Abstract
The heparan sulfate (HS) proteoglycan perlecan is a major component of basement membranes, plays a key role in extracellular matrix (ECM) structure, interacts with growth factors and adhesion molecules, and regulates the adhesion, differentiation and proliferation of vascular cells. Atherosclerosis is characterized by chronic inflammation and the presence of oxidized materials within lesions, with the majority of protein damage present on ECM, rather than cell, proteins. Weakening of ECM structure plays a key role in lesion rupture, the major cause of heart attacks and strokes. In this study peroxynitrite, a putative lesion oxidant, is shown to damage perlecan structurally and functionally. Exposure of human perlecan to peroxynitrite decreases recognition by antibodies raised against both the core protein and heparan sulfate chains; dose-dependent formation of 3-nitrotyrosine was also detected. These effects were modulated by bicarbonate and reaction pH. Oxidant exposure resulted in aggregate formation, consistent with oxidative protein crosslinking. Peroxynitrite treatment modified functional properties of perlecan that are dependent on both the protein core (decreased binding of human coronary artery endothelial cells), and the HS chains (diminished fibroblast growth factor-2 (FGF-2) receptor-mediated proliferation of Baf-32 cells). The latter is consistent with a decrease in FGF-2 binding to the HS chains of modified perlecan. Immunofluorescence of advanced human atherosclerotic lesions provided evidence for the presence of perlecan and extensive formation of 3-nitrotyrosine epitopes within the intimal region; these materials showing marked co-localization. These data indicate that peroxynitrite induces major structural and functional changes to perlecan and that damage to this material occurs within human atherosclerotic lesions.
Abbreviations: ABTS, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); dONOO, decomposed peroxynitrite; ECM, extracellular matrix; FGF-2, fibroblast growth factor 2; HCAEC, human coronary artery endothelial cells; HS, heparan sulfate; HSPG, heparan sulfate proteoglycan; MTT, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan; 3-nitroTyr, 3-nitrotyrosine; ONOO-, peroxynitrous acid anion; ONOOH, peroxynitrous acid; TCA, trichloroacetic acid
Keywords: Atherosclerosis, Extracellular matrix, Perlecan, Peroxynitrite, Heparan sulfate proteoglycans, Plaque rupture, Cell adhesion, Cell proliferation, Inflammation
Introduction
Perlecan is a large multi-domain heparan sulfate proteoglycan (HSPG), which is found in basement membranes and the pericellular environment [1]. The perlecan protein core (mass 470 kDa) consists of five distinct domains. Domains II – V display homology to the low-density lipoprotein receptor, laminin A chain, neuronal cell adhesion molecule and epidermal growth factor [2]. The N-terminal domain, domain I, is the main region of glycosaminoglycan substitution, with a cluster of three attachment sites; an additional two potential attachment sites exist on domain V [3,4]. Endothelial cell-derived perlecan is substituted exclusively by heparan sulfate (HS) at the domain I attachment sites [5]; chondroitin sulfate and keratan sulfate variants have also been isolated from chondrogenic and epithelial cell sources [6]. Perlecan is the major HSPG expressed in the vascular wall and plays a key role in vascular homeostasis via the stabilization and organization of vascular extracellular matrix (ECM), and the regulation of adhesion, differentiation and proliferation of vascular cells [4,7]. These functions are mediated by interactions of its protein core and HS chains with a variety of extracellular matrix molecules, growth factors and adhesion molecules. Homozygous-null mutations in the perlecan gene are embryonically lethal and associated with cardiovascular malformations and rupture of the heart and large blood vessels [8].
Atherosclerosis is a multi-factorial disease characterized by the accumulation of lipids in the artery wall and chronic inflammation [9]. Lesions contain large numbers of activated monocytes and macrophages that are capable of generating reactive oxidants, and it has been demonstrated that human atherosclerotic lesions, of all degrees of severity, contain oxidized lipids and proteins. The levels of these materials are significantly elevated, and antioxidant levels significantly decreased, compared to healthy tissue (reviewed [10]). Previous studies have shown that the majority of oxidative damage to proteins detected in atherosclerotic lesions is associated with ECM, rather than intracellular, proteins [11]. The contribution of oxidative damage to vascular ECM to the progression of atherosclerosis is currently unclear, however it may be of considerable importance. Modification of the ECM of the artery wall is believed to play a key role in both the development of atherosclerosis [12], and the subsequent rupture of lesions, which is the major cause of heart attacks and strokes [13]. The composition of the ECM within the artery wall alters during lesion development, and is markedly different at site of lesion erosion and rupture, with alterations in the type and distribution of proteoglycans, collagens, and hyaluronan [14–16]. These changes in composition and properties may contribute to endothelial cell loss and dysfunction, altered vascular smooth muscle cell phenotypes and a decrease in the mechanical stability of the lesion cap, thereby increasing the propensity of lesions to undergo rupture. Notably, perlecan has been shown to play a key role in modulating smooth muscle cell proliferation and migration, a key feature of developing lesions and a major response to vascular injury [17].
Considerable evidence supports a role for peroxynitrite (ONOO-/ONOOH; this equilibrium mixture is termed peroxynitrite from hereon) in the initiation of oxidation in atherosclerotic lesions and ECM modification (reviewed in [18]). Peroxynitrite is generated as a result of the diffusion-controlled reaction (k ca. 7 × 109 M-1 s-1 [19]) of superoxide anion radicals (O2•-; generated by the respiratory burst of activated leukocytes) with nitric oxide radical (•NO, generated by the inducible nitric oxide synthase enzyme of macrophages) [20]. Other sources of O2•- and •NO present at sites of inflammation, such as in atherosclerotic lesions that may also contribute to peroxynitrite formation, include the activities of xanthine oxidase [21] and endothelial nitric oxide synthase [22]. Considerable data supports an increased rate of generation of these radicals, and hence peroxynitrite, at sites of inflammation, including within the diseased artery wall [20]. Thus extensive antibody staining for 3-nitrotyrosine (3-nitroTyr), a stable modification to Tyr residues formed on exposure to peroxynitrite and other reactive nitrogen species, has been detected throughout human atherosclerotic lesions [23], and high- and low-density lipoproteins isolated from atherosclerotic lesions contain elevated levels of 3-nitroTyr when compared to circulating lipoproteins [24,25].
Perlecan is likely to be an important target for damage by peroxynitrite in the vascular wall, however the effects of this oxidant on its structure and function are unknown. Data obtained with isolated glycosaminoglycans, and intact ECM, indicate that HS chains of perlecan are potential targets for modification and fragmentation by peroxynitrite. Thus, peroxynitrite can fragment isolated glycosaminoglycan chains (e.g. [26–28]), with this occurring in a site-specific manner as a result of damage being induced by both hydroxyl (HO.) and carbonate (CO3-.) radicals (but not to any great extent by the peroxynitrite anion or nitrogen dioxide radical; NO2.) [27,28]. Treatment of cell culture-derived matrix or isolated arterial matrix with peroxynitrite results in the release of matrix fragments that include both protein and carbohydrate components [29], consistent with damage to perlecan. Concomitant generation of 3-nitroTyr in ECM proteins was observed [29], however the susceptibility of perlecan to this modification and the potential roles of protein versus HS modification in the degradation of this target is unclear. Perlecan is also implicated as a target for the myeloperoxidase-derived oxidants HOCl and HOBr [11,30,31], and recent studies have established that HOCl can selectively modify and functionally impair the cell adhesive function of the protein core, without impairing the ability of its heparan sulfate chains to promote FGF-2-dependent cellular proliferation [32].
To further elucidate the potential role of damage to perlecan by peroxynitrite in vascular disease, the mechanisms and functional consequences of exposure of human arterial endothelial cell-derived perlecan (the major proteoglycan component of the arterial subendothelial matrix), to peroxynitrite have been examined in detail. In particular, these studies have sought to resolve the role of damage to the protein versus the HS chains, the potential role of bicarbonate and reaction pH in modulating these reactions, and whether such oxidant damage alters the biological activities of this important vascular macromolecule.
Materials and Methods
Chemicals
Solutions were prepared using water purified through a four-stage Milli Q system (Millipore-Waters) treated with washed Chelex resin (Bio-Rad) to remove contaminating trace metal ions. pH control was achieved using phosphate buffer (0.1 M), with pH adjustments made using sodium monophosphate (0.1 M), sodium diphosphate (0.1 M), or small quantities of concentrated HCl or NaOH.
Peroxynitrite was synthesized as previously [33]. Stock concentrations were determined spectrophotometrically using ε302 nm 1670 M-1 cm-1 [33]. Stock solutions of peroxynitrite anion (pH ca. 12) were prepared by dilution of the synthesized material into 0.1 M NaOH. Due to the high concentration and pH of the stock solutions, small volumes were added to strongly buffered solutions to minimize pH changes; the reported pH values are determined for the final reaction mixtures. “Decomposed” peroxynitrite (dONOO) was prepared by incubation overnight at 37 °C in 0.1 M phosphate buffer, pH 6.
Cell culture
Human coronary artery endothelial cells (HCAECs) were cultured in HCAEC Growth Medium (Cell Applications) at 37 °C in a humidified atmosphere containing 5% CO2. Cell-conditioned medium was aspirated from near confluent flasks of cells and stored at -80 °C, for subsequent perlecan purification. For cell adhesion studies, cells were cultured to approximately 90% confluence, washed with PBS (pH 7.4) containing 1 mM MgCl2/1 mM CaCl2 then incubated with 1% trypsin for 3 min at 37 °C. Cell-conditioned media was added to neutralize trypsin. Cells were washed twice with Medium-199 (Sigma) containing 1% BSA and resuspended in Medium-199 containing 1% BSA at a cell concentration of 2.5 × 105 cells mL-1.
BaF3 cell culture
The ability of perlecan to promote cellular proliferation in response to exogenous fibroblast growth factor-2 (FGF-2) was investigated using a heparan sulfate proteoglycan-deficient myeloid cell line (BaF3) expressing the FGF receptor isotype 1c (FGFR1c), essentially as described previously [34]. Briefly, BaF3 cells (105 cells mL-1) were incubated in RPMI-1640 medium with perlecan (1.25 nM) or heparin (0.3 – 30 nM) in the presence of FGF-2 (0.3 nM) for 72 h at 37 °C and proliferation was then determined by the MTT assay using CellTiter 96® AQueous One Solution (10% v/v, 6 h, 37 °C).
Perlecan isolation and purification
Perlecan was purified from HCAEC conditioned medium by DEAE chromatography followed by anti-perlecan (CSI-071, AbCam) affinity chromatography as described previously [5,34,35]. Perlecan core protein concentrations were determined using a Coomassie Plus microplate assay with BSA standards. Perlecan concentrations were calculated using a core protein molecular mass of 470 kDa.
Oxidant and endoglycosidase treatment of surface-adsorbed and soluble perlecan
Perlecan (10 - 32 nM, 4.7 μg mL-1, 50 μL) was absorbed from PBS onto wells of 96-well microtitre plates (high-binding polystyrene, Greiner BioOne; or polyvinyl chloride, BD Biosciences) for 16 h at 22 °C. This surface-adsorbed material was incubated with fresh and decomposed peroxynitrite (1 – 10 μM) for 20 min at 22 °C. Oxidant/perlecan molar ratios in these experiments were 100 – 1000 (equivalent to 0.21 - 2.1 μmoles oxidant per mg protein) with surface absorption assumed to be quantitative. Enzymatic digestion of perlecan was carried out using heparinase III (0.025 U mL-1; Ibex) in PBS, pH 7.2, for 16 h at 37 °C. Soluble perlecan (100 – 300 nM) was subject to oxidation by peroxynitrite, and enzymatic digestion with heparinase III, in a similar manner.
Enzyme-linked immunosorbent assays (ELISAs)
Wells of microtitre plates containing surface absorbed perlecan were blocked with 0.1% casein in PBS and probed with mouse monoclonal antibodies (mAbs) against HS (HepSS-1, 2 μg mL-1; 10E4, 2 μg mL-1; JM403, 4 μg mL-1; Seikagaku), perlecan domain I (CSI-076, 3.3 μg mL-1; AbCam), perlecan domain III (7B5, 2 μg mL-1; Zymed Laboratories) perlecan domain V (CSI-074, 2 μg mL-1; AbCAm); and 3-nitroTyr (HM11, 2 μg mL-1, Zymed Laboratories). Detection of the immune complexes was performed using a biotinylated anti-mouse secondary antibody (1:1000, GE Biosciences) with streptavidin-conjugated horseradish peroxidase (1:500, GE Biosciences) and ABTS (KPL).
Measurement of protein carbonyls
Protein carbonyl formation was measured using a Protein Carbonyl Enzyme Immuno-Assay kit (Biocell Laboratories Inc.). Samples of soluble perlecan treated with peroxynitrite were concentrated by trichloroacetic acid precipitation, and derivatized with 2,4-dinitrophenylhydrazine (DNPH) prior to measurement of protein carbonyls by ELISA as described previously [36].
SDS-PAGE and immunoblotting
SDS-PAGE was carried out using 3-8% NuPAGE® Tris acetate gels according to the manufacturer's instructions (Invitrogen), 2 μg protein was loaded per well and HiMark® molecular mass standards (Invitrogen) were used for reference. Gels were visualized using a combined stains all-silver staining method [37].
For Western blotting studies, proteins were electroblotted onto nitrocellulose membranes using an iBlot® transfer apparatus (Invitrogen). Membranes were blocked with 1% casein in PBS / 0.1% Tween then probed with appropriate mouse mAbs (CSI-076, 2 μg mL-1; 10E4, 0.5 μg mL-1; 3-nitroTyr HM11, 1 μg mL-1). Detection of immune complexes was performed using horseradish peroxidase-conjugated anti-mouse Ig antibodies, ECL reagents and a ChemiDoc XRS image acquisition system.
Cell adhesion assay
Adhesion of endothelial cells to polystyrene microtitre plates coated with perlecan (20 nM, 9.4 μg mL-1) or fibronectin (20 nM, 10 μg mL-1) was quantified after 2 h incubation with HCAECs (2.5 × 105 cells mL-1, 50 μL) at 37 °C by staining with crystal violet as described previously [5].
Cell proliferation assay
Proliferation of Baf-32 cells was monitored using the MTT assay. Briefly Baf-32 cells in RPMI medium (4 × 105 cells mL-1, 200 μL) were mixed with native perlecan, or that subjected to oxidation by peroxynitrite at oxidant/perlecan molar ratios of 100 and 1000 for 20 min at 22 °C (5 nM, 200 μL) and 0.3 nM FGF-2 in RPMI 1640 medium (400 μL), plated onto a 96-well plate for 72 h at 37 °C in a humidified atmosphere containing 5% CO2. MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) and an electron coupling agent, phenazine methosulfate were added to each well and incubated for 6 h at 37 °C. The absorbance at 490 nm was measured and used to calculate cell proliferation.
FGF-2 binding
Binding of [125I]-labeled FGF-2 (50 μl; ca. 120 000 cpm well-1) to surface-absorbed perlecan (32 nM, 15 μg mL-1) was quantified by gamma counting as described previously [5].
Analysis of human atherosclerotic lesions
Aortae and aortae abdominalis were obtained from three autopsy subjects who died of cerebral hemorrhage. The morphology of the aortae ranged from microscopically normal to pronounced atheroma containing calcium inclusions; the lesions were classified as types I/II and III/IV using the Stary classification system [38]. For classification of lesion types, lesions were stained with Oil-red O. The samples were obtained within 12 h of death, as described previously [39] and immediately frozen in a cryostat (Microm, Walldorf, Germany, Microm HM 500 OM), supported by tissue freezing medium (Tissue Tec OCT-compound, Miles, Elkhard, Ind., USA). Serial cryosections (5 μm) were collected on glass slides, air dried for 2 h at 21 °C, fixed in acetone for 5 min at 21 °C and stored at -40 °C until analyzed [40]. The following monoclonal or polyclonal antibodies were used as primary antibodies: anti-nitrotyrosine (rabbit IgG, Millipore, 1:75 dilution), anti-human perlecan (mouse mAb, clone 7B5, perlecan domain III, Zymed Laboratories, 200 µg/ml, 1:10 dilution) anti-human CD14 (mouse mAb recognizing monocytes/macrophages, Serotec, 1:50 dilution), non-immune rabbit IgG (Sigma) and non-immune mouse IgG (Sigma). The following polyclonal antibodies (Jackson Dianova) were used as detection antibodies: goat anti-rabbit cyanine-3 (Cy-3)-labelled IgG (1:300 dilution) and goat anti-mouse Cy-2-labeled IgG (1:300 dilution).
For double immunofluorescence, the sections were re-hydrated in PBS (pH 7.4), blocked with UV ultra block (Lab Vision) for 10 min and incubated for a further 30 min with anti-nitrotyrosine antibody diluted with antibody diluent (Dako). The samples were then rinsed in PBS, and incubated with a Cy-3-labeled goat anti-rabbit antibody for 30 min. The sections were subsequently incubated with antibodies against perlecan or CD14 followed by incubation with Cy-2-labeled goat anti-mouse antibody [41]. The sections were incubated with DAPI (Partec; 1:1000 dilution) for 5 min, mounted with Moviol (Calbiochem-Novabiochem, La Jolla, USA) and analyzed using a confocal laser scanning microscope in sequential mode (Leica SP2, Leica Lasertechnik GmbH, Heidelberg, Germany), using the 405 nm laser line for the excitation of DAPI, the 488 nm laser line for the excitation of Cy-2, and the 543 nm line for Cy-3 respectively. Detection emission settings were: 410 - 470 nm (blue staining) for DAPI, 500 - 540 nm (green staining) for Cy-2 and 560 - 660 nm (red staining) for Cy-3. All incubation steps were performed in a dark moist chamber at 21 °C. Controls experiments were performed by omission of the primary antibodies or by replacing the primary antibodies by non-immune mouse IgG or normal rabbit IgG.
Computational modeling of the reaction of CO3•- with the protein core and HS chains of perlecan
Modeling of the reaction of CO3•- with perlecan was carried out using the SimBiology feature of Matlab (The Math Works Inc, Natick, MA) using published abundance data for the amino acid and HS composition of perlecan [42] and the following second-order rate constants (M-1 s-1 at 22 °C, pH 7): 7 × 105 for disaccharide subunits of heparan sulfate (based on data for the related polymer hyaluronan [43]), 5.0 × 106 for Tyr (based on second order rate constants for reaction of CO3•- with phenol [44]), 7.2 × 108 for Trp (data for Gly-Trp [45]), 3.6 × 107 for Met [45], 4.3 × 106 for His (data for Gly-His [45]), 1.3 × 106 for cystine (based on data for GSSG [45]) and 9 × 104 for Arg [45]. Rate constants for reaction of CO3•- with other amino acids are < 1 × 104 M-1 s-1 [45] and were not incorporated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism, PC version 4 (GraphPad Software). One- and two-way ANOVA were performed with post-hoc statistical analysis using the Tukey's multiple comparison test and Bonferroni post-tests, respectively. Statistical significance was assumed at P < 0.05.
Results
Modification of the core protein and HS chains of intact perlecan by peroxynitrite
Perlecan was purified from HCAEC and the presence of a full-length protein core (470 kDa) and exclusive substitution by HS was confirmed as described previously [32]. The effects of exposure of perlecan to peroxynitrite was explored by examining both the alteration in the binding of antibodies that recognize specific epitopes on the protein core (domains I, III and V) and the HS chains, and specific oxidation products (3-nitroTyr and carbonyls) that may result from oxidative damage. Studies were carried out at both pH 7.4 and lower pH values (6, 6.5 and 7) that mimic those found at sites of inflammation [46,47].
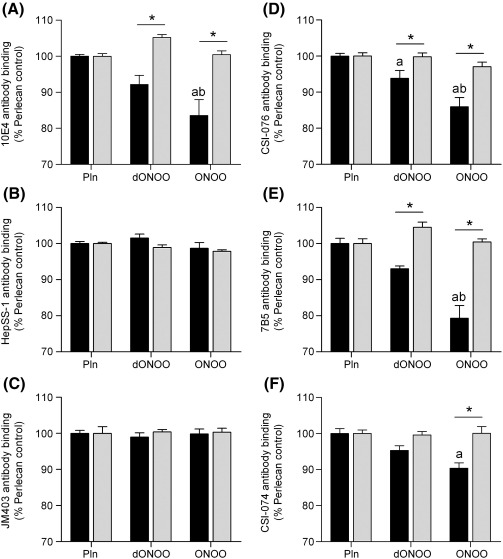
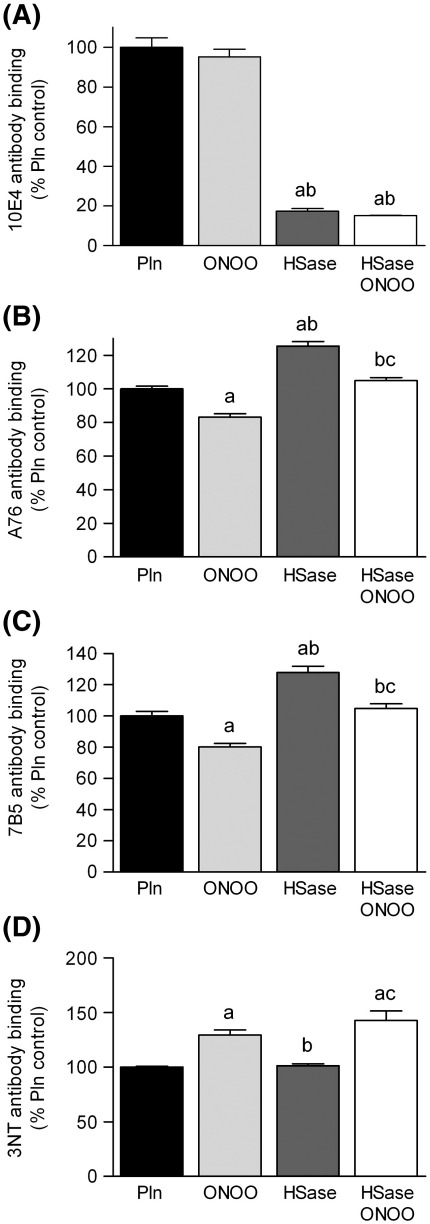
Treatment of surface-adsorbed perlecan with peroxynitrite at a 1000-fold molar excess over the perlecan protein concentration (2.1 μmol oxidant per mg protein) at pH 6.5 caused a significant loss (ca. 20%) in recognition of HS by antibody 10E4 whilst decomposed peroxynitrite (dONOO) did not (Fig. 1A). In contrast, this oxidant treatment did not impair recognition of HS by antibodies JM403 and HepSS-1, which recognize different HS structures to antibody 10E4 (Figs. 1B, C). A significant loss of epitope recognition (10 – 20%) was also observed with the peroxynitrite-treated perlecan using antibodies raised against core protein domain I (CSI-076, Fig. 1D), domain III (7B5, Fig. 1E) and domain V (CSI-074, Fig. 1F). A significant loss in domain I epitope recognition was also observed with dONOO, though the magnitude of this effect was significantly less than with the active oxidant (Fig. 1D). This may be due to the formation of HNO2 on peroxynitrite decomposition; this compound is known to induce slow nitrosative deamination of amine functions on proteins [48]. As the anion form of peroxynitrite reacts rapidly with CO2 to give an adduct species (ONOOCO2-) with this potentially modulating in vivo reactions [49], studies were also carried out in the presence of bicarbonate (NaHCO3, 25 mM, a concentration that results in physiological CO2 levels). Inclusion of this material in the incubation medium abrogated the effects of dONOO and peroxynitrite on recognition of HS and protein core by all the antibodies investigated (Fig. 1).
Fig. 1.
Effect of peroxynitrite exposure on the recognition of native epitopes on perlecan by monoclonal antibodies. Surface absorbed perlecan (10 nM) was treated with buffer (Pln), decomposed peroxynitrite (dONOO, 10 μM) or peroxynitrite (ONOO, 10 μM) at 22 °C for 20 min in 0.1 M phosphate buffer, pH 6.5 in the absence (black bars) and presence (light grey bars) of NaHCO3 (25 mM). The perlecan was then probed by ELISA using monoclonal antibodies (mAbs) against HS epitopes: (A) mAb 10E4; (B) mAb HepSS-1; (C) mAb JM403 and protein core epitopes: (D) perlecan domain I (mAb CSI-076); (E) perlecan domain III (mAb 7B5); and (F) perlecan domain V (mAb CSI-074). Data are expressed as a percentage of the untreated proteoglycan ELISA signal, and are means ± SEM of values obtained from triplicate wells from n ≥ 3 independent experiments. Data were analyzed by 2-way ANOVA, with statistical significance assumed at p < 0.05; a different to perlecan control, b different to dONOO, * significantly different to experiments carried out in the absence of NaHCO3.
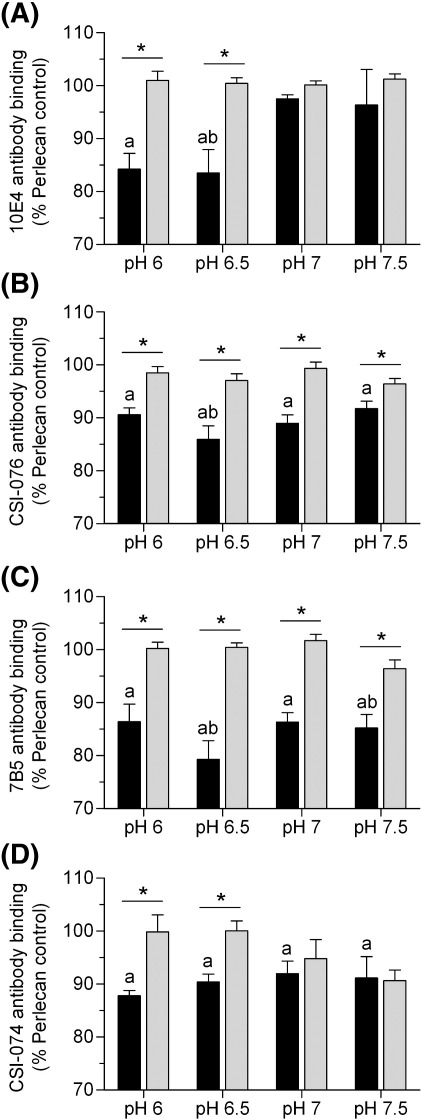
As the chemistry of peroxynitrite (and formation of downstream reactive products such as HO., CO3-. NO2. and HNO2) is influenced by pH, the effects of reaction pH (6 – 7.5) on antibody recognition of perlecan exposed to a 1000-fold molar excess of peroxynitrite were investigated to mimic conditions at sites of inflammation (Fig. 2). Peroxynitrite caused a significant decrease in recognition of HS by antibody 10E4 at both pH 6 and pH 6.5, but at higher values no changes in antibody binding were observed (Fig. 2A), and no changes in recognition were observed with the other HS antibodies (JM403 and HepSS-1) at any of the pH values tested (data not shown). In contrast, loss of recognition of the protein core was observed at all pH values with the antibodies against domain I (Fig. 2B), domain III (Fig. 2C) and domain V (Fig. 2D). The greatest loss in antibody binding occurring at pH 6.5 for domain I and III and at pH 6 for domain V. The presence of bicarbonate (25 mM) abrogated the changes in antibody binding induced by peroxynitrite at all the pH values examined.
Fig. 2.
Effect of reaction pH on peroxynitrite-induced modifcation of perlecan. Surface absorbed perlecan (10 nM) was treated with peroxynitrite (10 μM) at 22 °C for 20 min in 0.1 M phosphate buffer, pH 6 – 7.5 in the absence (black bars) and presence (light grey bars) of NaHCO3 (25 mM). The perlecan was then probed by ELISA using mAbs against HS epitopes; (A) mAb 10E4 and protein core epitopes; (B) perlecan core (mAb CSI-076), (C) perlecan domain III (mAb 7B5) and (D) perlecan domain V (mAb CSI-074). Data are expressed as % normal ELISA signal and are means ± SEM of values obtained from triplicate wells with n ≥ 3. Data were analyzed by 2-way ANOVA, with statistical significance assumed at p < 0.05, a different to perlecan control, b different to dONOO, * effect of NaHCO3.
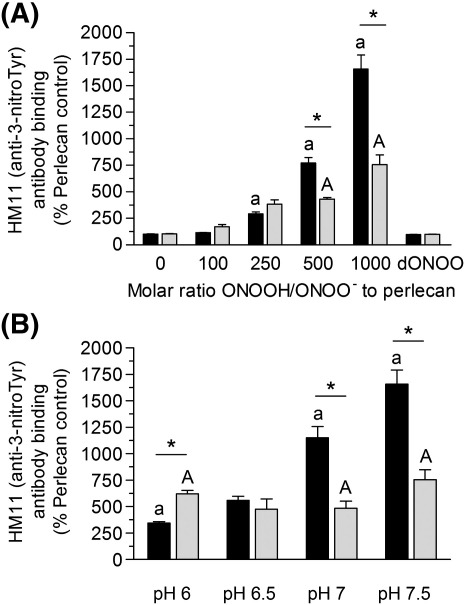
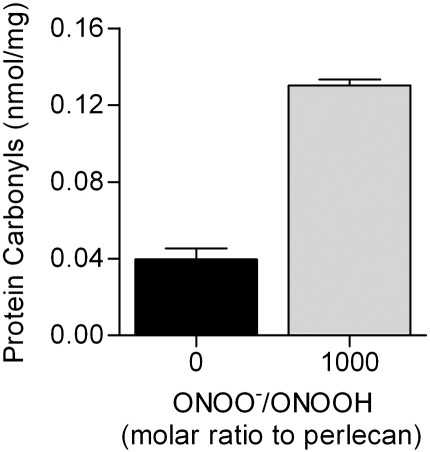
3-NitroTyr, a long-lived product arising from oxidation of Tyr residues by reactive nitrogen species, is widely used as a marker of peroxynitrite exposure [50]. Exposure of surface-adsorbed perlecan to 100 – 1000-fold molar excesses of peroxynitrite at pH 7.5, resulted in a concentration-dependent increase in 3-nitroTyr formation as detected by ELISA (Fig. 3A). Only background levels of 3-nitroTyr were detected with dONOO. In the presence of bicarbonate (25 mM), exposure to increasing concentrations of peroxynitrite resulted in a concentration-dependent increase in 3-nitroTyr levels, however the levels of this product were significantly lower than in the absence of bicarbonate (Fig. 3A). The yield of 3-nitroTyr on perlecan after exposure to a 1000-fold molar excess of peroxynitrite increased with pH, with a 3-fold increase above background detected at pH 6, and a 17-fold increase at pH 7.5 (Fig. 3B). Inclusion of bicarbonate (25 mM) resulted in higher 3-nitroTyr levels compared to its absence at pH 6, whilst at pH 7 and above this trend was reversed (Fig. 3B). Similar levels of 3-nitroTyr were detected (5 – 7.5 fold higher than background) across the pH range in the presence of 25 mM NaHCO3 (Fig. 3B). Exposure of perlecan to a 1000-fold molar excess of peroxynitrite at pH 7 also resulted in elevated levels of protein carbonyls (ca. 3-fold greater), a generic marker of oxidative damage, compared to native protein (Fig. 4).
Fig. 3.
Formation of the tyrosine oxidation product 3-nitroTyr on perlecan exposed to peroxynitrite: (A) effect of oxidant concentration, and (B) effect of reaction pH. Surface absorbed perlecan (10 nM) was treated in the absence (black bars) and presence (light grey bars) of NaHCO3 (25 mM) for 20 min at 22 °C with (A) 0.1 M phosphate buffer, pH 7.5 containing 1 – 10 μM peroxynitrite (100 – 1000 molar excess of oxidant over protein) or dONOO (10 μM) or (B) 0.1 M phosphate buffer, pH 6 – 7.5 containing 10 μM peroxynitrite. The perlecan was then probed by ELISA using a mAb against 3-nitroTyr (HM11). Data are expressed as a % of the control ELISA signal obtained with untreated perlecan and are means ± SEM of values obtained from triplicate wells with n ≥ 3. The absolute absorbance (at 405 nm) for the controls with no perlecan were ∼ 0.055, and for native perlecan ∼ 0.09; those for the peroxynitrite-treated perlecan were up to 1.6 absorbance units. These data indicate that the level of 3-nitroTyr on the native perlecan was very low. Data were analyzed by 2-way ANOVA, with statistical significance assumed at p < 0.05; a/A: different to native perlecan in the absence or presence of NaHCO3 respectively, * effect of NaHCO3.
Fig. 4.
Formation of protein carbonyls, a generic marked of protein oxidation, on perlecan exposed to peroxynitrite. Perlecan (100 nM) was exposed to peroxynitrite (100 μM) in 0.1 M phosphate buffer, pH 7 for 20 min at 22 °C. Protein carbonyl formation was measured by ELISA.
Structural consequences of perlecan-modification by peroxynitrite
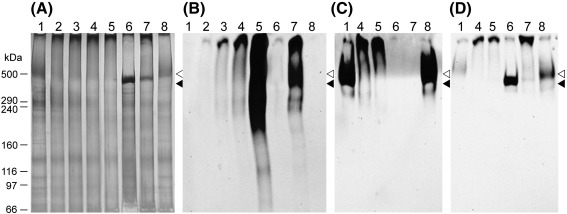
Perlecan migrates as a diffuse band at ≥ 470 kDa on 3-8% NuPAGE gels as detected by both Stains-all/silver staining (Fig. 5A; lane 1) and recognition by the protein core domain III antibody 7B5 and the HS antibody 10E4 (Figs. 5B, C; lane 1). Exposure of perlecan to increasing molar excesses of peroxynitrite at pH 7 resulted in the formation of aggregated material that only migrated slightly into the gel, consistent with oxidative crosslinking of the protein core (Fig. 5A, lanes 2-4). These materials were also recognized by protein core and HS antibodies (Figs. 5B, C; lane 4). In contrast, perlecan treated with dONOO showed similar electrophoretic mobility to the untreated material (Figs. 5A, B, C; lane 8). Western blot analysis using the 3-nitroTyr antibody showed that peroxynitrite treatment induced a dose-dependent increase in antibody staining (Fig. 5D; lanes 2-4), with this predominantly observed at the same gel position as the aggregated protein (Fig. 5A; lanes 2-4). No staining for 3-nitroTyr was detected on treatment of perlecan with dONOO (Fig. 5B, lane 8). In the presence of bicarbonate the extent of recognition of 3-nitroTyr was significantly increased, with the antibody recognizing material with a broad range of molecular masses (Fig. 5D; lane 4 vs. lane 5).
Fig. 5.
Structural consequences of perlecan modification by peroxynitrite. Perlecan (330 nM) in 0.1 M phosphate buffer, pH 7 (lane 1) was exposed for 20 min at 22 °C to peroxynitrite at molar ratios of 250 (lane 2), 500 (lane 3), 1000 (lane 4), 1000 in the presence of 25 mM bicarbonate (lane 5), heparinase III for 16 h at 37 °C (lane 6), heparinase III followed by 1000-fold molar excess of peroxynitrite (lane 7), and dONOO (lane 8), prior to separation on 3-8% Tris-acetate gels under reducing conditions for 1 h at 150 V and subsequent western blotting to nitrocellulose. (A) Stains-all/silver stain of gel. (B) Western blot probed for 3-nitroTyr formation with mAb against 3-nitroTyr (HM11). (C) Western blot probed for heparan sulfate epitopes with mAb 10E4. (D) Western blot probed for perlecan domain III with mAb 7B5. Position of molecular mass markers are shown for reference: ◁ perlecan heparan sulfate, ◀ perlecan protein core.
Potential role of the HS chains is modulating the extent of core protein damage induced by peroxynitrite
The potential role of the HS chains on perlecan in modifying oxidative damage was investigated by comparing native and heparinase III-treated perlecan, as the latter enzyme removes the HS moieties from the protein core. Loss of the HS chains on such treatment (16 h incubation at 37 °C) was confirmed with the HS specific antibody 10E4, with this treatment resulting in a 80% loss of recognition by the antibody compared to non-treated proteoglycan (Fig. 6A). In contrast recognition of the perlecan core protein by domain I (CSI-076) and domain III (7B5) antibodies was increased by ca. 20%, probably due to an increased exposure of epitope sites (Fisg. 6 B, C). Subsequent exposure of the heparinase III-treated perlecan to peroxynitrite (1000-fold molar excess) at pH 6.5 had no effect on recognition of perlecan by antibody 10E4, as expected (Fig. 6A), but decreased antibody recognition of the perlecan core protein domain I (antibody CSI-076) and domain III (antibody 7B5) by ca. 20% (Figs. 6B, C) with this loss in recognition being of a similar extent to that observed with the parent proteoglycan. No significant difference in 3-nitroTyr detection was observed between intact perlecan and heparinase III-treated perlecan on exposure to peroxynitrite (Fig. 6D).
Fig. 6.
Effect of removal of the heparan sulfate (HS) chains of perlecan on modification induced by peroxynitrite. Surface absorbed perlecan (10 nM, Pln) was treated with 0.025 IU mL-1 heparinase III (HSase) for 16 h at 37 °C prior to exposure to peroxynitrite (10 μM, ONOO) in 0.1 M phosphate buffer, pH 6.5 for 20 min at 22 °C. The perlecan was then probed by ELISA using (A) mAb 10E4 (B) perlecan core (mAb CSI-076), (C) perlecan domain III (mAb 7B5) and (D) 3-nitroTyr (HM11). Data are expressed as % normal ELISA signal and are means ± SEM of values obtained from 6 wells, representative data shown. Data were analyzed by 1-way ANOVA, with statistical significance assumed at p < 0.05, a different to perlecan control, b different to peroxynitrite, c different to perlecan exposed to heparinase III.
Heparinase III treatment of perlecan prior to electrophoresis resulted in a shift in the main protein band to ca. 470 kDa (Fig. 5A; lane 6), with this band recognized by the protein core domain III antibody 7B5 (Fig. 5B; lane 6) but not the HS antibody 10E4 (Fig. 5C; lane 6), This is consistent with the generation of the full-length, unsubstituted (HS-free) perlecan protein core. Exposure of the HS-free perlecan to a 1000-fold molar excess of peroxynitrite resulted in decreased staining of the band at ca. 470 kDa, and an increase in the amount of aggregated protein (Fig. 5A; lanes 6 vs. 7). The high-molecular-mass band attributed to aggregated protein showed significant 3-nitroTyr formation, which was greater than that observed with intact perlecan exposed to the same concentration of peroxynitrite (Fig. 5B; lanes 4 vs. 7).
Computational modeling of the reaction of CO3•- with the protein core and HS chains of perlecan
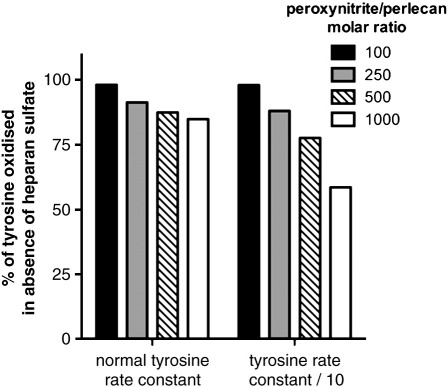
Computational modeling of the effect of HS chains on the extent of reaction of CO3•- with Tyr residues on the protein core of perlecan was performed using literature abundance data and second-order rate constants (see Materials and Methods). The kinetic model was constructed to predict the oxidation (consumption) of Tyr residues by CO3•- for HS-free perlecan (10 nM) and native perlecan (10 nM) with peroxynitrite (0.1 – 10 μM) in the presence of bicarbonate (25 mM) (cf. Fig. 3A). The consumption of Tyr residues in native perlecan (i.e. with HS chains present as a competing target) was expressed as a percentage of that predicted for the HS-free perlecan (i.e. no competing HS chains) at varying oxidant excesses. The yield of CO3•- in this system was taken as 35% of the peroxynitrite concentration [50]. This modeling predicts (Fig. 7) that the HS chains compete with the Tyr residues on the protein core, with this effect becoming more significant with greater oxidant excesses, due to the majority of reaction at low excesses occurring with Trp residues. Modeling was also carried out with a lower rate constant for reaction of CO3•- with tyrosine (5 × 105 M-1 s-1) to mimic potential steric and electronic hindrance of the reaction of the oxidant radical with buried Tyr side-chains (cf. data for a diminished reactivity of Trp residues in peptides compared to free Trp [51]). Under these conditions the competition of the HS chains for reaction with CO3•- was more marked (Fig. 7), as evidenced by an even greater decrease in the extent of Tyr consumption in the native perlecan compared to the HS-free perlecan at identical oxidant doses.
Fig. 7.
Computational modelling of the reaction of CO3•- with the protein core and heparan sulfate chains of perlecan. Modeling of the reaction of CO3•- with native and HS-free perlecan (10 nM) was carried out using literature abundance data and second-order rate constants (see Materials and Methods) at various molar excesses (0.1 – 10 μM) of perlecan and assuming a 35% yield of CO3•-. Data are presented as the predicted consumption of Tyr residues in native perlecan, expressed as a percentage of the predicted consumption of Tyr residues in HS-free perlecan, using both the literature rate constant for reaction of CO3•- with phenol, and a 10-fold lower value to simulate steric and electronic effects on the reaction of CO3•- with buried Tyr residues. In both systems, the model predicts less loss of Tyr residues in the presence of HS chains than in their absence (i.e. the HS chains act as a competitive target for CO3•-), with this effect being more marked at higher oxidant excesses.
Exposure of perlecan to peroxynitrite modulates its functional properties
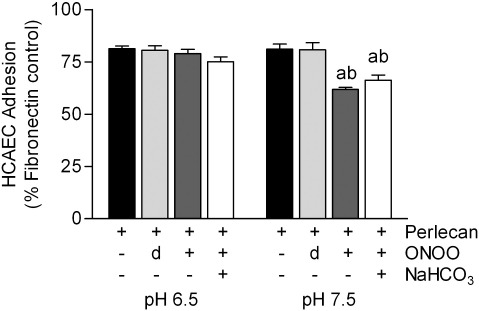
The functional consequences of exposure of perlecan to peroxynitrite was examined by quantifying both its ability to bind human endothelial cells (HCAEC), a protein core-dependent process, and the promotion of FGF-2-dependent cellular proliferation, which is dependent on the presence of intact HS chains. Binding of HCAEC to the perlecan protein core was inhibited by pre-incubation of the perlecan with a 1000-fold molar excess of peroxynitrite at pH 7.5 (Fig. 8) in both the presence and absence of 25 mM NaHCO3. At pH 6.5, these treatments had no effect on cell adhesion.
Fig. 8.
Effect of perlecan modification by peroxynitrite on human coronary artery endothelial cell adhesion. Surface absorbed fibronectin (20 nM) and perlecan (20 nM) were exposed to peroxynitrite (ONOO, 20 μM) or dONOO (20 μM) in 0.1 M phosphate buffer, pH 6.5 or pH 7.5, in the absence and presence of NaHCO3 (25 mM) for 20 min at 22 °C. The adhesion of endothelial cells (HCAECs) was measured by staining of bound cells with crystal violet and expressed as a percentage of adhesion to surface-adsorbed fibronectin. Data are means ± SEM of values obtained from six separate wells from a single assay, representative of three. Data were analyzed by 2-way ANOVA, with statistical significance assumed at p < 0.05, a different to perlecan control, b different to dONOO.
Perlecan stimulated the proliferation of FGFR1c expressing Baf-32 cells in response to FGF-2 and this stimulation was partially abrogated by prior treatment of the perlecan with peroxynitrite at pH 7.5 (Fig. 9A), with a decrease in cell proliferation of 13% and 20% with a 100-fold and 1000-fold molar excess of peroxynitrite respectively; this was statistically significant with the 1000-fold excess. Exposure of perlecan to peroxynitrite, at either pH 6.5 or 7.5, resulted in a decrease in its ability to bind FGF-2 (Fig. 9B), with this effect being significant at pH 7.5.
Fig. 9.
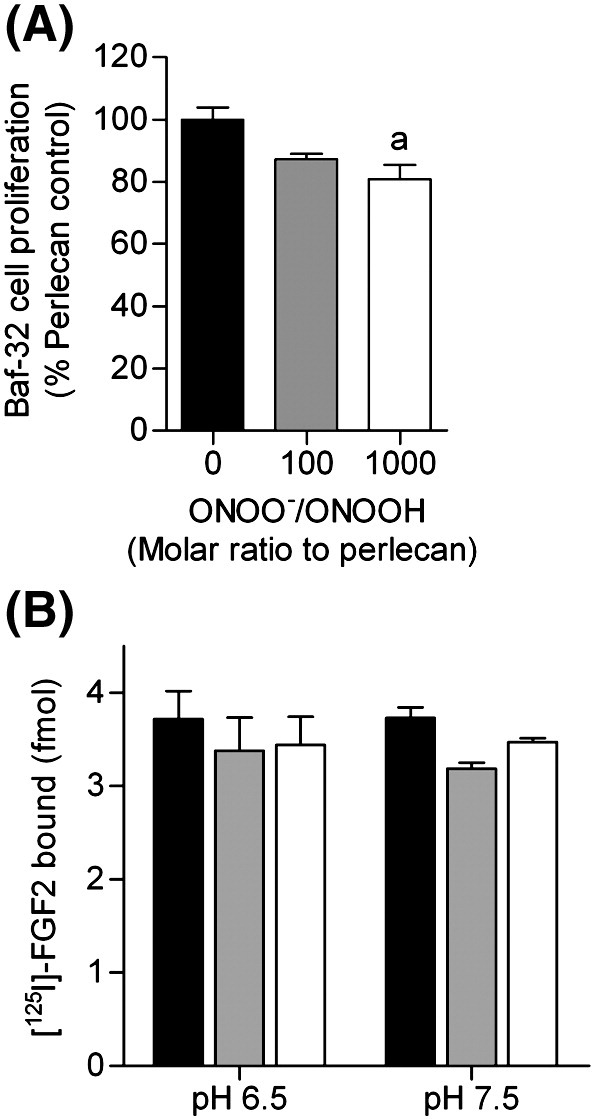
Effect of perlecan modification by peroxynitrite on Baf-32 cell proliferation and binding of FGF-2. (A) BaF3 cells (expressing FGF receptor 1c) were incubated with perlecan and FGF2 and the relative amount of growth was measured as described in the methods section. (B) Surface absorbed perlecan (32 nM, black bars) was exposed to peroxynitrite (ONOO, 32 μM, white bars) or dONOO (32 μM, light grey bars) in 0.1 M phosphate buffer, pH 6.5 or pH 7.5 for 20 min at 22 °C. The extent of [125I]-labeled FGF-2 binding after 2 h incubation at 22 °C was quantified by gamma counting. a significantly different to untreated perlecan control.
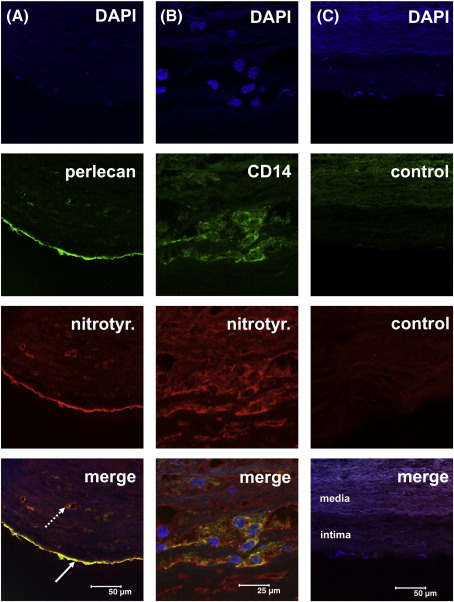
Detection of perlecan and 3-nitrotyrosine epitopes in human atherosclerotic lesions by double immunofluorescence
Perlecan, which is a component of the basement membrane, was detected underneath the endothelial cells lining the artery wall, as expected (Fig. 10A). In arteries with advanced atherosclerotic lesions (type III/IV) 3-nitrotyrosine epitopes were readily detected in the basement membrane intima, in the vasa vasorum, and at lower levels throughout the media and adventia. The signals from the perlecan and 3-nitrotyrosine showed marked co-localization in these lesions (Fig. 10A). In type I/II lesions the intensity of the signals arising from 3-nitrotyrosine epitopes was faint, but that from perlecan was readily detected (data not shown). The role of tissue monocytes macrophages in the formation of the 3-nitrotyrosine epitopes was examined by use of a monoclonal anti-CD14 antibody. 3-Nitrotyrosine could not only be detected in association with perlecan, but was frequently found to colocalize with CD14-positive cells located with the intima of atherosclerotic plaques from type III/IV lesions (Fig. 10B).
Fig. 10.
Double immunofluorescence staining for perlecan, 3-nitrotyrosine epitopes and CD14-positive cells in human atherosclerotic lesion sections. Frozen sections (5 μm) of human atherosclerotic lesions were incubated with monoclonal or polyclonal primary antibodies: anti-nitrotyrosine (rabbit IgG), anti-human perlecan (mouse mAb, clone 7B5, perlecan domain III) anti-human CD14 (mouse mAb), non-immune rabbit IgG and non-immune mouse IgG, and subsequently the following detection antibodies: goat anti-rabbit cyanine-3 (Cy-3)-labelled IgG or goat anti-mouse Cy-2-labeled IgG. DAPI was used to image cell nuclei. Images were acquired as described in the Materials and Methods. A) Heavily thickened intima of an artery with a type III/IV lesion. Epitopes for 3-nitrotyrosine (red signal) show marked co-localization with perlecan (green) present in the basement membrane of the endothelium (arrow) as well as with those of the vasa vasorum (dotted arrow). B) The signal for 3-nitrotyrosine (red) was frequently co-localized with CD14-positive cells (macrophages, green). The red signal for nitrotyrosine underneath the bulk of macrophages results from multiple oblique sections through a vas vasorum. C) In sections where the antibodies for perlecan/CD14 and for 3-nitrotyrosine were replaced with non-immune mouse IgG (control, green) and non-immune rabbit IgG (control, red), no staining in the intima was observed. The faint background staining in the media (also observed when no primary or secondary antibodies were applied to the sections) results from elastic membranes located in this area. Scale as indicated in the bottom images.
Discussion
In this study the effects of peroxynitrite, an oxidant reported to be formed in atherosclerotic lesions [23–25] and at other sites of inflammation [52], on the major basement membrane HSPG perlecan, have been examined. It has been shown that this oxidant modifies both the core protein structure, as evidenced by decreased recognition by multiple antibodies that recognize specific epitopes and the formation of both 3-nitroTyr and protein carbonyls, and the HS chains (decreased recognition by an antibody that recognizes specific HS sequences [53,54]). These changes occur in a dose-dependent manner and are modulated by the reaction pH and the presence of bicarbonate. The specific core protein epitopes recognized by the core protein and HS antibodies have been characterized previously [53–56]. Evidence for the in vivo formation of peroxynitrite-modified perlecan has been obtained by double immunofluorescence studies of advanced human atherosclerotic lesions, where intense co-localization of perlecan and 3-nitrotyrosine epitopes has been detected in the intimal region. These epitopes also co-localize with CD14-positive cells, implicating tissue macrophages – a known source of peroxynitrite [50] - in the generation of this damaged material. The localization of damaged perlecan with CD14-positive cells may be of particular importance as macrophage-rich sites are associated with weakening of the cap of atherosclerotic lesions and subsequent rupture [57]. Modified perlecan has also been reported previously in a study of human asthmatic tissue samples [58], but the nature and source of the modifications were not examined.
The three HS antibodies used in the ELISA studies to probe the effect of peroxynitrite on perlecan structure recognize distinct domain structures in this polysaccharide. HepSS-1 recognizes predominately N-sulfated domains, JM403 binding is dependent on N-unsubstituted glucosamine residues and 10E4 recognizes a sequence containing N-acetylated and N-sulfated glucosamine and an N-unsubstituted glucosamine residue [53,54]). The selective loss of 10E4 recognition, but not that of the other two antibodies suggests that structures present within the sequence recognized by 10E4 may be particularly susceptible to damage by peroxynitrite. These data are consistent with previous studies which have shown that isolated glycosaminoglycan chains are damaged and cleaved by peroxynitrite-derived radicals, and in particular HO. (and CO3-. when the reactions are carried out in the presence of bicarbonate) [27,28]. The results obtained in the current study are also consistent with previous studies in which cell culture-derived ECM samples were treated with peroxynitrite; in these experiments damage to both the protein and carbohydrate components was detected, though it was unclear whether the carbohydrate damage arose from free (e.g. hyaluronan) or protein-bound (proteoglycan or glyco-protein) species [29].
Damage by peroxynitrite to globular proteins [59], and isolated glycosaminoglycans [27,28], has been shown previously to be pH dependent in the absence of bicarbonate. This is believed to arise from the greater reactivity of ONOOH (or species derived from it, such as HO. and NO2.) when compared to ONOO-. This is consistent with the data obtained here for the HS chains (Fig. 2), with a greater loss of recognition of the epitopes recognized by antibody 10E4 at pH 6 and 6.5, than at pH 7 or 7.4 (Fig. 2). In contrast, there was no detectable pH dependence of the loss of recognition of the protein core of perlecan by antibodies CSI-076, 7B5 and CSI-074 (Fig. 2), although higher yields of 3-nitroTyr were detected at higher pH values (Fig. 3, black bars). These differences may reflect alterations in the extent of attack at the HS chains relative to the protein, as the pH of the reaction systems changes, with a greater extent of reaction occurring at the HS chains compared to the protein core at lower pH values (6.0, 6.5) and the inverse at higher pH's. The higher levels of 3-nitroTyr detected at higher pH values are consistent with the greater ease of oxidation of Tyr residues due to the higher concentrations of the (deprotonated) phenolate anion.
Previous studies have provided evidence for enhanced damage to globular proteins in the presence of bicarbonate, with this being manifested as an enhancement in the yield of 3-nitroTyr (e.g. [60]). This has been proposed to arise, at least in part, from the formation of the peroxynitrite-carbonate adduct (ONOOCO2-). The formation of this species is pH dependent, as it requires ONOO-, and subsequent decomposition yields both NO2. and CO3-. [60,61]. The higher yield of 3-nitroTyr has been ascribed to the selective oxidation of Tyr residues by CO3-., with the resulting phenoxyl radical undergoing dimerization with NO2. to give 3-nitroTyr [61]. In contrast to these previous reports, with perlecan the presence of bicarbonate diminished the extent of damage. Thus, the losses of antibody recognition induced by peroxynitrite were lower in the presence of bicarbonate than in its absence (Figs. 1 and 2), and higher yields of 3-nitroTyr were detected in the absence of bicarbonate, compared to its presence (Fig. 3). These differences between globular proteins and perlecan may arise, at least in part, from the relatively high reactivity of CO3-. with the HS chains (cf. k 7 × 105 dm3 mol-1 s-1 for reaction with the related glycosaminoglycan, hyaluronan [43]) compared to reaction with most protein side chains, with the exception of Tyr and Trp residues [62]. The presence of these alternative targets for CO3-. (and to a lesser extent NO2., which reacts with hyaluronan at a lower rate [63]) appears to divert a significant proportion of the initial oxidant from damage to the protein core, to the HS chains; this conclusion is supported by the computational modeling data carried out with native and HS-free perlecan (Fig. 7).
A major biological function of perlecan, present as a component of ECM, is its interaction with growth factors and adhesion molecules, with this playing a major role in the adhesion, differentiation and proliferation of associated cells. Thus arterial wall proteoglycans play a major role in cell adhesion and function [64], processes that are perturbed during the development of atherosclerosis. Adhesion of human endothelial and vascular smooth muscle cells to human perlecan has been investigated, with this shown to be dependent on the protein core [5]. Attempts to determine the site of attachment (through the use of recombinant perlecan domains) have been unsuccessful [5]; in contrast, with mouse perlecan cell interaction occurs via β1 and β3 integrins, with binding partially dependent on a RGD motif (absent in human perlecan) localized to the C-terminus domain V [65]. In the light of this previous data, and the observed modification of the protein core of perlecan by peroxynitrite, it was of interest to determine whether oxidant exposure modulated this function of perlecan. There was a slight but significant reduction in the amount of cell adhesion to the protein core when it was treated with peroxynitrite at pH 7.5, but not at pH 6.5. Decomposed oxidant did not have this effect. Interestingly, treatment with peroxynitrite at pH 7.5 in the presence of bicarbonate, did not reverse the effect of peroxynitrite (Fig. 8).
A second major function of perlecan is the binding of growth factors [3,34]. Fibroblast growth factor-2 (FGF-2) associates with perlecan through its HS chains [34,66] with interaction occurring via short, highly-sulfated regions of the HS chains linked to domain I of the protein core [7,56]. This association may protect FGF-2 from proteolytic degradation [67]. Previous studies have reported that subtle changes in HS structure can modulate growth factor binding and have profound biological effects [34]. Treatment with peroxynitrite at pH 6.5 had no significant effect on the ability of the HS to signal via the FGF receptors, whereas at pH 7.5 a small, but significant decrease was observed (Fig. 9). This decrease is consistent with the observed decrease is recognition of 10E4 epitopes by approximately 20%. The ability of perlecan to stimulate FGF-2-dependent cellular proliferation not only requires a capacity to bind FGF-2, but also to form a ternary complex with the cognate cell surface receptor [34]. In experiments with FGFR-1 expressing Baf-32 cells, this biological activity of perlecan was significantly impaired (Fig. 9). Together these data indicate that oxidation of the HS chains on perlecan may be of functional significance.
Overall these studies show that peroxynitrite, an oxidant widely believed to be generated at sites of inflammation, can modify the structure of the HS proteoglycan perlecan, by altering both the protein core and the HS chains, in a concentration-, pH- and bicarbonate-dependent manner. Damage to the HS chains by peroxynitrite was greater at pH 6 and 6.5 than at pH 7 or 7.4, with this modification being markedly inhibited by the presence of bicarbonate. In contrast, damage to the protein core, as evidenced by loss of native epitope recognition did not show this pH effect, and higher yields of 3-nitroTyr were detected at pH 7.5 and 7 compared to lower pH values. This damage to the protein was also markedly diminished by the presence of bicarbonate. Decomposed peroxynitrite showed only minor effects. These changes to the protein and HS chains resulted in higher levels of protein carbonyls, and the formation of both lower- and higher-molecular mass species, consistent with fragmentation and (to a greater extent) aggregation of the proteoglycan. These oxidant-mediated effects resulted in altered human endothelial cell binding to the proteoglycan and altered cell proliferation and FGF2 binding. These functional changes may be of significance in vivo at sites of peroxynitrite formation. This conclusion is supported by the marked co-localization of 3-nitrotyrosine epitopes with perlecan and CD14-positive (macrophage) cells in the intima of advanced human atherosclerotic lesions.
Acknowledgments
We thank the National Heart Foundation (GO8S 3769), the Australian Research Council (through the ARC Centres of Excellence: CE0561607, and Discovery Programs: DP0988311) and the Austrian Science Fund (FWF, P19074-B05) for financial support, Ms. Anastasia Nilasaroya for performing the cell proliferation experiments, Mr. Bill Cheng for isolating the perlecan and Dr. G. Höfler (Institute of Pathology, Center for Theoretical-Clinical Medicine 1, Medical University of Graz, Graz, Austria) for providing the human tissues samples.
References
- 1.Iozzo R.V., Cohen I.R., GrÀssel S., Murdoch A.D. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 1994;302:625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch A., Dodge G., Cohen I., Tuan R., Iozzo R. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J. Biol. Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- 3.Whitelock J.M., Iozzo R.V. Heparan sulfate: A complex polymer charged with biological activity. Chem. Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo R.V. Basement membrane proteoglycans: from cellar to ceiling. Nat. Rev. Mol. Cell. Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 5.Whitelock J.M., Graham L.D., Melrose J., Murdoch A.D., Iozzo R.V., Underwood P.A. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 6.Knox S., Fosang A.J., Last K., Melrose J., Whitelock J. Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan. FEBS Lett. 2005;579:5019–5023. doi: 10.1016/j.febslet.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 7.Melrose J., Hayes A.J., Whitelock J.M., Little C.B. Perlecan, the "jack of all trades" proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays. 2008;30:457–469. doi: 10.1002/bies.20748. [DOI] [PubMed] [Google Scholar]
- 8.Costell M., Carmona R., Gustafsson E., Gonzalez-Iriarte M., Fassler R., Munoz-Chapuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ. Res. 2002;91:158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Stocker R., Keaney J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 11.Woods A.A., Linton S.M., Davies M.J. Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem. J. 2003;370:729–735. doi: 10.1042/BJ20021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima Y., Fujii H., Sumiyoshi S., Wight T.N., Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 13.Davies M.J., Thomas A.C. Plaque fissuring - the cause of acute myocardial-infarction, sudden ischemic death, and crescendo angina. Br. Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raines E.W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolodgie F.D., Burke A.P., Wight T.N., Virmani R. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Curr. Opin. Lipidol. 2004;15:575–582. doi: 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kolodgie F.D., Burke A.P., Farb A., Weber D.K., Kutys R., Wight T.N., Virmani R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler. Thromb. Vasc. Biol. 2002;22:1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 17.Kinsella M.G., Tran P.K., Weiser-Evans M.C., Reidy M., Majack R.A., Wight T.N. Changes in perlecan expression during vascular injury: role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler. Thromb. Vasc. Biol. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- 18.Rees M.D., Kennett E.C., Whitelock J.M., Davies M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Huie R.E., Padmaja S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 20.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar T.M. Peroxynitrite formation from the simultaneous reduction of nitrite and oxygen by xanthine oxidase. FEBS Lett. 2004;562:129–133. doi: 10.1016/S0014-5793(04)00218-2. [DOI] [PubMed] [Google Scholar]
- 22.Vásquez-Vivar J., Kalyanaraman B., Martásek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic. Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 23.Beckman J.S., Ye Y.Z., Anderson P.G., Chen J., Accavitti M.A., Tarpey M.M., White C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 24.Pennathur S., Bergt C., Shao B., Byun J., Kassim S.Y., Singh P., Green P.S., McDonald T.O., Brunzell J., Chait A., Oram J.F., O'Brien K., Geary R.L., Heinecke J.W. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 25.Leeuwenburgh C., Hardy M.M., Hazen S.L., Wagner P., Ohishi S., Steinbrecher U.P., Heinecke J.W. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem. 1997;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 26.Li M., Rosenfeld L., Vilar R.E., Cowman M.K. Degradation of hyaluronan by peroxynitrite. Arch. Biochem. Biophys. 1997;341:245–250. doi: 10.1006/abbi.1997.9970. [DOI] [PubMed] [Google Scholar]
- 27.Kennett E.C., Davies M.J. Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: evidence for a hydroxyl-radical-like mechanism. Free Radic. Biol. Med. 2007;42:1278–1289. doi: 10.1016/j.freeradbiomed.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Kennett E.C., Davies M.J. Glycosaminoglycans are fragmented by hydroxyl, carbonate, and nitrogen dioxide radicals in a site-selective manner: implications for peroxynitrite-mediated damage at sites of inflammation. Free Radic. Biol. Med. 2009;47:389–400. doi: 10.1016/j.freeradbiomed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Kennett E.C., Davies M.J. Degradation of extracellular matrix by peroxynitrite/peroxynitrous acid. Free Radic. Biol. Med. 2008;45:716–725. doi: 10.1016/j.freeradbiomed.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Klebanoff S.J., Kinsella M.G., Wight T.N. Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase-H2O2-chloride system. Am. J. Pathol. 1993;143:907–917. [PMC free article] [PubMed] [Google Scholar]
- 31.Rees M.D., McNiven T.N., Davies M.J. Degradation of extracellular matrix and its components by hypobromous acid. Biochem. J. 2007;401:587–596. doi: 10.1042/BJ20061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rees M.D., Whitelock J.M., Malle E., Chuang C.Y., Iozzo R.V., Nilasaroya A., Davies M.J. Myeloperoxidase-derived oxidants selectively disrupt the protein core of the heparan sulfate proteoglycan perlecan. Matrix Biol. 2010;29:63–73. doi: 10.1016/j.matbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uppu R.M., Pryor W.A. Synthesis of peroxynitrite in a two-phase system using isoamyl nitrite and hydrogen peroxide. Anal. Biochem. 1996;236:242–249. [PubMed] [Google Scholar]
- 34.Knox S., Merry C., Stringer S., Melrose J., Whitelock J. Not all perlecans are created equal - interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J. Biol. Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- 35.Knox S., Melrose J., Whitelock J. Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins. Proteomics. 2001;1:1534–1541. doi: 10.1002/1615-9861(200111)1:12<1534::aid-prot1534>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg H.A., Warner K.J. The staining of acidic proteins on polyacrylamide gels: Enhanced sensitivity and stability of ''stains-all'' staining in combination with silver nitrate. Anal. Biochem. 1997;251:227–233. doi: 10.1006/abio.1997.2252. [DOI] [PubMed] [Google Scholar]
- 38.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Rosenfeld M.E., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 39.Marsche G., Hammer A., Oskolkova O., Kozarsky K.F., Sattler W., Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J. Biol. Chem. 2002;277:32172–32179. doi: 10.1074/jbc.M200503200. [DOI] [PubMed] [Google Scholar]
- 40.Malle E., Marsche G., Panzenboeck U., Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins. Arch. Biochem. Biophys. 2006;445:245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Hammer A., Desoye G., Dohr G., Sattler W., Malle E. Myeloperoxidase-dependent generation of hypochlorite-modified proteins in human placental tissues during normal pregnancy. Lab. Invest. 2001;81:543–554. doi: 10.1038/labinvest.3780263. [DOI] [PubMed] [Google Scholar]
- 42.Rees M.D., Pattison D.I., Davies M.J. Oxidation of heparan sulphate by hypochlorite: role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem. J. 2005;391:125–134. doi: 10.1042/BJ20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Assaf S., Navaratnam S., Parsons B.J., Phillips G.O. Chain scission of hyaluronan by carbonate and dichloride radical anions: Potential reactive oxidative species in inflammation? Free Radic. Biol. Med. 2006;40:2018–2027. doi: 10.1016/j.freeradbiomed.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 44.Chen S.N., Hoffman M.Z., Parsons G.H. Reactivity of the carbonate radical toward aromatic compounds in aqueous solution. J. Phys. Chem. 1975;79:1911–1912. [Google Scholar]
- 45.Chen S.N., Hoffman M.Z. Rate constants for the reaction of the carbonate radical with compounds of biochemical interest in neutral aqueous solution. Radiat. Res. 1973;56:40–47. [PubMed] [Google Scholar]
- 46.Hackam D.J., Rotstein O.D., Zhang W.J., Demaurex N., Woodside M., Tsai O., Grinstein S. Regulation of phagosomal acidification - Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-ATPases. J. Biol. Chem. 1997;272:29810–29820. doi: 10.1074/jbc.272.47.29810. [DOI] [PubMed] [Google Scholar]
- 47.Jankowski A., Scott C.C., Grinstein S. Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 2002;277:6059–6066. doi: 10.1074/jbc.M110059200. [DOI] [PubMed] [Google Scholar]
- 48.Deng H. Nitrite anions induce nitrosative deamination of peptides and proteins. Rapid Commun. Mass Spectrom. 2006;20:3634–3638. doi: 10.1002/rcm.2776. [DOI] [PubMed] [Google Scholar]
- 49.Lymar S.V., Hurst J.K. Rapid reaction between peroxonitrite ion and carbon dioxide: Implications for biological activity. J. Am. Chem. Soc. 1995;117:8867–8868. [Google Scholar]
- 50.Radi R., Peluffo G., Alvarez M.N., Naviliat M., Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 51.Chen S.N., Hoffman M.Z. Reactivity of the carbonate radical in aqueous solution. Tryptophan and its derivatives. J. Phys. Chem. 1974;78:2099–2102. [Google Scholar]
- 52.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 53.van den Born J., Salmivirta K., Henttinen T., Ostman N., Ishimaru T., Miyaura S., Yoshida K., Salmivirta M. Novel heparan sulfate structures revealed by monoclonal antibodies. J. Biol. Chem. 2005;280:20516–20523. doi: 10.1074/jbc.M502065200. [DOI] [PubMed] [Google Scholar]
- 54.Mani K., Cheng F., Sandgren S., van den Born J., Havsmark B., Ding K., Fransson L.-A. The heparan sulfate-specific epitope 10E4 is NO-sensitive and partly inaccessible in glypican-1. Glycobiology. 2004;14:599–607. doi: 10.1093/glycob/cwh067. [DOI] [PubMed] [Google Scholar]
- 55.Murdoch A., Liu B., Schwarting R., Tuan R., Iozzo R. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J. Histochem. Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- 56.Whitelock J.M., Murdoch A.D., Iozzo R.V., Underwood P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 57.Koenig W., Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 58.Malmstrom J., Larsen K., Hansson L., Lofdahl C.G., Norregard-Jensen O., Marko-Varga G., Westergren-Thorsson G. Proteoglycan and proteome profiling of central human pulmonary fibrotic tissue utilizing miniaturized sample preparation: a feasibility study. Proteomics. 2002;2:394–404. doi: 10.1002/1615-9861(200204)2:4<394::AID-PROT394>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez B., Ferrer-Sueta G., Freeman B.A., Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J. Biol. Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 60.Gow A., Duran D., Thom S.R., Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch. Biochem. Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 61.Andrekopoulos C., Zhang H., Kalivendi S., Kalyanaraman B. Bicarbonate enhances α-synuclein oligomerization and nitration: Intermediacy of carbonate radical anion and nitrogen dioxide radical. Biochem. J. 2003;378:435–447. doi: 10.1042/BJ20031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neta P., Huie R.E., Ross A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:1027–1284. [Google Scholar]
- 63.Al-Assaf S., Navaratnam S., Parsons B.J., Phillips G.O. Chain scission of hyaluronan by peroxynitrite. Arch. Biochem. Biophys. 2003;411:73–82. doi: 10.1016/s0003-9861(02)00724-5. [DOI] [PubMed] [Google Scholar]
- 64.Hultgardh-Nilsson A., Durbeej M. Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr. Opin. Lipidol. 2007;18:540–545. doi: 10.1097/MOL.0b013e3282ef77e9. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi K., Madri J., Yurchenco P. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J. Cell Biol. 1992;119:945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Folkman J., Klagsbrun M., Sasse J., Wadzinski M., Ingber D., Vlodavsky I. A heparin-binding angiogenic protein–basic fibroblast growth factor–is stored within basement membrane. Am. J. Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 67.Saksela O., Moscatelli D., Sommer A., Rifkin D. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]