Abstract
Objectives
Few epidemiological studies have examined differences in the prevalence of risk factors and comorbidities in patients with systolic heart failure (HF), as compared to those with diastolic HF.
Methods
We analyzed data from 1,426 residents of the Worcester (MA) metropolitan area hospitalized at all 11 greater Worcester medical centers for acute HF during 1995 and 2000 who had data available on ejection fraction (EF) findings during hospitalization. The analysis was conducted based on the presence of either normal (diastolic HF), as compared to reduced (systolic HF) EF, using an EF cutpoint of ≥50%.
Results
The average age of study patients was 71 years, 56% were women, and 43% had diastolic HF. Patients with diastolic HF were more likely to be older, female, obese, and to have higher systolic blood pressures and lower heart rates at the time of hospital presentation than patients with systolic HF. In contrast, patients with systolic HF had a greater prevalence of diabetes, previous myocardial infarction, and a history of alcohol abuse as compared to patients with diastolic HF. In multivariate analyses, the strongest metabolic correlates of diastolic HF were obesity, hypertension, and clustered metabolic risk factors; diabetes was associated with the occurrence of systolic HF.
Conclusions
The results of our population-based investigation demonstrate that multiple risk factors and comorbidities are present in patients with systolic and diastolic HF. Consideration of these comorbidities and risk factors should be taken into account in distinguishing patients with diastolic HF from those with systolic HF and in their optimal management.
Keywords: Epidemiology, congestive heart failure, ejection fraction, echocardiography
Introduction
The prevalence of heart failure (HF) in developed countries is rapidly increasing, partially due to the aging of these industrialized societies. In 2005, HF affected more than 5 million Americans with an estimated incidence of more than 600,000 new cases occurring annually (1–3). Findings from several recent studies suggest an increase in the prevalence of patients with HF and preserved left ventricular ejection fraction (EF) (4,5). This condition is recognized as diastolic HF (1).
While the presence of systolic and diastolic HF are related to an increased risk of adverse outcomes, the underlying pathophysiologies of these clinical syndromes are thought to be markedly different (5,6). These findings have been based on several studies that have found the highest prevalence rates of diastolic HF to be observed in the elderly, as well as in obese and/or hypertensive women without prevalent myocardial infarction (7,8).
While acute myocardial infarction is considered to be the most common cause of HF with reduced EF (systolic HF) (6), recently observed increases in the prevalence of diastolic HF are thought to be the result of increases in average life expectancy and greater prevalence of hypertensive cardiac hypertrophy and obesity, perhaps mediated through a chronic cardiac overload state (6,9).
The majority of studies examining the epidemiology of diastolic HF have been conducted in the ambulatory setting with wide variation in population demographic and clinical characteristics as well as inclusion criteria (10–12). Limited data exist on patients hospitalized with decompensated HF, particularly from the more broadly generalizable perspective of a community-wide investigation.
The objectives of the present study were to describe the prevalence and clustering of a variety of cardiac risk factors and important comorbidities in ‘real world’ patients with diastolic HF compared to those with systolic HF in patients hospitalized with decompensated HF at all central Massachusetts medical centers. Data from the population-based Worcester Heart Failure Study were utilized for purposes of the present investigation (13–15).
Methods
The study population consisted of 4,537 adult male and female residents of all ages from the Worcester (MA) metropolitan area hospitalized for possible acute HF at all 11 greater Worcester medical centers during the 2 study years of 1995 and 2000 (13–15).
The medical records of greater Worcester residents hospitalized at all area medical centers with primary and/or secondary International Classification of Disease (ICD)-9 discharge diagnoses consistent with the possible presence of HF were reviewed in a standardized manner (13,15). Patients with a discharge diagnosis of HF (ICD-9 code 428) comprised the primary diagnostic rubric reviewed for the identification of cases of possible HF. In addition, the medicalrecords of patients with discharge diagnoses of rheumatic HF (code 398.9), hypertensive heart and renal disease (codes 402 and 404, respectively), acute cor pulmonale (code 415), other diseases of the endocardium (code 424), cardiomyopathy (code 425.4), pulmonary heart disease and congestion (codes 416.9 and 514, respectively), acute lung edema (code 518.4), edema(code 782.3), and dyspnea and respiratory abnormalities (code 786) were reviewed by trained study physicians and nurses to identify patients who also may have had new-onset HF. Confirmation of the diagnosis of HF, based on use of the Framingham criteria, included the presence of 2 major criteria or presence of 1 major and 2 minor criteria (16).
Patients who developed HF during hospital admission for another acute illness (e.g., acute myocardial infarction), or after an interventional procedure or surgery (e.g., percutaneous coronary intervention (PCI)) were excluded from the study sample. Presence of a prior history of cardiovascular disease was determined based on the review of information contained in hospital medical charts.
For purposes of the present analysis, only patients with an available echocardiogram during the index hospitalization for acute HF and who had a quantitative analysis of ejection fraction (EF) were included.
Data Collection
Information on patient’s demographic factors, medical history, clinical characteristics, presenting symptoms, physical examination findings and laboratory test results was collected through the review of medical records by trained physician and nurse data abstractors. This included information about patient’s age, sex, race, prevalent cardiovascular disease (e.g., myocardial infarction (MI), stroke/TIA), body mass index (BMI), presenting symptoms, physical examination findings, laboratory findings (e.g., serum levels of creatinine, glucose) and clinical characteristics (e.g., blood pressure, heart rate).
Echocardiographic examinations were performed at the time of hospital admission and reported by cardiologists at all greater Worcester hospitals. Data on left ventricular ejection fraction were abstracted from patients’ medical charts and included in the study database.
Definition of Cardiac Risk Factors
Hypertension, type 2 diabetes, and dyslipidemia were considered to be present based on the review of data contained in patient’s hospital medical records. Obesity was defined as a body mass index (BMI)≥30 Kg/m2, following current guidelines (17). Glomerular filtration rate (GFR) was estimated using the Modification Diet in Renal Disease (MDRD) formula (18). Patients were defined as having either normal renal function (GFR ≥60ml/min per 1.73 m2) or renal dysfunction (GFR<60ml/min per 1.73 m2) based on this formula. The clustered metabolic phenotype was defined as the concurrent presence of at least 3 metabolic risk factors (among diabetes, obesity, hypertension, and dyslipidemia).
Risk factor control was assessed based on anthropometric and laboratory data obtained at the time of hospital admission. In patients with a prior diagnosis of hypertension, poor blood pressure control was defined when the systolic blood pressure exceeded 140mmHg; in patients with a previous history of diabetes, poor glycemic control was defined when the blood glucose level at the time of hospital admission (i.e., non fasting) exceeded 180mg/dL.
Data Analysis
Individuals with a normal EF (≥50%) were defined as having diastolic HF while those with a reduced EF (<50%) were defined as having systolic HF. Comparison of the characteristics of patients with diastolic HF and systolic HF was performed through the use of chi-square and t-tests for categorical and continuous variables, respectively.
Given the well-documented association between ischemic heart disease and reduced left ventricular EF, a subgroup analysis was performed in patients without a history of MI with the specific aim of identifying a possible association between metabolic risk factors and type of HF, in the absence of the confounding effects of prior MI.
Multivariate logistic regression models were performed, controlling for age, sex, and prevalent cardiovascular disease, for purposes of identifying metabolic correlates of systolic and diastolic HF. Odds ratios (OR) and accompanying 95% confidence intervals (CI) of factors associated with the occurrence of either systolic or diastolic HF were calculated in a standard manner.
Results
Study sample characteristics
Of the 4,537 patients included in our study sample, 1,725 (38%) underwent an echocardiographic examination during hospitalization. Of these, 1,426 (83% of patients with an available echocardiogram or 32% of the total study sample) had data available on EF to diagnosis the presence of systolic or diastolic HF. As compared to the total study sample, patients with available EF findings were slightly younger (75 vs. 77 years) but had similar distributions of sex, BMI, heart rate, serum glucose, hemoglobin, and plasma creatinine findings.
Diastolic HF was diagnosed in 612 patients, representing 43% of the analyzed study population. Patients diagnosed with diastolic HF were more likely to be older, female, and present with higher systolic blood pressures and a higher BMI at the time of hospitalization, and lower heart rates (p<0.05) (Table 1). Patients with systolic HF had significantly higher levels of serum potassium, and a significantly lower estimated GFR, as compared to patients with diastolic HF.
Table 1.
Characteristics of Patients According to Type of Heart Failure (HF)
| Diastolic HF (n=612) | Systolic HF (n=814) | P Value | |
|---|---|---|---|
| Demographic and Clinical Factors | |||
| Age (mean, yrs) | 76.4±11.7 | 74.1±12.2 | <0.001 |
| Men (%) | 31.7 | 53.8 | <0.001 |
| Systolic BP (mean, mmHg) | 151.5±33.5 | 144.7±31.5 | <0.001 |
| Diastolic BP (mean, mmHg) | 77.8±20.4 | 79.8±19.3 | 0.07 |
| Heart Rate (mean, bpm) | 89.3±23.2 | 93.3±23.5 | <0.001 |
| BMI (mean, Kg/m2) | 28.4±7.9 | 27.4±7.1 | <0.05 |
| Clinical Symptoms (%) | |||
| Dyspnea on exertion | 94.3 | 95.6 | 0.27 |
| Orthopnea | 35.6 | 43.0 | <0.005 |
| Paroxysmal nocturnal dyspnea | 14.9 | 23.6 | <0.001 |
| Nocturnal cough | 50.8 | 49.8 | 0.69 |
| Fatigue | 9.2 | 12.0 | 0.08 |
| Palpitations | 8.0 | 8.5 | 0.75 |
| Anorexia/weight loss | 14.9 | 14.6 | 0.90 |
| Peripheral edema | 72.5 | 70.4 | 0.37 |
| Laboratory Findings (mean) | |||
| Serum glucose (mg/dL) | 160.7±73.7 | 166.7±83.0 | 0.16 |
| Serum creatinine (mg/dL) | 1.46±1.13 | 1.66±1.24 | <0.05 |
| Serum sodium (mg/dL) | 137.4±7.2 | 137.3±5.2 | 0.49 |
| Serum potassium (mg/dL) | 4.4±0.7 | 4.5±0.7 | <0.05 |
| Hemoglobin (mg/dL) | 12.1±2.9 | 12.4±2.3 | 0.17 |
| Estimated GFR (ml/min/1.73 m2) | 56.6±27.9 | 52.3±25.0 | <0.005 |
BP= blood pressure
BMI= body mass index
GFR = glomerular filtration rate
Virtually all patients with decompensated HF presented to greater Worcester hospitals with dyspnea on exertion and peripheral edema, without significant differences in these clinical signs and symptoms noted between our two primary comparison groups. Patients with systolic HF were more likely to present with orthopnea and paroxysmal nocturnal dyspnea as compared to patients with diastolic HF (Table 1).
Prevalence of Cardiac Risk Factors and Cardiovascular Comorbidities
Patients with diastolic HF had a significantly greater prevalence of obesity, hypertension, and clustered metabolic abnormalities compared to patients with systolic HF (Table 2). In contrast, patients with systolic HF had a higher prevalence of prior diabetes and history of alcohol abuse.
Table 2.
Prevalence of Coronary Risk Factors and Prior Cardiovascular Events According to Type of Heart Failure (HF)
| Diastolic HF (n=612) | Systolic HF (n=814) | P Value | |
|---|---|---|---|
| Cardiac Risk Factors (%) | |||
| Obesity | 34.6 | 26.6 | <0.005 |
| Dyslipidemia | 21.4 | 23.7 | 0.30 |
| Hypertension | 69.0 | 63.5 | <0.05 |
| Diabetes | 33.8 | 40.3 | <0.05 |
| Clustered metabolic risk factors | 22.3 | 17.9 | 0.05 |
| Renal dysfunction | 59.7 | 63.8 | 0.11 |
| COPD | 30.9 | 26.2 | 0.052 |
| Cigarette smoking | 9.2 | 11.8 | 0.11 |
| Alcohol abuse | 11.3 | 16.6 | <0.005 |
| Prevalent Cardiovascular Comorbidities (%) | |||
| Heart failure | 59.3 | 63.4 | 0.12 |
| Myocardial infarction | 34.0 | 53.7 | <0.001 |
| Stroke | 12.1 | 13.1 | 0.55 |
| Transient ischemic attack | 3.9 | 4.7 | 0.49 |
| Pulmonary embolus | 1.8 | 1.6 | 0.77 |
| Peripheral vascular disease | 15.7 | 20.5 | <0.05 |
| No prior cardiovascular event | 27.1 | 18.9 | <0.005 |
COPD = Chronic obstructive pulmonary disease
The distribution of prior cardiovascular events was also significantly different between our 2 primary comparison groups. Slightly more than one quarter (27%) of patients hospitalized with diastolic HF did not have a history of prior cardiovascular disease compared with 18% of patients with systolic HF. More than one half of patients with systolic HF had a prior history of MI compared with only 34% of patients with diastolic HF (p<0.001). Patients with systolic HF were also more likely to have reported a history of previously diagnosed peripheral vascular disease in comparison to patients with diastolic HF (Table 2). Relatively similar differences in the characteristics of patients with diastolic HF, as compared to those with systolic HF, were noted when we carried out separate analyses in men and women and in those of different age strata.
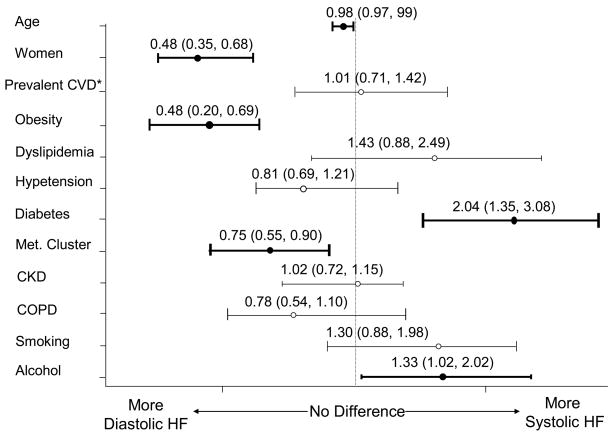
To reduce the potential confounding effect of a history of prior MI in examining the association of various coronary risk factors with type of HF, we carried out a subgroup analysis in the 781 patients without previous documentation of MI. Similar proportions of patients with systolic HF (52%) and diastolic HF (48%) were observed in this subgroup of patients compared to the overall study sample. Risk factors significantly associated with the presence of systolic HF in this subgroup of patients were histories of prior diabetes and alcohol abuse, whereas patients with diastolic HF were characterized by a higher prevalence of obesity, women, and clustered metabolic abnormalities (Figure 1).
Figure 1.
Unadjusted odds ratios for diastolic HF vs systolic HF (including anthropometrics, cardiovascular risk factors, and prevalent history of cardiovascular disease (CVD) other than MI); odds ratio and 95% confidence interval (in parenthesis) are shown.
Multivariate Correlates of Systolic or Diastolic HF
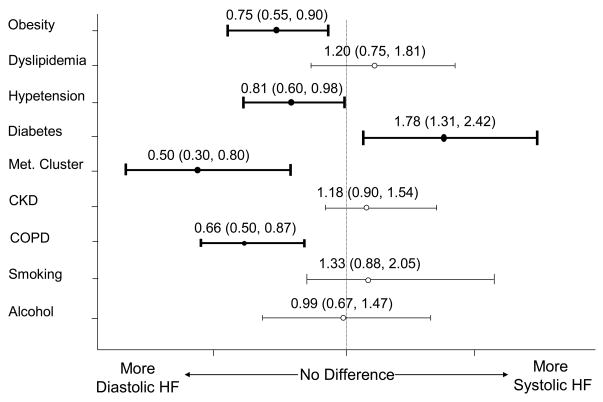
Our univariate observations were further confirmed by the results of our multivariate adjusted regression analyses performed in the entire study sample of 1,426 patients (Figure 2). Independent of the effects of age, sex, and prevalent cardiovascular disease (MI, pulmonary embolus, peripheral vascular disease, transient ischemic attack, stroke, or heart failure), patients with systolic HF were more likely to present with a history of diabetes. On the other hand, patients with diastolic HF were more likely to present with an adverse metabolic risk profile, characterized by the presence of obesity, hypertension, COPD, and a greater frequency of clustered metabolic abnormalities.
Figure 2.
Multivariate Logistic Regression Results (adjusted for differences in age, sex, and history of prevalent cardiovascular disease) for cardiovascular risk factors and diastolic HF vs. systolic HF (N=1,426); odds ratio and 95% confidence interval (in parenthesis) are shown.
In comparing differences in the control of several important coronary risk factors, in over one half of patients with a prior diagnosis of diabetes, irrespective of type of HF, plasma glucose levels were not well controlled at the time of hospital admission (52% of patients with systolic HF and 51% of patients with diastolic HF). In contrast, a significantly higher prevalence of poorly controlled hypertension was observed in the presence of diastolic HF as compared to systolic HF (62% vs 56% respectively; p<0.05).
We performed an additional multivariate logistic regression analysis comparing patients with an EF ≥55% (diastolic HF: n=418) and patients with severely reduced EF (≤35%: systolic HF; n=475). This post-hoc analysis was performed with the specific aim of overcoming the potentially confounding effect of patients having possible concurrent systolic and diastolic HF, and to minimize any potential bias related to the choice of an arbitrary value of EF to separate patients with diastolic HF from those with systolic HF. The results of this regression analysis revealed findings similar to those reported in the entire study sample who were dichotomized into those with systolic as compared to those with diastolic HF at an EF cutpoint of 50%. In particular, a significant association was noted for clustered metabolic risk factors and COPD with diastolic HF, while diabetes was confirmed as the only independent metabolic correlate of systolic HF (all p<0.05).
Discussion
The objective of the present observational study was to describe differences in the frequency of important comorbidities and metabolic risk factors in a community-wide population of patients hospitalized for acute HF. Evidence that the prevalence of risk factors in patients with systolic HF is significantly different from that of patients with diastolic HF strengthens the notion that markedly different pathophysiological pathways might lead to similar clinical symptoms and outcomes in patients with HF. In addition, the identification of specific metabolic clusters associated with HF might improve the ability of clinicians to identify patients at higher risk for diastolic rather than systolic HF and guide the use of different treatment strategies in these patient populations.
Our principal findings were that while systolic HF is associated with a high prevalence of previous cardiovascular disease, especially MI, in more than one quarter of patients with diastolic HF the current hospitalization for HF represents their first overt cardiovascular event. In addition, we found that systolic HF was independently associated with risk factors known to be related to the underlying pathophysiology of coronary artery disease such as diabetes; in contrast, diastolic HF was observed more often in the very elderly, in women, and in patients with either individual or clustered metabolic abnormalities. Moreover, in patients without a history of MI, systolic HF was associated with higher rates of diabetes. On the other hand, diastolic HF was more often noted in patients with clustered metabolic risk factors and a cardiac overload state, resulting from the concurrent presence of obesity, hypertension, and COPD.
The majority of the published data that has examined the frequency of various risk factors and cardiovascular comorbidities in patients with decompensated HF has been derived from patients enrolled in clinical trials with more narrowly defined inclusion criteria and potentially limited clinical applicability. In contrast, a limited number of epidemiological surveys of patients with HF, including the Acute Decompensated Heart Failure National Registry (ADHERE)(19) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) (20), have provided relatively contemporary and broader insights into the clinical characteristics of patients hospitalized with acutely decompensated HF.
The results of the present study confirm previous reports from the ADHERE study and the OPTIMIZE-HF registry showing that patients with diastolic HF are usually older, more likely to be women, and are more likely to present with uncontrolled hypertension and obesity than patients with systolic HF (20,21). However, in contrast with the findings of previous reports, diabetes was noted significantly more often in patients with systolic HF than in those with diastolic HF.
Our findings suggest that the association between diabetes mellitus and HF might be mediated by the presence of underlying coronary artery disease, despite the absence of prior MI (itself a common macrovascular complication of diabetes mellitus), resulting in the development of systolic HF. Previous reports have suggested that since diabetes mellitus, hypertension, central obesity, and coronary heart disease often coexist, these factors may act synergistically to produce left ventricular dysfunction, sustain its progression, and precipitate the onset of acute HF (21,22). However, while features associated with increased cardiac volume load (e.g., obesity, hypertension, and clustered metabolic abnormalities) were strongly associated with preserved EF in the present study, diabetes was independently associated with the occurrence of systolic HF. A possible explanation of this finding is that, in our patient population, isolated diabetes was more often noted in patients with systolic HF, while in the presence of diastolic HF, diabetes was associated with other metabolic abnormalities in nearly all patients.
Data from previous studies have shown that patients with diastolic HF are clinically indistinguishable from those with left ventricular systolic HF and the presence of symptoms or abnormal physical examination findings at the time of hospital admission have been shown to be similar despite differences in underlying pathophysiology (20,21). In the present study, we only partially confirm these findings. Despite the clinical similarity between acutely decompensated patients with HF with and without a reduced EF, patients with systolic HF were more likely to present to greater Worcester hospitals with orthopnea and paroxysmal nocturnal dyspnea as compared to patients with diastolic HF.
It is important to note that the differences observed in the present study with regards to the frequency of various risk factors and acute clinical symptoms might be the result of different cutpoints used to distinguish patients with diastolic HF from those with systolic HF. In fact, while the diagnosis of diastolic HF was defined as an EF ≥50%, previous reports have used different cutoff values (40% or 45%) to discriminate between these patient groups. However, in our post-hoc analysis, we found similar associations between various cardiac risk factors and type of HF when applying different cutpoints for the definition of systolic HF (EF≤35%) and diastolic HF (EF≥55%), supporting the hypothesis that an EF cutpoint of 50% might effectively distinguish patients with diastolic HF from those with systolic HF.
Study Limitations and Strengths
As with all observational studies, there are a number of potential limitations that must be kept in mind in interpreting the study results. One limitation is the potential for selection bias since only one third of patients admitted for acute HF underwent echocardiography and had EF findings available. Of note, patients with EF data did not appreciably differ in clinical and laboratory characteristics as compared to patients who did not have this information available. In addition, while data on EF were collected, indices of left ventricular geometry and diastolic function were not available for further analysis. Another potential limitation is the lack of data regarding brain natriuretic peptide (BNP), as it was not part of the standard assessment of HF during the years under study. However, it should be noted that while BNP is effective in differentiating cardiac HF from non-cardiac HF, its ability to discriminate systolic from diastolic HF has been shown to be very poor (23). In addition, the majority of our study population was Caucasian and our findings may not be generalizable to other racial groups. A major strength of the current study is that we included a population-based sample of patients who had objective assessment of their left ventricular function by echocardiography at the time of their initial hospitalization for decompensated HF.
Conclusions
The distribution of coronary risk factors and comorbidities is significantly different in ‘real world’ patients with systolic HF and diastolic HF. Independent of differences between patients with systolic HF and diastolic HF in terms of age, sex, and prevalent cardiovascular disease, systolic HF is strongly associated with diabetes, while obesity, hypertension, and clustered metabolic abnormalities are more frequently detected in patients with diastolic HF. These findings provide insights into the clinical profile of patients with systolic HF compared to those with diastolic HF which may help in facilitating the diagnosis of these clinical syndromes, in understanding the differing pathophysiologies of these clinical syndromes, and in enhancing the treatment of these patients.
Acknowledgments
Grant support for this project was provided by the National Heart, Lung, and Blood Institute (R37 HL69874).
This study was made possible through the cooperation of the administration, medical records, and cardiology departments of participating Worcester metropolitan area hospitals.
Reference List
- 1.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S. 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Aurigemma GP. Diastolic heart failure--a common and lethal condition by any name. N Engl J Med. 2006;355:308–310. doi: 10.1056/NEJMe068128. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–1574. doi: 10.1016/0735-1097(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 6.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 8.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 9.Bench T, Burkhoff D, O’Connell JB, Costanzo MR, Abraham WT, St John Sutton M, et al. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57–64. doi: 10.1007/s11897-009-0010-z. [DOI] [PubMed] [Google Scholar]
- 10.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 11.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Badano LP, Albanese MC, De Biaggio P, Rozbowsky P, Miani D, Fresco C, et al. Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr. 2004;17:253–261. doi: 10.1016/j.echo.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167:490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RJ, Goldberg JH, Pruell S, Yarzebski J, Lessard D, Spencer FA, et al. Delays in seeking medical care in hospitalized patients with decompensated heart failure. Am J Med. 2008;121:212–218. doi: 10.1016/j.amjmed.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: A community-wide perspective. Am J Med. 2005;118:728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 16.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 17.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults– The Evidence Report. National Institutes of Health. Obes Res. 1998;6(suppl):51S–209S. [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 21.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure: traits among urban blacks. Arch Intern Med. 1988;148:2013–2016. [PubMed] [Google Scholar]
- 22.Tsuyuki RT, McKelvie RS, Amold JM, Avezum A, Jr, Barretto AC, Carvalho AC, et al. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med. 2001;161:2337–2342. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- 23.Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, et al. Breathing Not Properly Multinational Study Investigators. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–2017. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]