Abstract
America’s obesity epidemic has gathered much media attention recently. A rise in the percent of the population who are obese coincides with an increase in the widespread use of non-caloric artificial sweeteners, such as aspartame (e.g., Diet Coke) and sucralose (e.g., Pepsi One), in food products (Figure 1). Both forward and reverse causalities have been proposed [1,2]. While people often choose “diet” or “light” products to lose weight, research studies suggest that artificial sweeteners may contribute to weight gain. In this mini-review, inspired by a discussion with Dr. Dana Small at Yale’s Neuroscience 2010 conference in April, I first examine the development of artificial sweeteners in a historic context. I then summarize the epidemiological and experimental evidence concerning their effects on weight. Finally, I attempt to explain those effects in light of the neurobiology of food reward.
Keywords: artificial sweeteners, non-caloric, obesity, food reward, postingestive effects, saccharin, aspartame, sucralose, acesulfame K, neotame, sugar, weight gain
Sweeteners
We owe the discovery of several artificial sweeteners to a few brave scientists who violated the code of laboratory hygiene and tasted their samples, often inadvertently [3]. Saccharin, the oldest artificial sweetener, was discovered by Constantine Fahlberg at Johns Hopkins in 1879 [4] while working on coal tar derivatives. For decades after its debut, saccharin remained a specialty product for diabetics on stores’ medicinal shelves [5]. A sugar shortage during World War II and shift of esthetics toward favoring a thin figure encouraged women to turn to artificial substitutes as well [6]. Around this time, the wording on diet soda bottles subtly changed from “for use only in people who must limit sugar intake” to “for use in people who desire to limit sugar intake” [7]. Saccharin is about 300 times sweeter than sucrose but has a bitter aftertaste. Cyclamate, which was discovered in 1937 by Michael Sveda at the University of Illinois [8], was often blended with saccharin to improve the taste. Both compounds were deemed “generally recognized as safe” in the 1958 Food Additives Amendment to the Federal Food, Drug, and Cosmetics Act. After the Food and Drug Administration (FDA) banned cyclamate in 1969 because of its carcinogenic potentials, concern about saccharin’s safety also intensified. Eventually, the FDA announced its intention to ban saccharin in 1977. Avid consumer protests led to a moratorium from Congress on the final ban decision. A warning label was nonetheless required on all saccharin products. Subsequent studies refuted the link between cyclamate and cancer [9]. Bladder cancer associated with saccharin ingestion was also found to be specific to rodent physiology [9]. Cyclamate continues to be marketed in about 50 countries, including Canada. The saccharin warning label was removed in 2000.
Even though saccharin stayed on the U.S. market, regular artificial sweetener users were relatively few until the next generation of compounds arrived (Figure 1, orange line) [2]. Rigorous safety testing preceded FDA approval for those new artificial sweeteners. In 1965, James Schlatter at Searle discovered aspartame [10]. He was trying to make new ulcer drugs. Aspartame consists of two amino acids, phenylalanine and aspartate, linked to a methanol backbone (Figure 2). Unlike the other artificial sweeteners that are usually excreted unchanged, aspartame can be metabolized. Therefore, it is not strictly non-caloric (4 Kcal/g) and forbidden in people with phenylketonuria [9]. Aspartame is about 200 times sweeter than sucrose. Due to the small amount ingested at a time, its caloric contribution is negligible. The FDA approved aspartame first for use in dry foods in 1981, then as a general sweetener in 1996. Monsanto bought Searle and converted it into NutraSweet in 1984. The patent on aspartame expired in 1992. Amid competition from generic manufacturers, NutraSweet engineered neotame, which was approved in 2002 [11]. Neotame is the most potent sweetener on the market, at 7,000 times the sweetness of sucrose.
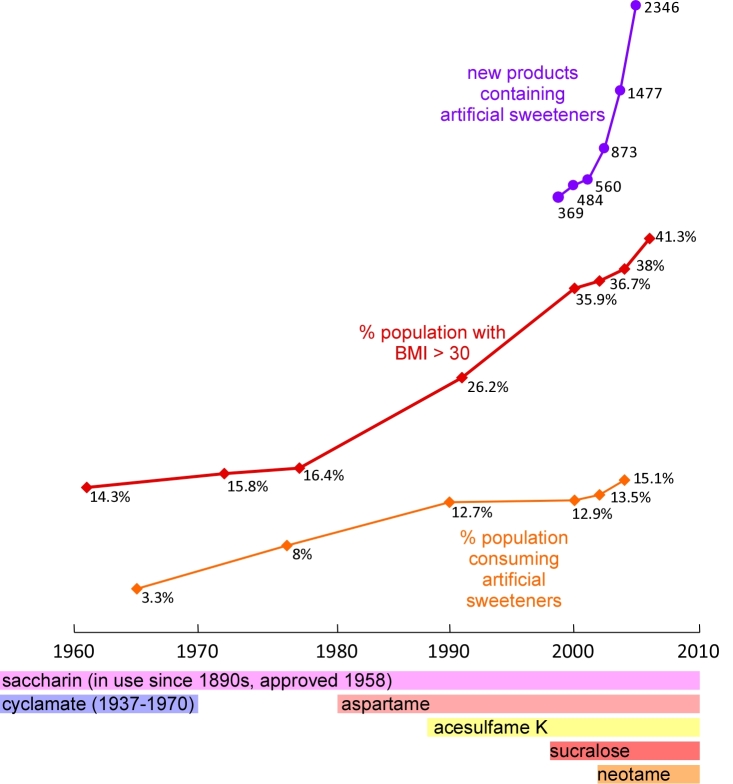
Figure 1.
Time line of artificial sweetener use and obesity trends in the United States. Blue line: changes in the percentage of the population who are obese (BMI >30) from 1961 to 2006. Source: National Health and Nutrition Examination Survey [57]. Orange line: changes in the percentage of the population who are regular artificial sweetener users from 1965 to 2004. Source: National Household Survey [2]. Purple line: changes in the number of new artificial sweetener containing food products introduced to the American market from 1999 to 2004. Source: Mintel Market Analysis [14]. Bars below the time axis indicates the type and availability of artificial sweeteners in the United States over time. Source: Kroger et al [9].
Figure 2.
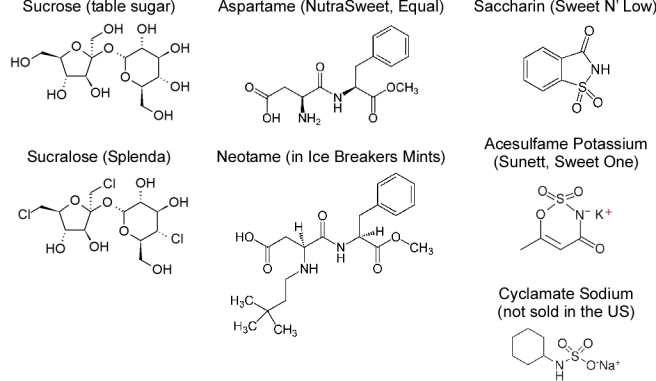
Structures of sucrose, a natural caloric sweetener, and various artificial sweeteners. Source: Kroger et al. [9] and Brown et al [25].
Acesulfame potassium resembles saccharin and cyclamate in structure and taste (Figure 2). Karl Clauss at Hoechst discovered it in 1967 [12]. The FDA approved its use in dry foods in 1988 and as a general sweetener in 2003.
The most recent structural advance came in 1979, when Shashikant Phadnis, a graduate student working for Tate & Lyle, discovered sucralose [3]. It is synthesized from sucrose by substituting chlorine for three of its hydroxyl groups, generating 600 times the sweetness (Figure 2). It was approved in 1999. Sucralose sales amounted to £148 million in 2008, generating 23 percent of Tate & Lyle’s total operating profit [13].
The last decade saw an explosive increase in the number of food products containing non-caloric artificial sweeteners. More than 6,000 new products were launched in the United States between 1999 and 2004 (Figure 1, purple line) [14]. Currently, an ingredient search on foodfacts.com yields 3,648 products containing one or more of the five FDA approved artificial sweeteners. Sucralose is the most popular (1,500 products), followed by acesulfame potassium (1,103 products) and aspartame (974 products). Artificial sweeteners are most commonly used in carbonated drinks. They also are found in a variety of other products, from baby food (e.g., Pedialyte) to frozen food (e.g., Lean Pockets). With such a diverse selection, it is more likely that people will encounter artificially sweetened items when making the day-to-day choices on food and beverages. The National Household Nutritional Survey estimated that as of 2004, 15 percent of the population regularly were using artificial sweetener [2]. IRI Consumer Report stated that 65 percent of American households bought at least one sucralose-containing product in 2008. Therefore, the total number of artificial sweetener consumers, either regular or sporadic, is probably much greater.
Do artificial sweeteners affect weight?
Intuitively, people choose non-caloric artificial sweeteners over sugar to lose or maintain weight. Sugar provides a large amount of rapidly absorbable carbohydrates, leading to excessive energy intake, weight gain, and metabolic syndrome [15,16,17]. Sugar and other caloric sweeteners such as high fructose corn syrup have been cast as the main culprits of the obesity epidemic. Whether due to a successful marketing effort on the part of the diet beverage industry or not, the weight conscious public often consider artificial sweeteners “health food” [6]. But do artificial sweeteners actually help reduce weight?
Surprisingly, epidemiologic data suggest the contrary. Several large scale prospective cohort studies found positive correlation between artificial sweetener use and weight gain. The San Antonio Heart Study examined 3,682 adults over a seven- to eight-year period in the 1980s [18]. When matched for initial body mass index (BMI), gender, ethnicity, and diet, drinkers of artificially sweetened beverages consistently had higher BMIs at the follow-up, with dose dependence on the amount of consumption. Average BMI gain was +1.01 kg/m2 for control and 1.78 kg/m2 for people in the third quartile for artificially sweetened beverage consumption. The American Cancer Society study conducted in early 1980s included 78,694 women who were highly homogenous with regard to age, ethnicity, socioeconomic status, and lack of preexisting conditions [19]. At one-year follow-up, 2.7 percent to 7.1 percent more regular artificial sweetener users gained weight compared to non-users matched by initial weight. The difference in the amount gained between the two groups was less than two pounds, albeit statistically significant. Saccharin use was also associated with eight-year weight gain in 31,940 women from the Nurses’ Health Study conducted in the 1970s [20].
Similar observations have been reported in children. However, childhood studies often were complicated by the more dynamic growth-associated diet changes. Consumption of both sugar-sweetened and artificially sweetened soda increased and milk consumption decreased with age [21]. A strict differentiation between artificial sweetener users and non-users was not possible. A two-year prospective study involving 166 school children found that increased diet soda consumption was associated with higher BMI Z-scores at follow-up, indicating weight gain [22]. The Growing Up Today Study, involving 11,654 children aged 9 to 14 also reported positive association between diet soda and weight gain for boys [23]. For each daily serving of diet beverage, BMI increased by 0.16 kg/m2. The correlation was not significant for girls. The National Heart, Lung, and Blood Institute Growth and Health Study followed 2,371 girls from age 9 to 19 for 10 years [24]. Both diet and regular soda drinking was associated with increase in total daily energy intake. Soda intake also predicted the greatest increase in BMI, although the correlation between diet soda and BMI was not significant. A cross-sectional study looking at 3,111 children and youth found diet soda drinkers had significantly elevated BMI [21].
In addition, consensus from interventional studies suggests that artificial sweeteners do not help reduce weight when used alone [2,25]. BMI did not decrease after 25 weeks of substituting diet beverages for sugar-sweetened beverages in 103 adolescents in a randomized controlled trial, except among the heaviest participants [26]. A double blind study subjected 55 overweight youth to 13 weeks of a 1,000 Kcal diet accompanied by daily capsules of aspartame or lactose placebo. Both groups lost weight, and the difference was not significant. Weight loss was attributed to caloric restriction [27]. Similar results were reported for a 12-week, 1,500 Kcal program using either regular or diet soda [28]. Interestingly, when sugar was covertly switched to aspartame in a metabolic ward, a 25 percent immediate reduction in energy intake was achieved [29]. Conversely, knowingly ingesting aspartame was associated with increased overall energy intake, suggesting overcompensation for the expected caloric reduction [30]. Vigilant monitoring, caloric restriction, and exercise were likely involved in the weight loss seen in multidisciplinary programs that included artificial sweeteners [31,32].
Experimental studies on artificial sweeteners and energy
Preload experiments generally have found that sweet taste, whether delivered by sugar or artificial sweeteners, enhanced human appetite. Aspartame-sweetened water, but not aspartame capsule, increased subjective appetite rating in normal weight adult males [33]. Aspartame also increased subjective hunger ratings compared to glucose or water [34]. Glucose preload reduced the perceived pleasantness of sucrose, but aspartame did not [34]. In another study, aspartame, acesulfame potassium, and saccharin were all associated with heightened motivation to eat and more items selected on a food preference list [35]. Aspartame had the most pronounced effect, possibly because it does not have a bitter aftertaste. Unlike glucose or sucrose, which decreased the energy intake at the test meal, artificial sweetener preloads either had no effect [33,35] or increased subsequent energy intake [36,37]. Those findings suggest that the calorie contained in natural sweeteners may trigger a response to keep the overall energy consumption constant.
Human research must rely on subjective ratings and voluntary dietary control. Rodent models helped elucidate how artificial sweeteners contribute to energy balance. Rats conditioned with saccharin supplement had significantly elevated total energy intake and gained more weight with increased body adiposity compared to controls conditioned with glucose [38]. Saccharin-conditioned rats also failed to curb their chow intake following a sweet pre-meal. When a flavor was arbitrarily associated with high or low caloric content, rats ate more chow following a pre-meal with the flavor predictive of low caloric content [39]. These studies pose a hypothesis: Inconsistent coupling between sweet taste and caloric content can lead to compensatory overeating and positive energy balance.
Neuronal responses to artificial sweeteners
What drives the desire to eat? Food reward shares brain circuitry with other pleasurable activities such as sex and drug administration [40,41]. It also shares the same behavioral paradigm with other forms of addiction: binging, withdrawal, craving, and cross-sensitization [41]. A period of abstinence greatly increased sucrose self-administration in rats, similar to binging behavior in humans [42].
Food reward consists of two branches: sensory and postingestive [41]. In humans, gustatory information perceived by taste receptors on the tongue ascends through the thalamus and eventually terminates in the anterior insula/frontal operculum and the orbitofrontal cortex [43,44]. Amygdala makes reciprocal connections along all levels of the gustatory pathway. Mesolimbic dopamine system is also crucial for the hedonic recognition of the stimulus and feeling of satisfaction following ingesting food with pleasant tastes [41,45,46,47].
The postingestive component depends on metabolic products of the food [48]. When food deprived, rats preferred glucose solution over saccharin solution, regardless of flavor that can be masked by adding quinine [49]. The postingestive effects contained both positive and negative neuronal signals separate from mechanical satiety [48]. For moderately concentrated nutrients, rats learned to prefer the food associated with regular feeding than “sham feeding,” in which the ingested food flowed out of the body through a gastric fistula. However, rats did not show preference if highly concentrated nutrients were used [48]. Hypothalamus has been shown to mediate the postingestive food reward [41,50]. Hypothalamus secretes various neuropeptides to regulate energy, osmotic balance, and feeding behavior.
The separation of brain areas in food reward is not exclusive, as dopaminergic activation was associated in sucrose preference in mice lacking sweet taste perception [51].
Increasing evidence suggests that artificial sweeteners do not activate the food reward pathways in the same fashion as natural sweeteners. Lack of caloric contribution generally eliminates the postingestive component. Functional magnetic imaging in normal weight men showed that glucose ingestion resulted in a prolonged signal depression in the hypothalamus. This response was not observed with sucralose ingestion [50]. Natural and artificial sweeteners also activate the gustatory branch differently. The sweet taste receptor, a heterodimer of two G protein coupled transmembrane receptors, contain several ligand-binding sites. For instance, aspartame and cyclamate, respectively, bind to each of the two monomers [52]. On the functional level, sucrose ingestion, compared to saccharin ingestion, was associated with greater activation of the higher gustatory areas such as the insula, orbitofrontal cortex, and amygdala [53].
These pilot investigations are consistent with a revised hypothesis: Sweetness decoupled from caloric content offers partial, but not complete, activation of the food reward pathways. Activation of the hedonic component may contribute to increased appetite. Animals seek food to satisfy the inherent craving for sweetness, even in the absence of energy need. Lack of complete satisfaction, likely because of the failure to activate the postingestive component, further fuels the food seeking behavior. Reduction in reward response may contribute to obesity. Impaired activation of the mesolimbic pathways following milkshake ingestion was observed in obese adolescent girls [45].
Lastly, artificial sweeteners, precisely because they are sweet, encourage sugar craving and sugar dependence. Repeated exposure trains flavor preference [54]. A strong correlation exists between a person’s customary intake of a flavor and his preferred intensity for that flavor. Systematic reduction of dietary salt [55] or fat [56] without any flavorful substitution over the course of several weeks led to a preference for lower levels of those nutrients in the research subjects. In light of these findings, a similar approach might be used to reduce sugar intake. Unsweetening the world’s diet [15] may be the key to reversing the obesity epidemic.
Acknowledgments
This article was inspired by a discussion with Dr. Dana Small at the Neuroscience 2010 Conference, which took place at Yale University on April 10, 2010. The author thanks Michaela Panter for editorial support and Steve Broner for helpful suggestions with the manuscript.
Abbreviations
- FDA
Food and Drug Administration
- BMI
body mass index
References
- Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106:1992–2000. doi: 10.1016/j.jada.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen D. Alternative Sweeteners. Biotechnology and food ingredients. New York: Van Nostrand Reinhold; 1991. [Google Scholar]
- Kauffman GB, Priebe PM. The discovery of saccharin: a centennial retrospect. Ambix. 1978;25:191–207. doi: 10.1179/amb.1978.25.3.191. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Saccharin: past, present, and future. J Am Diet Assoc. 1986;86:929–931. [PubMed] [Google Scholar]
- de la Peña C. Artificial sweetener as a historical window to culturally situated health. Ann NY Acad Sci. 2010;1190:159–165. doi: 10.1111/j.1749-6632.2009.05253.x. [DOI] [PubMed] [Google Scholar]
- Rubini ME. Noncaloric sweetening agents. Am J Clin Nutr. 1968;21:644–645. doi: 10.1093/ajcn/21.6.644. [DOI] [PubMed] [Google Scholar]
- Kaufman L. Michael Sveda, the Inventor Of Cyclamates, Dies at 87. New York Times. 1999 Aug
- Kroger M, Meister K, Kava R. Low-calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues. Comprehensive Reviews in Food Science and Food Safety. 2006;5:35–47. [Google Scholar]
- Mazur RH, Goldkamp AH, James PA, Schlatter JM. Structure-taste relationships of aspartic acid amides. Journal of Medicinal Chemistry. 1970;13:1217–1221. doi: 10.1021/jm00300a046. [DOI] [PubMed] [Google Scholar]
- Witt J. Discovery and development of neotame. World Rev Nutr Diet. 1999;85:52–57. doi: 10.1159/000059702. [DOI] [PubMed] [Google Scholar]
- Clauss K, Lück E, von Rymon Lipinski GW. [Acetosulfam, a new sweetener. 1. synthesis and properties (author's transl)] Z Lebensm Unters Forsch. 1976;162:37–40. doi: 10.1007/BF01104359. German. [DOI] [PubMed] [Google Scholar]
- Tate and Lyle Annual Report 2008. 2008
- Mintel. Ingredient Trends - US - December 2004 - Market Research Report. 2004
- Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11:1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC. et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- Saris WHM. Sugars, energy metabolism, and body weight control. Am J Clin Nutr. 2003;78:850S–857S. doi: 10.1093/ajcn/78.4.850S. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring, Md.) 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Garfinkel L. Artificial sweetener use and one-year weight change among women. Prev Med. 1986;15:195–202. doi: 10.1016/0091-7435(86)90089-7. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51:1100–1105. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- Forshee RA, Storey ML. Total beverage consumption and beverage choices among children and adolescents. Int J Food Sci Nutr. 2003;54:297–307. doi: 10.1080/09637480120092143. [DOI] [PubMed] [Google Scholar]
- Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr. 2005;24:93–98. doi: 10.1080/07315724.2005.10719449. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12:778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Thompson D, Affenito SG, Franko DL, Obarzanek E, Barton BA. et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148:183–187. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Brown RJ, de Banate MA, Rother KI. Artificial Sweeteners: A systematic review of metabolic effects in youth. [Epub 18 Jan 2010];Int J Pediatr Obes. doi: 10.3109/17477160903497027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- Knopp RH, Brandt K, Arky RA. Effects of aspartame in young persons during weight reduction. J Toxicol Environ Health A. 1976;2:417–428. doi: 10.1080/15287397609529443. [DOI] [PubMed] [Google Scholar]
- Williams CL, Strobino BA, Brotanek J. Weight control among obese adolescents: a pilot study. Int J Food Sci Nutr. 2007;58:217–230. doi: 10.1080/09637480701198083. [DOI] [PubMed] [Google Scholar]
- Porikos KP, Booth G, Van Itallie TB. Effect of covert nutritive dilution on the spontaneous food intake of obese individuals: a pilot study. Am J Clin Nutr. 1977;30:1638–1644. doi: 10.1093/ajcn/30.10.1638. [DOI] [PubMed] [Google Scholar]
- Mattes R. Effects of aspartame and sucrose on hunger and energy intake in humans. Physiol Behav. 1990;47:1037–1044. doi: 10.1016/0031-9384(90)90350-d. [DOI] [PubMed] [Google Scholar]
- Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65:409–418. doi: 10.1093/ajcn/65.2.409. [DOI] [PubMed] [Google Scholar]
- Rodearmel SJ, Wyatt HR, Stroebele N, Smith SM, Ogden LG, Hill JO. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move family study. Pediatrics. 2007;120:e869–e879. doi: 10.1542/peds.2006-2927. [DOI] [PubMed] [Google Scholar]
- Black RM, Leiter LA, Anderson GH. Consuming aspartame with and without taste: differential effects on appetite and food intake of young adult males. Physiol Behav. 1993;53:459–466. doi: 10.1016/0031-9384(93)90139-7. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Hill AJ. Paradoxical effects of an intense sweetener (aspartame) on appetite. Lancet. 1986;1:1092–1093. doi: 10.1016/s0140-6736(86)91352-8. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Carlyle JA, Hill AJ, Blundell JE. Uncoupling sweet taste and calories: comparison of the effects of glucose and three intense sweeteners on hunger and food intake. Physiol Behav. 1988;43:547–552. doi: 10.1016/0031-9384(88)90207-7. [DOI] [PubMed] [Google Scholar]
- Lavin JH, French SJ, Read NW. The effect of sucrose- and aspartame-sweetened drinks on energy intake, hunger and food choice of female, moderately restrained eaters. Int J Obes Relat Metab Disord. 1997;21:37–42. doi: 10.1038/sj.ijo.0800360. [DOI] [PubMed] [Google Scholar]
- King NA, Appleton K, Rogers PJ, Blundell JE. Effects of sweetness and energy in drinks on food intake following exercise. Physiol Behav. 1999;66:375–379. doi: 10.1016/s0031-9384(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Pierce WD, Heth CD, Owczarczyk JC, Russell JC, Proctor SD. Overeating by young obesity-prone and lean rats caused by tastes associated with low energy foods. Obesity (Silver Spring, Md.) 2007;15:1969–1979. doi: 10.1038/oby.2007.235. [DOI] [PubMed] [Google Scholar]
- Small DM. Toward an understanding of the brain substrates of reward in humans. Neuron. 2002;33:668–671. doi: 10.1016/s0896-6273(02)00620-7. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Small DM. Central gustatory processing in humans. Adv Otorhinolarygol. 2006;63:191–220. doi: 10.1159/000093761. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Ogawa H, Kaneda H, Ayabe-Kanamura S, Endo H, Saito S. Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chem Senses. 1999;24:201–209. doi: 10.1093/chemse/24.2.201. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S. et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse. 2007;61:748–756. doi: 10.1002/syn.20418. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Smeets PAM, de Graaf C, Stafleu A, van Osch MJP, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005;82:1011–1016. doi: 10.1093/ajcn/82.5.1011. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL. et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman DR. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav. 2004;83:421–429. doi: 10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Bertino M, Beauchamp GK, Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134–1144. doi: 10.1093/ajcn/36.6.1134. [DOI] [PubMed] [Google Scholar]
- Mattes RD. Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr. 1993;57:373–381. doi: 10.1093/ajcn/57.3.373. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1960-62 through 2005-2006. National Health and Nutrition Examination Survey