Abstract
Background
Weight gain is used by disease-management programs as a marker of heart failure decompensation, but little information is available to quantify the relationship between weight change in patients with heart failure and the risk for imminent hospitalization.
Methods and Results
We conducted a nested case-control study among patients with heart failure referred to a home monitoring system by managed care organizations. We matched 134 case patients with heart failure hospitalization to 134 control patients without heart failure hospitalization on the basis of age, sex, duration of home monitoring, heart failure severity, and baseline body weight. Compared with control patients, case patients experienced gradual weight gain beginning ≈30 days before hospitalization; changes in daily weight between case and control patients were statistically significant (P<0.001). Within the week before hospitalization, when weight patterns in case and control patients began to diverge more substantially, mean increases of more than 2 and up to 5 pounds, more than 5 and up to 10 pounds, and more than 10 pounds (relative to time of enrollment in the monitoring system) were associated with matched adjusted odds ratios for heart failure hospitalization of 2.77 (95% confidence interval 1.13 to 6.80), 4.46 (95% confidence interval 1.45 to 13.75), and 7.65 (95% confidence interval 2.22 to 26.39), respectively, compared with mean increases of 2 pounds or less.
Conclusions
Increases in body weight are associated with hospitalization for heart failure and begin at least 1 week before admission. Daily information about patients' body weight identifies a high-risk period during which interventions to avert decompensated heart failure that necessitates hospitalization may be beneficial.
Keywords: heart failure, prognosis, disease management, epidemiology
Systems to enhance remote monitoring of patients' clinical status have the potential to improve the care and outcomes of heart failure patients. These monitoring systems, which collect and transmit daily clinical information, are being used more frequently and in diverse settings.1–3 Moreover, the Centers for Medicare and Medicaid Services is implementing a widespread demonstration project involving >300 000 patients that mandates use of this surveillance strategy.4 The underlying clinical science defining the prognostic importance of surveillance information, however, has not been developed. For these systems to achieve their promise, the utility of the collected information must be defined. In particular, weight gain has long been regarded by clinicians as a marker of heart failure decompensation, but studies have not defined the pattern of daily weight change that precedes hospitalizations for heart failure.
Accordingly, we evaluated data from a group of heart failure patients who were weighing themselves daily as part of a disease-management remote-monitoring system. The goals of the present study were to describe trends in weight preceding hospitalization for heart failure and to determine whether increasing weight is a risk factor for imminent heart failure hospitalization. Equipped with such information, clinicians could make better management decisions to potentially avert hospitalization among their patients with decompensated heart failure.
Methods
Source Population
The present analysis is based on data collected over an 18-month period (January 1, 2003, to June 30, 2004) from patients with heart failure who were using the Alere (Reno, Nev) home monitoring system. This commercially available system includes an electronic scale placed in patients' homes and linked via a standard phone line (using a toll-free telephone number) to a computerized database monitored by trained cardiac nurses employed by Alere, Inc. Patients were referred to the system by their managed care organizations and did not incur any expense for participation. Typically, managed care organizations identify patients for participation on the basis of healthcare utilization (including emergency department and outpatient visits, as well as hospitalizations). To be eligible for this home monitoring system, patients had to speak English or Spanish, weigh <400 pounds, and be able to stand unassisted for 90 seconds for daily weighing. Patients were ineligible if they were receiving dialysis, intravenous chemotherapy, or paracentesis or if they had undergone organ transplantation. Patients were also excluded from the present study if they were missing >75% of daily weight measures or if they were hospitalized within 30 days after enrollment in the monitoring system (because patterns of weight gain preceding hospitalization could not be fully characterized in the event of hospitalization soon after enrollment in the monitoring system).
Case and Control Patients
Case patients were defined by the first occurrence of heart failure hospitalization during the 18-month follow-up period. (Hospitalizations were classified as “heart failure” or “non–heart failure” on the basis of patients' reports of the primary reason for admission.) For each case, we randomly selected a control patient from among all participants who had not been admitted to the hospital for decompensated heart failure up to the time of the matched case patient's hospitalization. To reduce bias, we did allow for subsequent hospitalization among control patients after the date of the case patient's hospitalization. Control patients were matched with case patients on the basis of the following criteria: same month in which patients started the monitoring system, similar baseline body weight (within 5 pounds), similar age (within 5 years), same sex, and same New York Heart Association (NYHA) class (class I to II and class III to IV). We included the latter 3 factors on the basis of previous studies that have identified age, sex, and NYHA class as primary determinants of worse outcomes among heart failure patients.5,6
During the 18-month study period, 10 525 patients were enrolled into the monitoring system. From this pool of potential subjects, 393 patients had at least 1 heart failure hospitalization, and 302 of these were successfully matched with corresponding control patients (without heart failure hospitalization) using the matching criteria outlined above. Compared with the 302 matched patients, the 91 unmatched patients were younger (average age 69 versus 74 years) and were more likely to have NYHA class I/II heart failure (16% versus 3%). Among the 302 matched case patients, we excluded 96 who were hospitalized within the first 30 days of enrollment into the monitoring system, 6 who were missing >75% of the daily weight measures, and 66 whose corresponding control patient did not meet inclusion criteria. Thus, we analyzed data for 134 case patients with a heart failure hospitalization and 134 control patients without a heart failure hospitalization during the same study period. Compared with the 268 patients who were included in the analyses, the 10 257 patients who were not included were slightly older (average age 72 versus 74 years) and were more likely to have NYHA class I/II heart failure (8% versus 2%). Otherwise, patients not included in the present analyses did not differ significantly according to sex, baseline weight, or length of participation in the home monitoring program.
Procedures
Patients enrolled in the home monitoring program were instructed to weigh themselves using the digital scale at the same time each morning after urinating, before eating or drinking, and wearing the same amount of clothing. Any weight gain of at least 5 pounds during the course of 3 days prompted an automated report that was faxed to the patient's physician, who could then institute a change in therapy or take other appropriate action. The median rate of missing data for daily weights was 4%, with an interquartile range of 0% to 15%, and did not differ between case and control patients.
Telephone interviews with patients were conducted by Alere staff on enrollment and disenrollment from the system. Patients were queried about medication use, comorbidities, and heart failure and non–heart failure hospitalizations. (Patients were asked what the primary reason for hospitalization was, and hospitalizations were then classified as “heart failure” or “non–heart failure” using this information.) Patients were also contacted when 2 consecutive days of using the monitoring system were missed, to enforce adherence or obtain information about hospitalization.
Statistical Analyses
For descriptive purposes, we compared medical conditions (hypertension, coronary artery disease, diabetes mellitus, stroke/transient ischemic attack, and chronic obstructive pulmonary disease) and use of medications (angiotensin-converting enzyme inhibitors and β-blockers) between case and control patients at the time of enrollment into the monitoring system using the Fisher exact test. We did not impute missing data for weight, and other fields in the analyses did not have missing data.
Daily weight change, calculated by subtracting baseline weight (first weight obtained on entry into the monitoring system) from weight on a given day, was compared between case and control patients. Visual inspection was used initially to evaluate timing of weight change among study participants. To assess the specificity of the results, daily weight changes before non–heart failure hospitalizations were also compared; we assembled 688 cases with non–heart failure hospitalizations and then matched an equal number of control patients. Case patients were identified by the first occurrence of non–heart failure hospitalization during the 18-month follow-up period. For each case, we randomly selected a control patient from among all participants who had not been admitted to the hospital up to the time of the matched case patient's hospitalization. Case and control patients were matched on the same criteria used in the analyses of heart failure hospitalizations (same month in which patients started the monitoring system, similar baseline body weight, similar age, same sex, and same severity of heart failure).
We evaluated the independent effect of antecedent weight change within a 1-week period before heart failure hospitalization (based on case patients) in both unadjusted and adjusted analyses using conditional logistic regression techniques for matched data.7 All comorbid conditions and medications listed in Table 1 were evaluated in the adjusted analyses: hypertension, coronary artery disease, diabetes mellitus, stroke/transient ischemic attack, chronic obstructive pulmonary disease, use of angiotensin-converting enzyme inhibitors, and use of β-blockers. Average weight change within 1 week before heart failure hospitalization was categorized as an increase of 2 pounds or less (including weight loss), >2 and up to 5 pounds, >5 and up to 10 pounds, and >10 pounds. For each of these categories, dummy variables were created (with “2 pounds or less” as the reference group) when entered into the conditional logistic regression model. We also evaluated average weight change within 1 week before hospitalization as a continuous variable in a separate model.
TABLE 1.
Baseline Characteristics of Study Population
| Total (n=268) |
Case Patients (n=134) |
Control Patients (n=134) |
P | |
|---|---|---|---|---|
| Mean age, y (SD) | 74 (11) | 74 (11) | 74 (11) | 0.96 |
| Sex, female, % | 55.2 | 55.2 | 55.2 | 1.00 |
| Duration of monitoring, d | 291 | 287 | 298 | 0.72 |
| NYHA class, % | ||||
| I | 0.8 | 0.8 | 0.8 | 1.00 |
| II | 0.8 | 0.8 | 0.8 | 1.00 |
| III | 97.4 | 96.3 | 98.5 | 0.45 |
| IV | 1.1 | 2.2 | 0.0 | 0.25 |
| Prescribed ACEI, % | 71.3 | 67.9 | 74.6 | 0.91 |
| Prescribed β-blocker, % | 48.9 | 55.2 | 42.5 | 0.05 |
| Hypertension, % | 52.6 | 50.0 | 55.2 | 0.83 |
| Coronary artery disease, % | 45.5 | 41.0 | 50.0 | 0.18 |
| Diabetes mellitus, % | 36.6 | 35.8 | 37.3 | 0.65 |
| Stroke/TIA, % | 9.7 | 9.0 | 10.5 | 0.74 |
| Chronic obstructive pulmonary disease, % | 10.5 | 11.9 | 9.0 | 0.28 |
ACEI indicates angiotensin-converting enzyme inhibitor; TIA, transient ischemic attack.
All analyses were conducted with SAS software version 9.1 (SAS Institute, Cary, NC). Alere Medical Inc provided the data but had no role in the design and conduct of the study; management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. The Yale Human Investigations Committee approved the study protocol.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
As shown in Table 1, the mean age of the present study population was 74 years; 55% of patients were female; the mean length of participation in the monitoring system was 291 days; and 97% had NYHA class III heart failure. Case and control patients were similar with regard to the use of angiotensin-converting enzyme inhibitors and the presence of comorbid conditions, but more case patients than control patients were prescribed β-blockers. Of the 134 cases, 125 patients had only 1 hospitalization for heart failure, 5 had 2 hospitalizations, and 4 had 3 hospitalizations. None of the control patients experienced hospitalization.
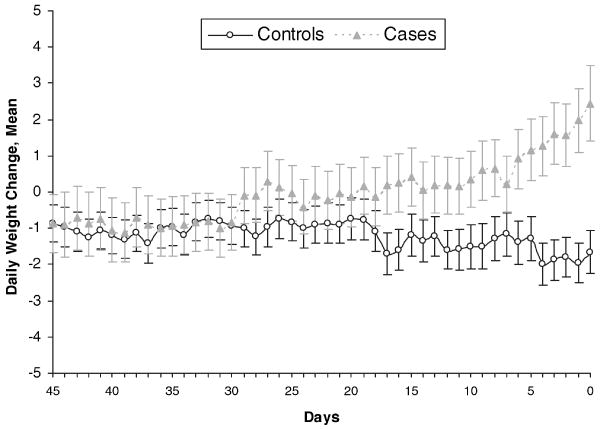
Figure 1 shows the daily weight changes during the time preceding heart failure hospitalization among case and control patients. Each group experienced a slight decline in average weight (−1 pound) from enrollment to 45 days before hospitalization. Patterns in daily weight change were relatively similar until ≈30 days before hospitalization, at which point the weight pattern of the case patients began to diverge from that of the control patients. At ≈1 week before hospital admission, the weight gain in the case patients began to increase markedly, whereas the control patients had relatively stable weight (average 1- to 2-pound loss compared with baseline weight). The difference in daily weight changes between case and control patients within 30 days before (case) hospitalization was statistically significant (P<0.001).
Figure 1.
Daily weight change before heart failure hospitalization: cases vs controls. n=268. “Days” on the x-axis denotes days before hospital admission in case patients. The difference in daily weight changes between case and control patients within 30 days before (case) hospitalization was statistically significant (P<0.001) on the basis of a generalized linear model with daily weight change as the dependent variable.
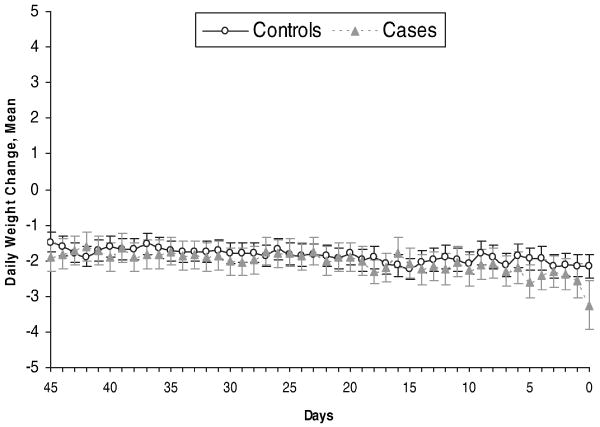
When non–heart failure hospitalization case and control patients were considered as a group, the average age was 73 years, 53% were female, and 94% had NYHA class III heart failure. Case and control patients were well matched on baseline characteristics (data not shown) with only 1 exception: The proportion of patients with NYHA class IV heart failure was greater in the case patients (2.9%) than in the control patients (0.9%; P=0.006). The patterns of weight change observed before non–heart failure hospitalization were similar in case and control patients; as shown in Figure 2, weights were relatively constant in both case and control patients before non–heart failure hospitalization.
Figure 2.
Daily weight change before non–heart failure hospitalization: cases vs controls. n=1376. “Days” on the x-axis denotes days before hospital admission in case patients.
Table 2 shows adjusted, matched odds ratios (ORs) of heart failure hospitalization based on weight gain within the prior 7 days, accounting for comorbid conditions and medication use (with weight gain of 2 pounds or less used to define the reference group). The 7-day interval was chosen on the basis of data presented in Figure 1, which demonstrated rapid weight gain among case patients during this time. Relative to the time of enrollment, a weight gain of >2 and up to 5 pounds was associated with an adjusted OR of 2.77 (95% confidence interval [CI] 1.13 to 6.80) for heart failure hospitalization. The strength of the association increased with increasing amounts of weight gain, so that a weight gain of >5 and up to 10 pounds had an adjusted, matched OR of 4.46 (95% CI 1.45 to 13.75) and a gain of >10 pounds had an adjusted, matched OR of 7.65 (95% CI 2.22 to 26.39) for heart failure hospitalization. The adjusted, matched OR of hospitalization for unknown weight (N=14) was 1.07 (95% CI 0.31 to 3.68). In a separate regression model that assessed weight as a continuous variable, the matched OR for heart failure hospitalization was 1.07 (95% CI 1.02 to 1.11) for each additional pound of weight gain.
TABLE 2.
Conditional Logistic Regression Models of Heart Failure Hospitalization (n=240)
| Weight Gain, lbs | Case Patients, n (%) | Control Patients, n (%) | Matched Unadjusted OR (95% CI) | Matched Adjusted OR (95% CI) | Adjusted P |
|---|---|---|---|---|---|
| ≤2 | 65 (54) | 92 (77) | Reference group | … | … |
| >2 up to 5 | 21 (18) | 16 (13) | 2.40 (1.05–5.45) | 2.77 (1.13–6.80) | 0.026 |
| >5 up to 10 | 17 (14) | 8 (7) | 3.81 (1.35–10.77) | 4.46 (1.45–13.75) | 0.009 |
| >10 | 17 (14) | 4 (3) | 5.65 (1.81–17.65) | 7.65 (2.22–26.39) | 0.001 |
Weight gain is during 1 week preceding hospitalization of case patients. Results were adjusted for comorbid conditions and the medications shown in Table 1.
Discussion
We have demonstrated that clinically important increases in body weight begin at least 1 week before hospitalization for heart failure. Moreover, during this time period, the risk of heart failure hospitalization increases in a monotonic fashion with increasing amounts of weight gain. In contrast, weight gain was not observed before hospitalization for causes other than heart failure. The present study represents basic clinical research that generates evidence to guide decision making for surveillance of weights in patients with heart failure.
The results of the present study can be viewed as a confirmation of “conventional wisdom” on weight gain preceding hospitalization for heart failure, because weight gain as a potential precipitant for hospitalization has not been demonstrated conclusively. The present results indicate that weight gain is an important risk factor for hospitalization within a relatively short time period. Prior studies have had mixed results with regard to the effectiveness of remote weight monitoring in improving outcomes for patients with heart failure.8 Because the weight-monitoring interventions in those studies were implemented with variable methods (eg, self-reported weight versus automatic transmission of weight as in the present study), frequency, and duration and with very different patient populations, a lack of consistent findings is perhaps not surprising. Although recent studies have used invasive measurements of thoracic impedance and pulmonary artery pressures,9 the published literature does not allow for direct comparisons with the present data.
Heart failure is the most frequent reason for hospitalization among older patients,10 with direct hospital costs of more than $15.4 billion each year.11 The burden that heart failure hospitalization imposes on patients and their caregivers is also substantial. The present results, which support current recommendations from professional groups about the importance of self-weighing for heart failure patients,12 indicate that clinicians have an opportunity to intervene (eg, by increasing diuretic doses) and possibly avert hospitalization.
The “real-world” applicability of the results of the present study is reflected by data that come from actual patients participating in a home monitoring system, rather than a clinical trial with strict inclusion-exclusion criteria and complex follow-up procedures. Similar to heart failure patients seen in clinical practice, the majority of study participants were elderly. The validity of the present results is strengthened by the lack of association between weight gain and hospital admission for reasons other than heart failure and by the small amount of missing data on body weight. Importantly, the present analyses were matched on, and adjusted for, potential confounding factors that could have introduced bias, including baseline body weight.
Participation in the home monitoring system was voluntary, and we do not have information about the number of patients who declined participation; it is possible that the present results were affected by patients' refusal to participate. Information was not available about type of heart failure (systolic versus diastolic) or presence of renal dysfunction, and it is possible that differences in these important clinical characteristics contributed to the weight patterns observed and hospitalization rates. Recent work suggests, however, that the rate of heart failure hospitalization is similar in patients with diastolic and systolic heart failure.13,14 Most patients in the present study had NYHA class III heart failure, which reflects the distribution of heart failure severity among patients participating in the monitoring system. Patients with NYHA class I and II heart failure may not have been deemed sick enough to justify the cost of the monitoring system, whereas those with NYHA class IV heart failure may have been deemed too sick to benefit from this system. Whether the present results may be generalized to patients with mild or severe heart failure is therefore uncertain.
Information was not available on how clinicians used the weight data, including attempts that may have been made to address weight gain (such as increasing diuretic dosage). It is possible that patients who experienced a significant weight gain but were not yet ill enough to require hospitalization had an increase in their diuretic dosage or received some other type of intervention. Such interventions would, however, bias the study toward a null finding. Alternatively, it is possible that weight gain played a role in the decision for hospitalization, but it seems unlikely that clinicians would hospitalize patients solely because of weight gain, without other signs and symptoms of clinical decompensation. Whatever impact clinicians' review of these data may have had on the results, significant differences between case and control patients in weight change were still observed. It is probable that the association between weight gain and hospitalization would be even stronger for patients in whom weight data were not being reviewed, and responded to with intervention, by clinicians.
The outcome in the present study (ie, heart failure hospitalization), was assessed by a nurse employed by Alere, Inc, during telephone interviews with patients. We do not have data to validate the accuracy of the outcome assessment, but a recent report suggests that concordance between self-reported and claims-based hospitalizations is high.15 Although all patients using the monitoring system were contacted via telephone by Alere staff to assess the outcomes used in the present study, we do not have data on the completeness of follow-up (ie, how often patients could not be reached to ascertain the outcome).
These data demonstrate that, on average, weight gain begins in a gradual fashion at least 1 week before heart failure hospitalization, but the present findings do not indicate that all admissions are preceded by weight gain. Some patients would have certainly experienced sudden decompensation with “flash” edema. For these episodes of decompensation, remote monitoring may prove less effective in averting hospitalization. In addition to weight gain, patients with heart failure may experience a range of other symptoms as manifestations of heart failure decompensation, including shortness of breath, fatigue, and leg swelling. The relative prognostic utility of such symptoms compared with body weight should be investigated in future studies.
Frequent monitoring of heart failure patients' clinical status, specifically their body weights, can alert clinicians to the early stages of heart failure decompensation. By focusing on weight changes, clinicians would be well positioned to implement interventions that could prevent decompensation of heart failure that necessitates hospitalization. To determine whether this type of monitoring system can improve outcomes, 2 study authors (SIC and HMK) are currently conducting a multicenter randomized, controlled trial that implements daily remote monitoring of body weight. Any weight gain >2 pounds triggers clinical assessment and potential intervention, including dietary advice, medication adjustment, and clinic or emergency department visit. The intervention is being implemented and all outcomes are being assessed during the 6 months after hospitalization for heart failure decompensation. More than 1600 patients from 20 sites across the country will be enrolled, which makes this one of the largest studies of remote monitoring to date. Systems to facilitate frequent monitoring of heart failure patients' clinical status have potential for improving the care and outcomes of patients with heart failure, but the emerging field of remote monitoring must be informed by evidence to guide clinicians in decision making.
Clinical Perspective.
Systems to enhance remote monitoring of patients' clinical status have the potential to improve the care and outcomes of patients with heart failure. Weight gain has long been recognized by clinicians as a marker of heart failure decompensation, but little information is available to quantify the relationship between weight change in patients with heart failure and the risk for imminent hospitalization. Accordingly, we evaluated data from a group of heart failure patients who were weighing themselves daily as part of a disease-management remote-monitoring system. Using a nested case-control study design, we matched 134 case patients with heart failure hospitalization to 134 control patients without heart failure hospitalization. The results demonstrate that increases in body weight are associated with hospitalization for heart failure and begin at least 1 week before admission. Within the week before hospitalization, the risk of heart failure hospitalization increases in a monotonic fashion with increasing amounts of weight gain. Any weight gain of >2 pounds is associated with increased risk of heart failure hospitalization. Our results indicate that weight gain is an important risk factor for hospitalization within a relatively short time period. Daily information about patients' body weight can alert clinicians to patients who are at high risk for hospitalization on the basis of weight gain. Equipped with this information, clinicians can implement interventions to try to avert hospitalization.
Acknowledgments
Sources of Funding: Dr Krumholz is currently the principal investigator of a National Heart, Lung, and Blood Institute–funded R01 (R01-HL080228) examining telemonitoring in patients with heart failure, which provides salary support for Dr Chaudhry. Dr Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
Footnotes
Guest Editor for this article was Gary Balady, MD.
Disclosures: Alere Medical Inc provided the data but had no role in the design and conduct of the study; management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr Krumholz serves on the Advisory Board for Alere Medical Inc but does not have equity in the company. Dr Krumholz has research contracts with the Colorado Foundation for Medical Care and the American College of Cardiology, serves on the advisory boards for Amgen and UnitedHealthcare, is a subject matter expert for VHA, Inc, and is editor-in-chief of Journal Watch Cardiology of the Massachusetts Medical Society. The remaining authors report no conflicts.
References
- 1.Chumbler NR, Vogel WB, Garel M, Qin H, Kobb R, Ryan P. Health services utilization of a care coordination/home-telehealth program for veterans with diabetes: a matched-cohort study. J Ambul Care Manage. 2005;28:230–240. doi: 10.1097/00004479-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Cavallerano AA, Cavallerano JD, Katalinic P, Blake B, Rynne M, Conlin PR, Hock K, Tolson AM, Aiello LP, Aiello LM. A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center: the Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro SE, Izumi S, Tanner CA, Moscato SR, Valanis BG, David MR, Gullion CM. Telephone advice nursing services in a US health maintenance organization. J Telemed Telecare. 2004;10:50–54. doi: 10.1258/135763304322764202. [DOI] [PubMed] [Google Scholar]
- 4.Super N. Medicare's Chronic Care Improvement Pilot Program: What Is Its Potential? Washington, DC: National Health Policy Forum; May 10, 2004. NHPF Issue Brief, No. 797. [PubMed] [Google Scholar]
- 5.Pulignano G, Del Sindaco D, Tavazzi L, Lucci D, Gorini M, Leggio F, Porcu M, Scherillo M, Opasich C, Di Lenarda A, Senni M, Maggioni AP. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry) Am Heart J. 2002;143:45–55. doi: 10.1067/mhj.2002.119608. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 7.Breslow NE, Day NE. Statistical methods in cancer research: volume I: the analysis of case-control studies. IARC Sci Pub. 1980;32:5–338. [PubMed] [Google Scholar]
- 8.Chaudhry SI, Phillips CO, Stewart SS, Riegel B, Mattera JA, Jerant AF, Krumholz HM. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail. 2007;13:56–62. doi: 10.1016/j.cardfail.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjellstrom B, Igel D, Abraham J, Bennett T, Bourge R. Trans-telephonic monitoring of continuous haemodynamic measurements in heart failure patients. J Telemed Telecare. 2005;11:240–244. doi: 10.1258/1357633054471795. [DOI] [PubMed] [Google Scholar]
- 10.Croft JB, Giles WH, Pollard RA, Casper ML, Anda RF, Livengood JR. National trends in the initial hospitalization for heart failure. J Am Geriatr Soc. 1997;45:270–275. doi: 10.1111/j.1532-5415.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 11.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics: 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 14.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–1518. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 15.Wolinsky FD, Miller TR, An H, Geweke JF, Wallace RB, Wright KB, Chrischilles EA, Liu L, Pavlik CB, Cook EA, Ohsfeldt RL, Richardson KK, Rosenthal GE. Hospital episodes and physician visits: the concordance between self-reports and Medicare claims. Med Care. 2007;45:300–307. doi: 10.1097/01.mlr.0000254576.26353.09. [DOI] [PMC free article] [PubMed] [Google Scholar]