Abstract
Objectives
Case finding is proposed as an important component of the forthcoming English National Clinical Strategy for chronic obstructive pulmonary disease (COPD) because of accepted widespread underdiagnosis worldwide. However the best method of identification is not known. The extent of undiagnosed clinically significant COPD in England is described and the effectiveness of an active compared with an opportunistic approach to case finding is evaluated.
Methods
A cross-sectional analysis was carried out using using Health Survey for England (HSE) 1995–1996 data supplemented with published literature. A model comparing an active approach (mailed questionnaires plus opportunistic identification) with an opportunistic-only approach of case finding among ever smokers aged 40–79 years was evaluated. There were 20 496 participants aged ≥30 years with valid lung function measurements. The main outcome measure was undiagnosed clinically significant COPD (any respiratory symptom with both forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 and FEV1 <80% predicted).
Results
971 (4.7%) had clinically significant COPD, of whom 840 (86.5%) did not report a previous diagnosis. Undiagnosed cases were more likely to be female, and smoked less. 25.3% had severe disease (FEV1 <50% predicted), 38.5% Medical Research Council (MRC) grade 3 dyspnoea and 44.1% were current smokers. The active case-finding strategy can potentially identify 70% more new cases than opportunistic identification alone (3.8 vs 2.2 per 100 targeted). Treating these new cases could reduce hospitalisations by at least 3300 per year in England and deaths by 2885 over 3 years.
Conclusions
There is important undiagnosed clinically significant COPD in the population, and the addition of a systematic case-finding approach may be more effective in identifying these cases. The cost-effectiveness of this approach needs to be tested empirically in a prospective study.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects 5–10% of the population worldwide,1 with a rising prevalence, and is a leading cause of mortality. In the UK, COPD accounts for 1.4 million general practitioner (GP) consultations and 1 million inpatient bed days annually, costing the National Health Service (NHS) >£800 million.2 Recently COPD has received increasing attention as an important condition in England, culminating in a National Clinical Strategy due to be published later this year.3 Global underdiagnosis, ranging between 45% and 85%,4–6 is widely reported, and recently the British Lung Foundation has led a drive to identify these ‘missing millions’. However, population screening using spirometry is not recommended7 because it would identify many people without clinically significant symptoms for whom there is little evidence of effective interventions.8 Evidence for progression of asymptomatic cases to clinically significant disease is also conflicting.
Nevertheless it is likely that there are people with unmet healthcare needs, who have clinically significant COPD, but are unknown to the health services. These patients may potentially benefit from known effective interventions (including inhaled treatment, pulmonary rehabilitation and smoking cessation),8 9 which could offer symptomatic relief, modify disease progression and improve quality of life. UK National Institute for Health and Clinical Excellence (NICE) guidelines recommend that a diagnosis of COPD should be considered in patients with chronic respiratory symptoms and risk factors.9 Such opportunistic case finding is likely to be sporadically implemented, but will miss patients not consulting their GP. There might be a case for more systematic targeted case finding such as indicated in the preliminary report for the forthcoming National Clinical Strategy.3 A few studies have explored potential approaches in a range of different target groups, but the best approach for identifying undiagnosed cases is not known as these reports have not included comparison groups.10–13
The Health Survey for England (HSE) offers the potential for assessing the degree of underdiagnosis of COPD and for modelling various case-finding strategies. Although not designed for this purpose, it is a large data set, representative of the English population and in 1995 and 1996 included both spirometry and questions about respiratory symptoms.
The aim of this study is first, to use the HSE to quantify the extent of undiagnosed clinically significant COPD in England, and describe the characteristics of this population; and secondly to model and compare two COPD case-finding approaches in primary care, and to identify critical points in the model using sensitivity analyses. By informing the choice of case-finding approaches, the model generated could be applied to similar countries in Europe and North America.
METHODS
Study design and participants
This was a cross-sectional analysis of data collected by the HSE (1995 and 1996).
The HSE is an annual survey which monitors the health of the nation. A general population sample is obtained by multistage stratified random sampling of private households in England14 15 with standardised home interviews and health assessments administered by trained interviewers/nurses. In 1995–1996, >32 000 adults participated. Data were obtained from the UK Data archive combining both years. Participants aged ≥30 years with valid lung function and height data were included.
Procedures
Information was obtained on demographic factors, lifestyle and health, including if they had diagnosed asthma, any (and which) longstanding illnesses and a range of respiratory symptoms. Smoking was defined as current, ex- and never regular smokers (regular defined as ≥1 cigarette per day). Pack-years were calculated for all participants.
Pulmonary function tests, without reversibility, were performed according to a standard protocol14 15 with a Vitalograph Escort spirometer (Fleisch pneumotachograph flow head) calibrated daily at normal room temperature.
Definition of COPD
In conformity with NICE guidelines,9 clinically significant COPD was defined as reporting of any respiratory symptom (exertional breathlessness, chronic cough, regular sputum, frequent winter bronchitis or wheeze) and evidence of airways obstruction on spirometry (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 and FEV1 <80% predicted (equivalent to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II16). There is controversy over the criteria which should be used to define COPD, therefore analyses were repeated with different spirometric criteria, including GOLD (FEV1/FVC <0.7), single LLN (FEV1/FVC below the lower limit of normal=5th percentile of the healthy never-smoking population, using the ECCS (European Community for Steel and Coal) reference equations)17 and double LLN (FEV1/FVC and FEV1 values both below the lower limit of normal).18
Participants with airways obstruction and reporting a diagnosis of asthma, with first episode of wheeze before the age of 30, were reclassified as ‘not having COPD’ to reduce misclassification of those with asthma. Those with clinically significant COPD who did not report having chronic bronchitis/emphysema were classified as being ‘undiagnosed’.
Development of a case-finding model
Target group
Initial analyses showed that the prevalence of clinically significant COPD among never smokers and in those aged <40 years was low. As there are also practical difficulties in measuring lung function in elderly patients, the chosen target group for case finding in our model was current or ex-smokers aged 40–79 years without a prior self-report of chronic bronchitis/emphysema. Although there are several screening tools published which include more complex methods for identifying undiagnosed COPD designed to increase specificity,19–21 none has been designed or validated with the clinical COPD definition used here. Consequently, this broad target group was chosen, allowing sensitivity analyses for more restrictive scenarios.
Model details
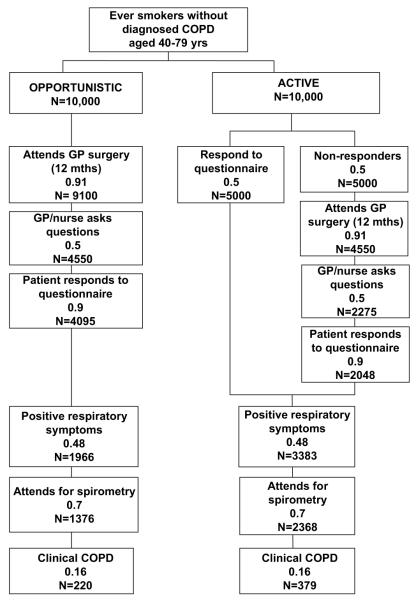
A simple model comparing an active case-finding approach with an opportunistic-only approach was developed using data from the HSE and published literature, and applied to a likely scenario in primary care with a hypothetical cohort of 10 000 patients (figure 1). Patients in the target group would be identified from general practice records. With an opportunistic-only approach, each patient’s notes would be flagged as a reminder for GPs/practice nurses to ask simple questions during any consultation about relevant respiratory symptoms. This would be compared with the ‘active’ approach, consisting of both an opportunistic component (as above) and the addition of a postal questionnaire with the same respiratory questions.
Figure 1.
Model comparing an active approach with opportunistic-only approach with case finding in chromic obstructive pulmonary disease (COPD). Figures are proportions (based on estimates from sources detailed in table 1) and calculated numbers based on a hypothetical cohort of 10 000 in each option.
Patients with positive respiratory symptoms (defined according to the NICE criteria as above) would be invited for spirometry and then classified as having COPD or not (definition as above). Assumptions for proportions entering each stage of the model were taken from the above HSE analyses or from the published literature (table 1). The model was based on a 1 year period, assuming the respiratory questions would be administered at one consultation.
Table 1.
Base case assumptions for model: among ever smokers aged 40–79 years without diagnosis of COPD
| Base case | Source/explanation | |
|---|---|---|
| Proportion of cohort having respiratory symptoms | ||
| Dyspnoea | 0.32 | HSE analysis |
| MRC grade 3 dyspnoea or worse | 0.13 | |
| Wheeze | 0.25 | |
| Chronic cough or phlegm | 0.21 | |
| Any of the above | 0.48 | |
| Proportion having childhood asthma | 0.04 | HSE analysis |
| Proportion with clinically significant COPD* among those with respiratory symptoms |
0.16 | HSE analysis |
| Proportion of COPD* with low FEV1 (% predicted) | ||
| 50–79% | 0.73 | HSE analysis |
| 30–49% | 0.23 | |
| <30% | 0.04 | |
| Proportion of COPD* with MRC grade 3 dyspnoea or worse | 0.39 | HSE analysis |
| Proportion of COPD* with either FEV1 <50% or MRC grade 3 dyspnoea | 0.51 | HSE analysis |
| Response rate to postal questionnaire/reminders | 0.5 | Response rates estimates from previous experience/literature vary between 50% and 90%22 23 |
| Probability that patients consult their GP/staff at least once per year | 0.91 over 12 months |
Patients aged 45–64 years consult on average 3.3 times per year24; patients with COPD consult 6.4 times per year.25 Overall estimate based on Poisson distribution, varying the prevalence of COPD between 1% and 20%. Assume patients will not be questioned more than once per year. |
| Proportion of consultations where questionnaire is administered opportunistically |
0.5 | Reported rates for atrial fibrillation screening with pulse 30–70%23 26 |
| Response rate to questionnaire in surgery | 0.9 | Response rates 98–100%11 12 27 |
| Proportion attendance for spirometry | 0.7 | Uptake rates 33–97%5 12 23 27 |
| Sensitivity of spirometry test (quality compared with specialised technician) | 1.0 | Possible 3% underdiagnosis28 but literature suggests overdiagnosis most common |
Clinically significant COPD is defined as respiratory symptoms and airways obstruction (National Institute for Health and Clinical Excellence criteria).
Estimates based on 1 year time period.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; GP, general practitioner; HSE, Health Survey for England; MRC, Medical Research Council.
Model outcomes
The main outcomes were: new cases detected as a percentage of patients targeted, difference in number of cases detected (per 100 targeted), proportion of total expected cases detected and numbers needed to target (NNT; calculated as 1/risk difference) for the active compared with the opportunistic approach. The proportion of new cases who would be expected to have stage III or IV disease (FEV1 <50% predicted) or Medical Reseach Council (MRC) grade 3 or worse dyspnoea was also calculated in order to quantify the proportion most able to benefit from disease-modifying treatment (treatment which can reduce exacerbations, hospitalisations or mortality).8 9
Sensitivity analyses
Sensitivity was assessed by considering the impact of varying each parameter individually within plausible ranges.
Alternative targeting strategies
The effects of alternative strategies were considered such as targeting different age groups, age–smoking combinations, symptom profiles and spirometric criteria. Estimates of input parameters were derived from the HSE.
Statistical analyses
Characteristics of undiagnosed and diagnosed patients with COPD were compared using χ2 tests.
RESULTS
Characteristics and prevalence of undiagnosed COPD
There were 20 496 participants aged ≥30 years with valid height and spirometry measurements, 9959 (48.6%) from 1995 and 10 537 (51.4%) from 1996 (table 2). Mean age was 51.8 years (SD 14.8) and 53.0% were female. A quarter of participants were current smokers, and a further 6187 (30.2%) were ex-smokers. A total of 2180 (10.6%) reported ever having been diagnosed with asthma, and 6.3% that their first episode of wheeze was before the age of 30. Although 8410 (41.0%) reported at least one of the included respiratory symptoms, only 971 (4.7%) also had airways obstruction, and were thus classified as having clinically significant COPD. Of these, only 131 (13.5%) reported chronic bronchitis/emphysema, suggesting that >85% were undiagnosed. Notably, although 291 (1.4%) reported a diagnosis of chronic bronchitis/emphysema, less than half of these demonstrated airflow obstruction.
Table 2.
Characteristics of participants
| Number of participants | 20496 | |
| 1995 | 9959 (48.6%) | |
| 1996 | 10537 (51.4%) | |
| Age (years) | ||
| 30–39 | 5340 (26.1%) | |
| 40–49 | 4822 (23.5%) | |
| 50–59 | 3821 (18.6%) | |
| 60–69 | 3371 (16.5%) | |
| 70–79 | 2365 (11.5%) | |
| ≥80 | 777 (3.8%) | |
| Sex | ||
| Male | 9643 (47.1%) | |
| Female | 10853 (53.0%) | |
| Smoking status | ||
| Current | 5191 (25.3%) | |
| Ex-regular | 6187 (30.2%) | |
| Never regular | 9115 (44.5%) | |
| Reported respiratory conditions | ||
| Reported asthma diagnosis | 2180 (10.6%) | |
| First wheezed before age 30 years |
1289 (6.3%) | |
| Reported diagnosis of chronic bronchitis or emphysema |
291 (1.4%) | |
| Other respiratory condition* | 489 (2.4%) | |
| Reported respiratory symptoms | ||
| Dyspnoea | 5425 (26.5%) | |
| MRC grade 3 or worse | 2223 (10.9%) | |
| Wheeze | 4422 (21.6%) | |
| Chronic cough | 2783 (13.6%) | |
| Chronic phlegm | 2181 (10.6%) | |
| Frequent winter bronchitis | 3447 (16.8%) | |
| Any of the above respiratory symptoms |
8410 (41.0%) | |
| Airways obstruction† | ||
| GOLD | 2872 (14.0%) | |
| NICE | 1305 (6.4%) | |
| Single LLN | 1796 (8.8%) | |
| Double LLN | 733 (3.6%) | |
| Clinically significant COPD†, ‡ | Reporting diagnosis of chronic bronchitis/emphysema |
|
| GOLD+symptoms | 91628 (7.9%) | 145 (8.9%) |
| NICE+symptoms | 971 (4.7%) | 131 (13.5%) |
| Single LLN+symptoms | 1072 (5.2%) | 113 (10.5%) |
| Double LLN+symptoms | 571 (2.8%) | 99 (17.3%) |
Excluding hayfever.
Prebronchodilator values with childhood asthmatics reclassified (see the Methods section).
Respiratory symptoms and airflow obstruction.
GOLD=FEV1/FVC <0.7; NICE=FEV1/FVC <0.7 and FEV1 <80% predicted; single LLN=FEV1/FVC <5th percentile of healthy never-smoking population; double LLN=FEV1/FVC <5th percentile and FEV1 <5th percentile.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LLN, lower limit of normal; MRC, Medical Research Council; NICE, National Institute for Health and Clinical Excellence.
Characteristics of participants with undiagnosed COPD
Table 3 compares characteristics of participants with diagnosed and undiagnosed clinically significant COPD. Although there was a greater proportion of females among undiagnosed cases, this was not significant (41.7% vs 35.9%, p=0.2); however, they were more likely to be never smokers (16.8% vs 6.9%, p=0.002). They were less likely to report each of the specific respiratory symptoms and their dyspnoea was less severe (38.5% had MRC grade 3 dyspnoea vs 69.5% of those reporting a diagnosis). Although airways obstruction in undiagnosed cases was milder overall (p<0.001), a quarter of these had severe airways obstruction (FEV1 <50% predicted) and therefore under current guidance would be eligible for disease-modifying inhalers.13 15 Those with milder stage disease would be eligible for bronchodilators or mucolytics for symptomatic relief,15 while those with MRC grade 3 dyspnoea would be eligible for pulmonary rehabilitation.15 In addition, 44.1% were current smokers, and would be eligible for smoking cessation therapy.
Table 3.
Comparison of the characteristics of diagnosed and undiagnosed clinically significant COPD in England
| Undiagnosed | Diagnosed | |
|---|---|---|
| Number of participants | 840 | 131 |
| Age (years) | ||
| 30–39 | 13 (1.6%) | 0 |
| 40–49 | 70 (8.3%) | 8 (6.1%) |
| 50–59 | 137 (16.3%) | 19 (14.5%) |
| 60–69 | 257 (30.6%) | 41 (31.3%) |
| 70–79 | 266 (31.7%) | 53 (40.5%) |
| ≥80 | 97 (11.6%) | 10 (7.6%) (p=0.2) |
| Sex | ||
| Male | 490 (58.3%) | 84 (64.1%) |
| Female | 350 (41.7%) | 47 (35.9%) (p=0.2) |
| Smoking status | ||
| Current | 370 (44.1%) | 53 (40.5%) |
| Ex-regular | 328 (39.1%) | 69 (52.7%) |
| Never regular | 141 (16.8%) | 9 (6.9%) (p=0.002) |
| Reported respiratory symptoms | ||
| Dyspnoea | 651 (77.5%) | 110 (84.0%) (p=0.09) |
| MRC grade 3 or worse | 323 (38.5%) | 91 (69.5%) (p<0.001) |
| Wheeze | 548 (65.2%) | 119 (90.8%) (p<0.001) |
| Chronic cough | 366 (43.6%) | 93 (71.0%) (p<0.001) |
| Chronic phlegm | 305 (36.3%) | 89 (67.9%) (p<0.001) |
| Frequent winter bronchitis | 428 (51.0%) | 105 (80.2%) (p<0.001) |
| Any of the above respiratory symptoms | 840 (100%) | 131 (100%) |
| Severity of airflow obstruction* | ||
| Stage (FEV1% predicted) | ||
| II (50–80%) | 628 (74.8%) | 55 (42.0%) |
| III (30–49%) | 181 (21.6%) | 52 (39.7%) |
| IV (<30%) | 31 (3.7%) | 24 (18.3%) (p<0.001) |
| Likely to benefit most from disease- modifying treatment (FEV1 <50% predicted or MRC grade 3 dyspnoea) |
417 (49.6%) | 104 (79.4%) (p<0.001) |
Stage according to GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; MRC, Medical Research Council.
Prevalence of undiagnosed clinically significant COPD increased with age, from 0.2% (30–39 years group), to 12.9% (≥80 years group). Prevalence was highest among smokers at all ages (figure 2), rising from age 40–45 years. Among ex-smokers, COPD prevalence remained at or below 1% until age 55 years, while for never smokers rates did not rise above 1% until age 60 years.
Figure 2.
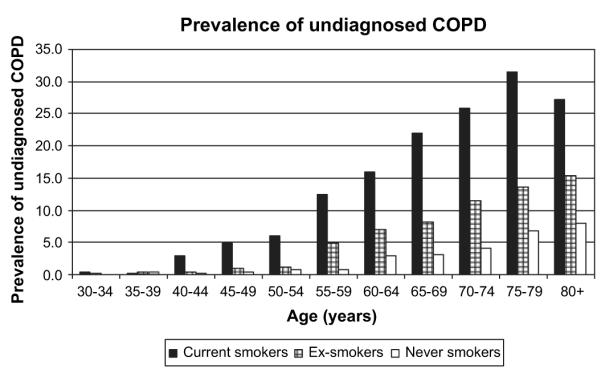
Prevalence of undiagnosed clinically significant chronic obstructive pulmonary disease (COPD) by age and smoking history in participants of the 1995–1996 Health Survey for England aged ≥30 years. (Clinically significant indicates respiratory symptoms and airways obstruction according to National Institute for Health and Clinical Excellence criteria.)
Comparing a model of an active approach against an opportunistic-only approach to case finding
Analysis of the HSE showed that 48% of the target group (ever smokers aged 40–79 years) reported relevant respiratory symptoms. Of these, 16% demonstrated airways obstruction, and therefore, for every 10 000 ever-smoking patients in this age group we would expect 768 undiagnosed cases (or 7.7 per 100).
Figure 1 details the flow of patients through both arms of the case-finding model taking into account other parameters such as response rates. The active approach to case finding would yield 70% more new cases than the opportunistic approach (3.8 vs 2.2 new cases per 100 ever smokers targeted), giving a rate difference of 1.6 per 100 targeted and identifying 49% of the expected cases. Sixty-three ever smokers would need to be actively targeted to identify one extra case of COPD, over and above the opportunistic approach. Of these new cases, 39.2% would have at least MRC grade 3 dyspnoea, and 26.8% stage III/IV disease (50.9% with either) and could benefit immediately from effective disease-modifying treatments.
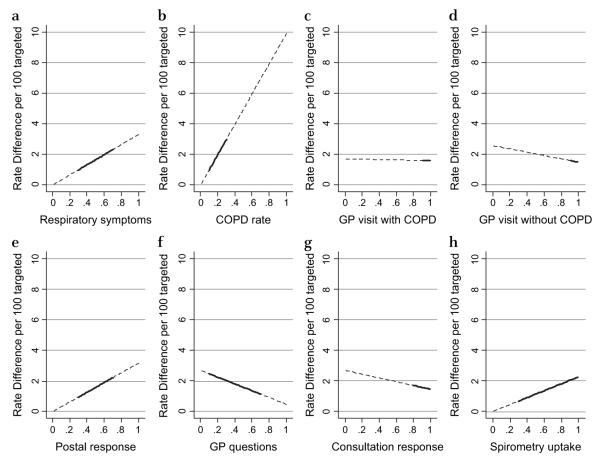
Sensitivity analyses
Table 4 and figure 3 illustrate the effect of varying key parameters on the relative benefit of the systematic approach, and highlight the plausible ranges. The model is sensitive to changes in the proportion of the target group with respiratory symptoms (a range of 30–70% alters the rate difference from 1.0 to 2.3 per 100) and to the proportion of those with respiratory symptoms having COPD on spirometry (a range of 10–30% alters the rate difference from 1.0 to 3.0 per 100 targeted). The key modifiable parameters are the response rate to postal questionnaires, the probability the respiratory questionnaire is administered opportunistically and, to some extent, the spirometry uptake rates. A variation in postal response of 30–70% would result in a rate difference of 1.0–2.2 per 100 targeted. In contrast, as practices administer more questionnaires, the advantage of the active approach is attenuated.
Table 4.
Sensitivity analysis
| Parameter | New cases (per 100 targeted) |
Rate difference (per 100 targeted) |
% additional cases detected with active approach |
NNT | Proportion of expected cases in the community detected with active approach |
|
|---|---|---|---|---|---|---|
| Active | Opportunistic only | |||||
| Base case | 3.8 | 2.2 | 1.6 | 70% | 63 | 0.49 |
| Model assumptions | ||||||
| Proportion with any respiratory symptom | ||||||
| 0.3 | 2.4 | 1.4 | 1.0 | 70% | 101 | 0.49 |
| 0.7 | 5.5 | 3.2 | 2.3 | 70% | 45 | 0.49 |
| COPD* in those with respiratory symptoms | ||||||
| 0.1 | 2.4 | 1.4 | 1.0 | 70% | 101 | 0.49 |
| 0.2 | 4.7 | 2.8 | 2.0 | 70% | 50 | 0.49 |
| 0.3 | 7.1 | 4.1 | 3.0 | 70% | 34 | 0.49 |
| Process assumptions | ||||||
| Questionnaire response rate (postal) | ||||||
| 0.3 | 3.2 | 2.2 | 1.0 | 43% | 105 | 0.41 |
| 0.7 | 4.4 | 2.2 | 2.2 | 101% | 45 | 0.58 |
| GP asks questionnaire in surgery | ||||||
| 0.1 | 2.9 | 0.4 | 2.5 | 561% | 41 | 0.38 |
| 0.2 | 3.1 | 0.9 | 2.2 | 255% | 45 | 0.41 |
| 0.3 | 3.3 | 1.3 | 2.0 | 154% | 49 | 0.41 |
| 0.7 | 4.2 | 3.1 | 1.2 | 37% | 87 | 0.55 |
| Spirometry uptake rate | ||||||
| 0.5 | 2.7 | 1.6 | 1.1 | 70% | 88 | 0.35 |
| 0.9 | 4.9 | 2.8 | 2.0 | 70% | 49 | 0.63 |
| Spirometry accuracy | ||||||
| 0.9 sensitivity | 3.4 | 2.0 | 1.4 | 70% | 70 | 0.44 |
| 0.8 sensitivity | 3.0 | 1.8 | 1.3 | 70% | 79 | 0.39 |
Numbers in bold are referred to in the text.
Clinically significant COPD defined as respiratory symptoms and airways obstruction (National Institute for Health and Clinical Excellence criteria).
COPD, chronic obstructive pulmonary disease; GP, general practitioner; NNT, number needed to target with the active approach to identify one additional new case over opportunistic only.
Figure 3.
Effect of varying key parameters on rate difference of active versus opportunistic case finding for chronic obstructive pulmonary disease (COPD). For each of the following eight parameters, the graphs indicate how changes in the estimates of the parameter (proportions from 0 to 1) affect the estimates of relative effectiveness of the active approach. Bold portions of the line indicate the most plausible range of interest. (a) Proportion of target population with specified respiratory symptoms. (b) Proportion of those with respiratory symptoms having clinically significant COPD. (c) Probability that patients with COPD visit their GP (general practitioner) at least once per year. (d) Probability that patients without COPD visit their GP at least once per year. (e) Uptake in response to postal questionnaire. (f) Probability GP/nurse will ask respiratory questions opportunistically. (g) Proportion of patients responding to questions administered at the surgery. (h) Uptake of spirometry.
Modelling alternative targeting strategies
Targeting those aged over 50 increases the efficiency of an active approach compared with an opportunistic-only approach (NNT 47 vs 65) although marginally less sensitive than targeting the full 40–79 year age range (44% vs 49% expected) (see online table).
Targeting current smokers aged ≥45 years and ex-smokers aged ≥55 years would improve the efficiency of the active approach without losing many cases (NNT=45; 47% total identified).
An active strategy without concurrent opportunistic case finding (ie, postal questionnaire only) would result in a difference of only 0.5 per 100 targeted over the base case, and identify one-third fewer cases than with the combined approach.
Although restricting the target group to those with dyspnoea only would identify patients with more severe disease (50.4% with MRC grade 3 dyspnoea vs 39.2% in the base case), the active approach would then have a relatively smaller benefit and pick up fewer undiagnosed cases than the base case.
Use of the single LLN criteria to define cases had little overall effect, although cases were generally milder. While double LLN decreased the yield in both arms, and reduced the advantage of the active approach, a higher proportion of more severe cases would be identified (59.0% eligible for disease-modifying treatment).
DISCUSSION
In this analysis of nationally representative data of >20 000 adults aged ≥30 years, we identified a substantial amount of undiagnosed clinically significant COPD (patients with both respiratory symptoms and airflow obstruction). Overall prevalence in this age group was 4.7%, consistent with internationally available data,1 but lower than many recent studies reporting spirometrically defined COPD only.4 6 29
A total of 86.5% of those with clinically significant COPD did not report previously diagnosed chronic bronchitis/emphysema. This is comparable with other studies, despite their broader diagnostic criteria.4–6 A substantial burden of undiagnosed disease is not confined to milder cases; over half would be eligible for combination or anticholinergic inhaler treattment to reduce hospitalisation and mortality or pulmonary rehabilitation, which is effective in improving quality of life. Milder cases would be eligible for symptomatic relief, smoking cessation, and influenza and pneumococcal vaccinations under current guidance,9 and reports of subgroup analyses also indicate that inhalers may reduce hospitalisation and mortality in these patients too.30 31 This clearly demonstrates the need for case finding.
Our analyses of the HSE suggest that case finding among responding ever smokers aged 40–79 years would result in a yield of 7.7%; this is somewhat lower than most previous reports11 13 27 but partly reflects the tighter requirement in our COPD case criteria for the concurrent presence of both symptoms and airflow obstruction. The yield would be further reduced if more stringent spirometric criteria, such as double LLN, were used. We have also demonstrated that the addition of active to opportunistic case finding could potentially identify 70% more cases. This result is unaltered by varying the definition of COPD. Direct mailing alone to eligible patients (without the back-up of opportunistic discovery for non-respondents) would only have small benefits over a reasonably well-implemented opportunistic approach.
In England, there are an estimated 10.9 million ever smokers aged 40–79 years without a diagnosis of COPD.32 33 Implementation of such an active case-finding approach could identify 403 073 new cases. Of these, 108 024 (26.8%) may be eligible for combination inhalers under current guidance. Assuming that patients with more severe undiagnosed COPD are hospitalised at a similar rate to those who are diagnosed, treatment with combination inhalers could reduce the number of annual hospitalisations by at least 3328 (combination treatment reduces on average 0.03 COPD-related hospitalisations per person30), 1401 more than with an opportunistic-only approach, and could also prevent 2885 deaths over 3 years.30 This is a conservative estimate, as patients could also benefit from pulmonary rehabilitation, smoking cessation and single inhalers, all with health benefits. Furthermore, even those with less severe disease (FEV1 >50% predicted) may benefit from combination inhalers or anticholinergic inhalers.
Our models show that modifying the target population for case finding (eg, smokers aged >45 and ex-smokers aged >55) could increase yield and improve the efficiency while detecting a similar proportion of all undiagnosed COPD.
Limitations
The estimates for most analyses were taken from the HSE 1995–1996. These data have the advantage of being representative of the English population and were obtained using standardised methodology, but the HSE was not designed for this purpose and therefore suffers from some limitations. The spirometry standards may not be as rigorous as those now recommended by the American Thoracic Society (ATS). Post-bronchodilator spirometry was not available, and assumptions made to help separate patients with COPD from those with asthma may have misclassified COPD in either direction. Participants were not explicitly questioned about whether they had ever been diagnosed with COPD; they were asked to report any longstanding illness which was coded as chronic bronchitis/emphysema or ‘other respiratory conditions’. The latter included ‘bad chest’ or ‘chesty cough’, which may signify a diagnosis of COPD. The reported diagnosis of COPD was likely to be underestimated; indeed the diagnosed prevalence of COPD in 1997 was 1.5%34 compared with the 1.0% reported in the 1995–1996 HSE population. However, even if the number of diagnosed cases of COPD were double, there would still have been >70% undiagnosed. This misclassification should not affect the case-finding model which focuses purely on those who had no previous diagnosis.
Smoking habits and the definitions of COPD may have changed since data collection 12 years ago, and the diagnosis of COPD in the UK may have improved with the advent of the Quality Outcomes Framework. However, in 1997, the diagnosed prevalence of COPD was ~1.5%34 and in 2006 1.4%.35 Even if the proportion of undiagnosed COPD varied, the case-finding model will remain valid.
Implications
We have demonstrated substantial undiagnosed COPD in the community of ~4% among adults ≥30 years in England. Identifying these cases would potentially have huge resource implications but could prevent a significant number of annual hospitalisations. The model is based on a simple spreadsheet and could be adapted to different settings and countries where rates of smoking, prevalence of respiratory symptoms and prevalence of airways obstruction might vary.
The model relies on estimates from the literature, though some of the process inputs could vary greatly. In order for the cost-effectiveness of both approaches to be fully evaluated, well-constructed primary studies are needed with examination of different scenarios. For example, the case for applying financial incentives to GPs to ask respiratory questions versus prioritising resources for ensuring optimal questionnaire response rate could be evaluated, as could the potential for more sensitive algorithms to identify patients at risk, and different methods of delivering spirometry.
In summary, our study adds to the evidence around case finding for COPD by confirming and quantifying the extent of undiagnosed clinically significant COPD and providing a simple model of two alternative case-finding approaches. This can feed into the development of case-finding strategies likely to be needed in the new National Clinical Strategy in England and also provides a flexible model which can be applied to other healthcare settings.
Supplementary Material
Acknowledgements
We are grateful to the UK Data Archive, University of Essex, and the Health Survey for England 1995, 1996, 2001 and 2007 for providing access to their data.
Footnotes
Supplementary tables are published online only. To view these files please visit the journal online (http://thorax.bmj.com).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–32. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 2.On the state of the Public Health Annual report of the chief medical officer. [accessed 5 May 2010]. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4115781.pdf.
- 3.Gruffydd-Jones K. A national strategy for the management of chronic obstructive pulmonary disease (COPD) in England: aiming to improve the quality of care for patients. Prim Care Resp J. 2008;17(Suppl 1):S1–8. doi: 10.3132/pcrj.2008.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data From the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 5.Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63:402–7. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- 6.Shahab L, Jarvis MJ, Britton J, et al. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61:1043–7. doi: 10.1136/thx.2006.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force Screening for chronic obstructive pulmonary disease using spirometry: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;148:529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 8.Wilt TJ, Niewoehner D, MacDonald R, et al. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639–53. doi: 10.7326/0003-4819-147-9-200711060-00009. [DOI] [PubMed] [Google Scholar]
- 9.The National Collaborating Centre for Chronic Conditions Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 10.Wilt T, Kim C-B, Kane R, et al. Use of spirometry for case finding, diagnosis and management of chronic obstructive pulmonary disease (COPD). Evidence Report/Technology assessment No 121 (prepared by the Minnesota Evidence-based practice Center under Contract No 290-02-0009) 2005;AHRQ Publication No. 05-E017-2. Agency for Healthcare Research and Quality; Rockville, MD: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Schayck CP, Loozen JMC, Wagena E, et al. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ. 2002;324:1370. doi: 10.1136/bmj.324.7350.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buffels J, Degryse J, Heyrman J, et al. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO study. Chest. 2004;125:1394–9. doi: 10.1378/chest.125.4.1394. [DOI] [PubMed] [Google Scholar]
- 13.Tinkelman D, Price D, Nordyke R, et al. COPD screening efforts in primary care: what is the yield? Prim Care Resp J. 2007;16:41–8. doi: 10.3132/pcrj.2007.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joint Health Surveys Unit of Social and Community Planning Research and University College London. Health survey for England, 1995 [computer file] 3rd edn. UK Data Archive [distributor]; Colchester, Essex: Mar 26, 2001. SN: 3796, 2001. [Google Scholar]
- 15.Joint Health Surveys Unit of Social and Community Planning Research and University College London. Health survey for England, 1996 [computer file] 3rd edn. UK Data Archive [distributor]; Colchester, Essex: Mar, 2001. SN: 3886, 2001. [Google Scholar]
- 16.Global Initiative for Chronic Lung Disease Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [accessed 5 May 2010]. Feb, 2009. http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=2003.
- 17.Quanjer P, Tammeling G, Cotes J, et al. Report working party Standardisation of lung function tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl 16):5–40. [PubMed] [Google Scholar]
- 18.Culver B. Interpretation of spirometry: we can do better than the GOLD standard. Respir Care. 2006;51:719–20. [PubMed] [Google Scholar]
- 19.Martinez F, Raczek A, Seifer F, et al. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS) COPD. 2008;5:85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price D, Tinkelman D, Halbert RJ, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73:285–95. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]
- 21.Calverley PMA, Nordyke R, Halbert RJ, et al. Development of a population-based screening questionnaire for COPD. COPD. 2005;2:225–32. [PubMed] [Google Scholar]
- 22.Jordan RE, Hawker JI, Ayres JG, et al. Effect of social factors on winter hospital admission for respiratory disease: a case control study of older people in the UK. Br J Gen Pract. 2008;58:e1–9. doi: 10.3399/bjgp08X302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzmaurice DA, Hobbs FDR, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. doi: 10.1136/bmj.39280.660567.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.QRESEARCH and The Information Centre for health and social care Trends in consultation rates in general practice 1995 to 2006: Analysis of the QRESEARCH database. [accessed 5 May 2010]. http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/general-practice/trends-in-consultation-rates-in-general-practice-1995–2006.
- 25.Britton M. The burden of COPD in the UK: results from the confronting COPD survey. Respir Med. 2003;97(Suppl 3):S71–9. doi: 10.1016/s0954-6111(03)80027-6. [DOI] [PubMed] [Google Scholar]
- 26.Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract. 2002;52:373–80. [PMC free article] [PubMed] [Google Scholar]
- 27.Vandevoorde J, Verbanck S, Gijssels L, et al. Early detection of COPD: a case finding study in general practice. Respir Med. 2007;101:525–30. doi: 10.1016/j.rmed.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Yawn BP, Enright PL, Lemanske RF, et al. Spirometry can be done in family physicians offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132:1162–8. doi: 10.1378/chest.06-2722. [DOI] [PubMed] [Google Scholar]
- 29.Buist AS, McBurnie M, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 30.Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 31.Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–8. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 32.National Centre for Social Research and University College London . Department of Epidemiology and Public Health. Health Survey for England, 2007 [computer file] UK Data Archive [distributor]; Colchester, Essex: Feb, 2009. SN: 6112, 2009. [Google Scholar]
- 33.Office for National Statistics Mid-2007 UK, England and Wales, Scotland and Northern Ireland population estimates. [accessed 5 May 2010]. http://www.statistics.gov.uk/statbase/Product.asp?vlnk=15106.
- 34.Soriano JB, Maier WC, Egger P, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax. 2000;55:789–94. doi: 10.1136/thorax.55.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health (England) Quality and outcomes framework. [accessed 5 May 2010]. http://www.dh.gov.uk/en/Healthcare/Primarycare/Primarycarecontracting/QOF/index.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.