), most likely due to the fact that BaP absorbed from water can undergo first-pass metabolism in gills (Andersson and Pärt, 1989; Stegeman et al., 1984). Consequently only a fraction of inducer absorbed by the gills would have reached the liver (Jönsson et al., 2006). Opposite findings were reported for rainbow trout caged at sites contaminated by a former PCB manufacturing plant in the Mud River system (a rural area in Kentucky, USA) (Brammell et al., 2010). The liver responded with transcriptional CYP1A1 induction in a PCB concentration-dependent manner, whereas induction in gills was fairly weak. Assuming that trout caged in the Mud River were primarily exposed to persistent PCBs and that trout caged in the Uppsala region were exposed mainly to PAHs, the different response patterns in gill and liver could be explained by differences in the rate of metabolism of inducers.
4.6. CYP1 mRNA expression patterns in gills as a monitoring tool
The endogenous substrates of the enzymes encoded by the newly cloned genes have not been determined, and it is not known whether they display EROD activity. In the caging experiment, the pattern of gill filament EROD activity closely matched the rbCYP1A1 mRNA expression pattern, and was fairly similar to the rbCYP1A3 mRNA expression pattern, but was different from the rbCYP1B1 and rbCYP1C mRNA expression patterns (Fig. 7). Consequently, when used as a biomarker it appeared that gill filament EROD activity primarily reflects the rbCYP1A response and to a lesser extent the rbCYP1B and rbCYP1C responses.
After laboratory exposure to PCB126 the response of the rbCYP1A and rbCYP1C genes was very similar, whereas the rbCYP1 subfamilies responded differently to environmental exposure. This suggests that the response to some inducers or pollutant mixtures is different between the rbCYP1 subfamilies. Furthermore, the mRNA expression patterns of the six genes varied with monitoring site. At Hålsjön, Ekoln, and the STP the patterns were similar, although the magnitude of the responses differed. The magnitude of induction probably reflects the local pollution load, supposedly implying that Hålsjön was the least contaminated and the STP the most contaminated site examined. In a previous study rainbow trout caged at the Uppsala STP outlet in Fyris River showed higher gill EROD induction than trout caged at other sites in the river (Abrahamson et al., 2007), suggesting the STP is a pollution source for AHR agonists.
Presumably, there were different contaminants in the water at the four caging sites. It is therefore an interesting finding that the expression pattern at the Marina was completely different from those at the other sites and that the response of the rbCYP1C genes differed from those of the rbCYP1As and rbCYP1B1. Hence, the atypical rbCYP1 mRNA expression pattern observed at the Marina suggests that fish caged at this site were exposed to chemicals that did not occur at the other sites.
In conclusion, mRNA expression patterns of multiple CYP1 genes in fish gills and liver could provide an improved model for monitoring of AhR-active pollutants in the aquatic environment. It will be important in future studies to determine whether such patterns could be used as a tool to characterize complex mixtures of pollutants.
Supplementary Material
Acknowledgement
We thank Gunnar Steinholtz for support and advice in the field study. This study was funded by the Swedish Foundation for Strategic Environmental Research (Mistra) through the MistraPharma program, the Swedish Research Council Formas, the Swedish Environmental Protection Agency, and Carl Trygger's Foundation. JVG was supported by NIH Grants to John Stegeman (R01-ES015912, and the Superfund Basic Research Program at Boston University 5-P42-ES007381). The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abnet CC, Tanguay RL, Hahn ME, Heideman W, Peterson RE. Two forms of aryl hydrocarbon receptor type 2 in rainbow trout (Oncorhynchus mykiss). Evidence for differential expression and enhancer specificity. J. Biol. Chem. 1999;274:15159–15166. doi: 10.1074/jbc.274.21.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson A, Andersson C, Jönsson ME, Fogelberg O, Örberg J, Brunström B, Brandt I. Gill EROD in monitoring of CYP1A inducers in fish: a study in rainbow trout (Oncorhynchus mykiss) caged in Stockholm and Uppsala waters. Aquat. Toxicol. 2007;85:1–8. doi: 10.1016/j.aquatox.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Allendorf FW, Thorgaard GH. Tetraploidy and the evolution of salmonid fishes. In: Turner BJ, editor. The Evolutionary Genetics of Fishes. New York: Plenum Press; 1984. pp. 1–53. [Google Scholar]
- Andersson T, Pärt P. Benzo[a]pyrene metabolism in isolated perfused rainbow trout gills. Mar. Environ. Res. 1989;28:3–7. [Google Scholar]
- Bailey GS, Poulter RT, Stockwell PA. Gene duplication in tetraploid fish: model for gene silencing at unlinked duplicated loci. Proc. Natl. Acad. Sci. U.S.A. 1978;75:5575–5579. doi: 10.1073/pnas.75.11.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijer K, Abrahamson A, Brunstrom B, Brandt I. CYP1A inhibition in fish gill filaments: A novel assay applied on pharmaceuticals and other chemicals. Aquat. Toxicol. 2010;96:145–150. doi: 10.1016/j.aquatox.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Berndtson AK, Chen TT. Two unique CYP1 genes are expressed in response to 3-methylcholanthrene treatment in rainbow trout. Arch. Biochem. Biophys. 1994;310:187–195. doi: 10.1006/abbi.1994.1156. [DOI] [PubMed] [Google Scholar]
- Brammell BF, McClain JS, Oris JT, Price DJ, Birge WJ, Elskus AA. CYP1A Expression in caged rainbow trout discriminates among sites with various degrees of polychlorinated biphenyl contamination. Arch. Environ. Contam. Toxicol. 2010 doi: 10.1007/s00244-009-9368-x. In press. [DOI] [PubMed] [Google Scholar]
- Buhler DR, Wang BJL. Rainbow trout cytochrome P450s: Purification, molecular aspects, metabolic activity, induction and role in environmental monitoring. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;121:107–137. doi: 10.1016/s0742-8413(98)10033-6. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch. Biochem. Biophys. 2005;436:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- David L, Blum S, Feldman MW, Lavi U, Hillel J. Recent duplication of the common carp (Cyprinus carpio L.) genome as revealed by analyses of microsatellite loci. Mol. Biol. Evol. 2003;20:1425–1434. doi: 10.1093/molbev/msg173. [DOI] [PubMed] [Google Scholar]
- Di Bello D, Vaccaro E, Longo V, Regoli F, Nigro M, Benedetti M, Gervasi PG, Pretti C. Presence and inducibility by beta-naphthoflavone of CYP1A1, CYP1B1 and phase II enzymes in Trematomus bernacchii, an Antarctic fish. Aquat. Toxicol. 2007;84:19–26. doi: 10.1016/j.aquatox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Easton MD, Luszniak D, Von der GE. Preliminary examination of contaminant loadings in farmed salmon, wild salmon and commercial salmon feed. Chemosphere. 2002;46:1053–1074. doi: 10.1016/s0045-6535(01)00136-9. [DOI] [PubMed] [Google Scholar]
- El-kady MA, Mitsuo R, Kaminishi Y, Itakura T. cDNA cloning, sequence analysis and expression of 3-methylcholanthrene-inducible cytochrome P450 1B1 in carp (Cyprinus carpio) Environ. Sci. 2004a;11:231–240. [PubMed] [Google Scholar]
- El-kady MA, Mitsuo R, Kaminishi Y, Itakura T. Isolation of cDNA of novel cytochrome P450 1B gene, CYP1B2, from Carp (Cyprinus carpio) and its induced expression in gills. Environ. Sci. 2004b;11:345–354. [PubMed] [Google Scholar]
- Gharbi K, Gautier A, Danzmann RG, Gharbi S, Sakamoto T, Hoyheim B, Taggart JB, Cairney M, Powell R, et al. A linkage map for brown trout (Salmo trutta): chromosome homeologies and comparative genome organization with other salmonid fish. Genetics. 2006;172:2405–2419. doi: 10.1534/genetics.105.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard CA, Goldstone JV, Said MR, Dickerson RL, Woodin BR, Stegeman JJ. The new vertebrate CYP1C family: cloning of new subfamily members and phylogenetic analysis. Biochem. Biophys. Res. Commun. 2005;331:1016–1024. doi: 10.1016/j.bbrc.2005.03.231. [DOI] [PubMed] [Google Scholar]
- Goldstone HM, Stegeman JJ. A revised evolutionary history of the CYP1A subfamily: Gene duplication, gene conversion, and positive selection. J. Mol. Evol. 2006;62:708–717. doi: 10.1007/s00239-005-0134-z. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, Goldstone HM, Morrison AM, Tarrant A, Kern SE, Woodin BR, Stegeman JJ. Cytochrome P450 1 genes in early deuterostomes (tunicates and sea urchins) and vertebrates (chicken and frog): origin and diversification of the CYP1 gene family. Mol. Biol. Evol. 2007;24:2619–2631. doi: 10.1093/molbev/msm200. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, Jönsson ME, Behrendt L, Woodin BR, Jenny MJ, Nelson DR, Stegeman JJ. Cytochrome P450 1D1: a novel CYP1A–related gene that is not transcriptionally activated by PCB126 or TCDD. Arch. Biochem. Biophys. 2009;482:7–16. doi: 10.1016/j.abb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, Stegeman JJ. Gene structure of the novel cytochrome P4501D1 genes in stickleback (Gasterosteus aculeatus) and medaka (Oryzias latipes) Mar. Environ. Res. 2008;66:19–20. doi: 10.1016/j.marenvres.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE1969. Procedures for detecting outlying observations in samples. Technometrics. 11:1–21. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hanson N, Persson S, Larsson A. Analyses of perch (Perca fluviatilis) bile suggest increasing exposure to PAHs and other pollutants in a reference area on the Swedish Baltic coast. J. Environ. Monit. 2009;11:389–393. doi: 10.1039/b817703a. [DOI] [PubMed] [Google Scholar]
- Hansson MC, Wittzell H, Persson K, von Schantz T. Unprecedented genomic diversity of AhR1 and AhR2 genes in Atlantic salmon (Salmo salar L.) Aquat. Toxicol. 2004;68:219–232. doi: 10.1016/j.aquatox.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Itakura T, El-Kady M, Mitsuo R, Kaminishi Y. Complementary DNA cloning and constitutive expression of cytochrome P450 1C1 in the gills of carp (Cyprinus carpio) Environ. Sci. 2005;12:111–120. [PubMed] [Google Scholar]
- Jönsson EM, Abrahamson A, Brunström B, Brandt I. Cytochrome P4501A induction in rainbow trout gills and liver following exposure to waterborne indigo, benzo[a]pyrene and 3,3',4,4',5-pentachlorobiphenyl. Aquat. Toxicol. 2006;79:226–232. doi: 10.1016/j.aquatox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Jönsson EM, Brandt I, Brunström B. Gill filament-based EROD assay for monitoring waterborne dioxin-like pollutants in fish. Environ. Sci. Technol. 2002;36:3340–3344. doi: 10.1021/es015859a. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3,'4,4',5-Pentachlorobiphenyl-induced expression of Cytochrome P450 1A, 1B and 1C Genes in Zebrafish. Toxicol. Appl. Pharmacol. 2007a;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebrafish exposed to 3,3’,4,4’,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007b;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Jørgensen SM, Kleveland EJ, Grimholt U, Gjoen T. Validation of reference genes for real-time polymerase chain reaction studies in Atlantic salmon. Mar. Biotechnol. 2006;8:398–408. doi: 10.1007/s10126-005-5164-4. [DOI] [PubMed] [Google Scholar]
- Leaver MJ, George SG. A cytochrome P4501B gene from a fish, Pleuronectes platessa. Gene. 2000;256:83–91. doi: 10.1016/s0378-1119(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Gillam EM, Everett SA, Shimada T. Molecular modelling of human CYP1B1 substrate interactions and investigation of allelic variant effects on metabolism. Chem.-Biol. Interact. 2003;145:281–295. doi: 10.1016/s0009-2797(03)00021-8. [DOI] [PubMed] [Google Scholar]
- Lewis DFV. 57 varieties: the human cytochromes P450. Pharmacogenomics. 2004;5:305–318. doi: 10.1517/phgs.5.3.305.29827. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maule AG, Gannam AL, Davis JW. Chemical contaminants in fish feeds used in federal salmonid hatcheries in the USA. Chemosphere. 2007;67:1308–1315. doi: 10.1016/j.chemosphere.2006.11.029. [DOI] [PubMed] [Google Scholar]
- McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdegela R, Myburgh J, Correia D, Braathen M, Ejobi F, Botha C, Sandvik M, Skaare JU. Evaluation of the gill filament-based EROD assay in African sharptooth catfish (Clarias gariepinus) as a monitoring tool for waterborne PAH-type contaminants. Ecotoxicology. 2006;15:51–59. doi: 10.1007/s10646-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Morrison HG, Oleksiak MF, Cornell NW, Sogin ML, Stegeman JJ. Identification of cytochrome P-450 1A (CYP1A) genes from two teleost fish, toadfish (Opsanus tau) and scup (Stenotomus chrysops), and phylogenetic analysis of CYP1A genes. Biochem. J. 1995;308(Pt 1):97–104. doi: 10.1042/bj3080097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, Melvin WT. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–3031. [PubMed] [Google Scholar]
- Ohno S, Wolf U, Atkin NB. Evolution from fish to mammals by gene duplication. Hereditas. 1968;59:169–187. doi: 10.1111/j.1601-5223.1968.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Ortiz-Delgado JB, Behrens A, Segner H, Sarasquete C. Tissue-specific induction of EROD activity and CYP1A protein in Sparus aurata exposed to B(a)P and TCDD. Ecotoxicol. Environ. Saf. 2008;69:80–88. doi: 10.1016/j.ecoenv.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råbergh CM, Vrolijk NH, Lipsky MM, Chen TT. Differential expression of two CYP1A genes in rainbow trout (Oncorhynchys mykiss) Toxicol. Appl. Pharmacol. 2000;165:195–205. doi: 10.1006/taap.2000.8941. [DOI] [PubMed] [Google Scholar]
- Sarasquete C, Segner H. Cytochrome P4501A (CYP1A) in teleostean fishes. A review of immunohistochemical studies. Sci. Total Environ. 2000;247:313–332. doi: 10.1016/s0048-9697(99)00500-8. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolowitz RM, Hahn ME, Stegeman JJ. Immunohistochemical localization of cytochrome P-450IA1 induced by 3,3',4,4'-tetrachlorobiphenyl and by 2,3,7,8-tetrachlorodibenzofuran in liver and extrahepatic tissues of the teleost Stenotomus chrysops (scup) Drug Metabol. Dispos. 1991;19:113–123. [PubMed] [Google Scholar]
- Smolowitz RM, Schultz ME, Stegeman JJ. Cytochrome P4501A induction in tissues, including olfactory epithelium, of topminnows (Poeciliopsis spp.) by waterborne benzo(a)pyrene. Carcinogenesis. 1992;13:2395–2402. doi: 10.1093/carcin/13.12.2395. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics (Oxford, England) 2006a;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. Phylogenetic models of rate heterogeneity: A high performance computing perspective. Proceedings of the Proceedings of 20th IEEE/ACM International Parallel and Distributed Processing Symposium (IPDPS2006); Rhodos, Greece. 2006b. [Google Scholar]
- Stegeman JJ, Woodin BR, Binder RL. Patterns of Benzo[a]pyrene Metabolism by Varied Species, Organs, and Developmental Stages of Fish. Natl. Cancer Inst. Monogr. 1984;65:371–377. [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ. Sci. Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 Messenger RNA Expression is Inducible by Benzo[a]pyrene in Fundulus heteroclitus Embryos and Adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Zanette J, Jenny MJ, Goldstone JV, Woodin BR, Watka LA, Bainy AC, Stegeman JJ. New cytochrome P450 1B1, 1C2 and 1D1 genes in the killifish Fundulus heteroclitus: Basal expression and response of five killifish CYP1s to the AHR agonist PCB126. Aquat. Toxicol. 2009;93:234–243. doi: 10.1016/j.aquatox.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.