Abstract
Active avoidance involving controlling and modifying threatening situations characterizes many forms of clinical pathology, particularly childhood anxiety. Presently our understanding of the neural systems supporting human avoidance is largely based on nonhuman research. Establishing the generality of nonhuman findings to healthy children is a needed first step towards advancing developmental affective neuroscience research on avoidance in childhood anxiety. Accordingly, this investigation examined brain activation patterns to threatening cues that prompted avoidance in healthy youths. During functional magnetic resonance imaging, fifteen youths (ages 9-13) completed a task that alternately required approach or avoidance behaviors. On each trial either a threatening ‘Snake’ cue or a ‘Reward’ cue advanced towards a bank containing earned points. Directional buttons enabled subjects to move cues away from (Avoidance) or towards the bank (Approach). Avoidance cues elicited activation in regions hypothesized to support avoidance in nonhumans (amygdala, insula, striatum and thalamus). Results also highlighted that avoidance response rates were positively correlated with amygdala activation and negatively correlated with insula and anterior cingulate activation. Moreover, increased amygdala activity was associated with decreased insula and anterior cingulate activity. Our results suggest nonhuman neurophysiological research findings on avoidance may generalize to neural systems associated with avoidance in childhood. Perhaps most importantly, the amygdala/insula activation observed suggests threat related responses can be maintained even when aversive events are consistently avoided, which may account for the persistence of avoidance-coping in childhood anxiety. The present approach may offer developmental affective neuroscience a conceptual and methodological framework for investigating avoidance in childhood anxiety.
Keywords: threat, fear, avoidance, anxiety, amygdala, insula, children, Money amount per se is not driving MT
Introduction
Avoidance can be adaptive or maladaptive depending on the individual, circumstances, and learning history. Excessive or maladaptive forms of avoidance designed to control/modify threatening external or internal (negative emotions, thoughts, bodily sensations) states characterize many different forms of adult psychopathology and substance abuse problems (Clark, 1986; Stewart, 1999; Blume, 2001; Brewin & Holmes, 2003; Klonsky et al., 2003; Brown et al., 2007; Koob & Kreek, 2007; Sinha, 2007, 2008; Li Chiang-shan & Sinha, 2008; Koob, 2009). Emerging evidence further suggests that pathological avoidance is a central feature of anxiety in children. For example, behaviorally inhibited children at-risk for anxiety disorders display avoidant reactions as early as infancy (Rapee et al., 2005) and anxious children nominate more avoidant responses to hypothetical threatening scenarios than controls (Barrett et al., 1996; Chorpita et al., 1996; Creswell et al., 2005). To understand both normal avoidance and its developmental translation to psychopathology, a fundamental understanding of the neurobiology of avoidance in healthy youth is required. Moreover, because much of our current understanding of the neurophysiology of avoidance is based on nonhuman research investigations are needed to assess the generality of nonhuman findings and observed development differences in avoidance to humans (e.g., Eclancher & Karli, 1980). In what follows, we briefly highlight one prominent theory of avoidance and hypothesized brain mechanisms and then describe an investigation which tested the generalization of a nonhuman neurophysiological model of avoidance to human youth.
Despite nearly 60 years of basic and clinical research, there is currently little agreement concerning the processes underlying active (volitional) avoidance. One common thread shared by theories of avoidance is an emphasis on Pavlovian and operant or instrumental learning processes. Two-Factor theories are perhaps the best known and propose that fear is conditioned to a cue (e.g., light) that precedes the occurrence of an unconditioned aversive stimulus (e.g., shock) via classical conditioning (factor 1) and termination of the threatening conditioned cue along with fear serves as a negative reinforcer for avoidance (factor 2) (Mowrer & Lamoreaux, 1946; Mowrer, 1947; Miller, 1948; Rescorla & Solomon, 1967). Essentially, environmental stimuli that predict unwanted and undesirable aversive events serve as conditioned threats that prompt avoidance behaviors, which are subsequently strengthened by removing such threats. However, the emphasis placed on removal of threatening cues as a reinforcer for avoidance has not received extensive empirical support (Sidman, 1953; Kamin, 1956; Kamin et al., 1963). Also, two-factor theories cannot account for the persistence of avoidance during extinction, where threat and avoidance should subside because the once threatening cue now predicts the absence of the aversive event (for reviews see Bolles, 1972; Herrnstein, 1969). Also problematic is the variable relationship among avoidance, cued threat and physiological measures of ‘fear.’ Some evidence shows during avoidance learning skin conductance responses, indexing ‘fear,’ decline over time (Lovibond et al., 2008) as the cue predicts the absence of the aversive event. However, other evidence shows that avoidance can increase reported fear and catastrophic thoughts (Eifert & Heffner, 2003) and increase skin conductance responses (Rose et al., 1995; Solomon et al., 1980). These latter findings parallel clinical findings that point out avoidance can be counterproductive and may paradoxically and unintentionally enhance, or at least maintain, negative experiences and anxiety (Craske et al., 1989; Cioffi & Holloway, 1993).
Despite its shortcomings, two-factor theories offer a framework for examining the interplay between Pavlovian and instrumental learning processes (Baron & Perone, 2001) and generating testable hypotheses about the brain mechanisms supporting normal and pathological avoidance in humans. One recently developed two-factor nonhuman neurophysiological model of avoidance that may guide research efforts in childhood anxiety is the Escape-From-Fear model (EFF; Cain & LeDoux, 2008). The strength of the EFF model is that it ties neurophysiological research findings on Pavlovian and instrumental learning to each factor in the model. The collaboration between these two distinct neural systems forms the motive circuit for avoidance: one involves amygdala-dependent fear conditioning and the second involves appetitive or instrumental conditioning. In the EFF model, pairing a cue with aversive future events produces a conditioned threat capable of eliciting fear. Subsequent presentation of the cued threat activates an “upstream process” involving the thalamus and sensory cortex that drives activation in the central nucleus of the amygdala and other arousal centers to produce a negative emotional state. Instrumental learning processes enter when avoidance terminates the cued threat, and negative emotional state, which involves a “downstream process” whereby incentive information flows from the lateral and basal amygdala to the nucleus accumbens and invigorates and guides behavior via projections to the ventral pallidum and downstream motor systems. The EFF model predicts that regional responses are largest during acquisition and decline once avoidance is learned, suggesting amydala activity should decline as conditioned fear or threat extinguishes, which is consistent with nonhuman lesion studies showing amygdala involvement in acquisition but not maintenance of instrumental avoidance (Roozendaal et al., 1993; Poremba & Gabriel, 1995, 1997, 1999). However, a potential drawback of the EFF model is its tie to two-factor theorizing which has failed to account for avoidance. Specifically, if amygdala activation and associated fear/threat subsides once avoidance is learned, what then maintains avoidance? Addressing this question is critical to understanding chronic avoidance-coping as is present in disorders such as childhood anxiety. Nevertheless, the neurophysiological model does provide an initial framework for assessing the generality of nonhuman findings and developing and testing hypotheses about neural mechanisms of avoidance in youths.
Developmental affective neuroscience research on the neural systems supporting avoidance in children faces a number of challenges. One challenge is modeling both the desired typography and function of an avoidance behavior. Avoidance may be inhibitory/evasive and function to direct oneself ‘away’ from an aversive event (e.g., running away, staying inside one's home). Avoidance may also be active and directed ‘towards’ an aversive event to prevent it (e.g., lever pressing to prevent electric shock, self injury to prevent unwanted thoughts). Consideration of the form and function of an avoidance behavior gains significance in light of evidence from nonhuman lesion studies that highlights differences in brain mechanisms supporting inhibitory and active avoidance (Winocur & Mills, 1969; Hogg et al., 1998; Lukoyanov & Lukoyanov, 2006). This investigation focused on active instrumental avoidance because many forms of psychopathology involve a response that functions to control or modify proximal or distal aversive external (e.g., canceling social engagements to prevent/escape negative evaluation) or internal events (e.g., engaging in self harm to prevent/escape negative thoughts/emotions). Another challenge is developing fMRI- and age-appropriate experimental preparations that employ ethical non-invasive aversive stimuli that will prompt and maintain avoidance. This investigation examined the utility of point loss (where points are paired with money) as an aversive stimulus. Numerous behavioral studies show money loss is effective in maintaining avoidance in adults and children and some evidence suggests pairing loss with a cue can produce a threat sufficient to generate conditioned fear in adults (Delgado et al, 2006). Within neuroimaging research, money may also be arranged to function as an appetitive (gain) or aversive (loss) stimulus within the same context, thereby eliminating potential confounds in brain activation associated with using different appetitive and aversive stimuli, such as money (a conditioned stimulus) and electric shock (an unconditioned stimulus), that may recruit different brain structures.
In this investigation, our primary aim was to evaluate whether the EFF model of avoidance is supported in healthy youths. This involved examining fMRI-derived brain responses to a threatening cue presented within a discriminated instrumental avoidance paradigm that used money loss as an aversive stimulus. Based on the EFF model, nonhuman lesion studies on avoidance (Winocur & Mills, 1969; Allen et al., 1972; Allen & Davison, 1973; Grossman et al., 1975; Poremba & Gabriel, 1997, 1999) and human neuroimaging studies on avoidance and aversion (Jensen et al., 2003; Simmons et al, 2004: Nitschke et al, 2006; Mobbs et al., 2007, 2009), our analyses focused on the amygdala, insula, striatum, thalamus and anterior cingulate. A secondary aim was to examine relations between individual levels of avoidance and approach behavior (i.e., rates of responding to avoidance and approach cues) and associated brain activation and examine interrelations among functionally determined regions of interest.
Material and Methods
Subjects
Fifteen healthy right-handed youths (ages 9-13, M=11.1 yrs (SD=1.6); 8 males) participated. Exclusion criteria for the study included: (a) symptoms suggestive of an Axis I psychiatric disorder based on parent report on the Child or Adolescent Symptom Inventory-4 (Gadow & Sprafkin, 1998a, 1998b), (b) the existence of a major systemic medical illness, (c) a history of serious head injury, or (d) having eye problems or difficulties in vision not corrected by the use of glasses or contact lenses, measured as vision of 30/20 or better with both eyes open using a hand-held eye-chart. All participants were recruited from community advertisements. The study was approved by the University of Pittsburgh Institutional Review Board. After a detailed description of the study and before participation, parents gave written informed consent for their child's participation in the study. Children gave written informed assent.
Neuroimaging task
The avoidance paradigm was developed through the collaboration of researchers at the Kennedy Krieger Institute and University of Pittsburgh. Prior to functional neuroimaging, subjects learned through trial and error to respond appropriately to several cue-response-outcome contingencies. Importantly, subjects received no instructions about the cue-response-outcome contingencies. Consequently, choices to avoid and approach emerged solely through contact with outcomes (point gains and losses). They were told that their task was to earn as many points as possible by using two available response buttons. Five trials of each contingency were completed during training, with contingency appropriate responding emerging within a few trials. Thus, this training period served to establish the conditioned properties of cues, contingency shape approach and avoidance behaviors and decrease the potential effects of performance anxiety associated with learning the task during imaging (i.e., Lovibond & Rapee, 1993).
Figure 1 highlights structural features of conditions (Approach, Avoidance and Control) and associated response contingencies. In general, each trial began with presentation of a visual cue, during which subjects were free to press or not press response buttons that controlled the direction of cues on the screen. Four to six seconds later a 2 s outcome prompt revealed the magnitude of point gain, loss or avoided loss in accordance with the current contingency. A variable 4-6 s intertrial interval signaled by a fixation stimulus followed outcomes. As shown in Figure 1, the Approach cue (described in instructions as a ‘Money’ cue) was presented and moved slowly towards the right side of the screen. Instructions emphasized learning to use the response buttons available to physically move the Money cue towards a hypothetical bank located (off-screen) on the right side of the screen to earn 50 points. For the Money cue, each right button press increased the speed at which the cue moved towards (right) the bank and left presses moved it away from the bank. Six right button presses were required to move the cue to the bank and produce a 50 point gain---a fixed-ratio 6 (FR6) schedule of reinforcement. Non-responding, emitting less than six right presses or moving the cue away (left) from the bank produced no point gain----because the cue would not have reached the bank. During the Avoidance condition, the Avoidance cue (described in instructions as a ‘money-eating Snake’) was presented and moved rapidly towards the bank. Instructions emphasized learning to use the response buttons available to keep the snake away from the bank and if the Snake reached the bank it would result in loss of 50 points. For the Snake cue, each left button press moved the rapidly advancing cue away from (left) the bank and right button presses advanced it towards the bank. Six left button presses (FR6) were required to avoid a 50 point loss; thus, responding was instrumental in terminating the programmed loss. Emitting less than six left presses, moving the cue into the bank or allowing the advancing cue to reach the bank on its own resulted in a 50 point loss. A baseline condition that involved no aversive or appetitive contingencies was included for comparison. This condition consisted of a control cue (“X”) that subjects were instructed to move either left or right using the response buttons. The outcome was a <<< >> stimulus and was presented after six responses. Failure to make six responses produced the prompt “Press Any Buttons for X”. Also included were Ambiguous trials where the Snake and Money cues were presented side-by-side. These trials were modeled in the analysis but as their psychological interpretation was ambiguous, results are not discussed in this first examination of neural correlates of avoidance of threat. During fMRI, fifteen trials of each condition were completed.
Figure 1. Approach-Avoidance task cues and response contingencies.
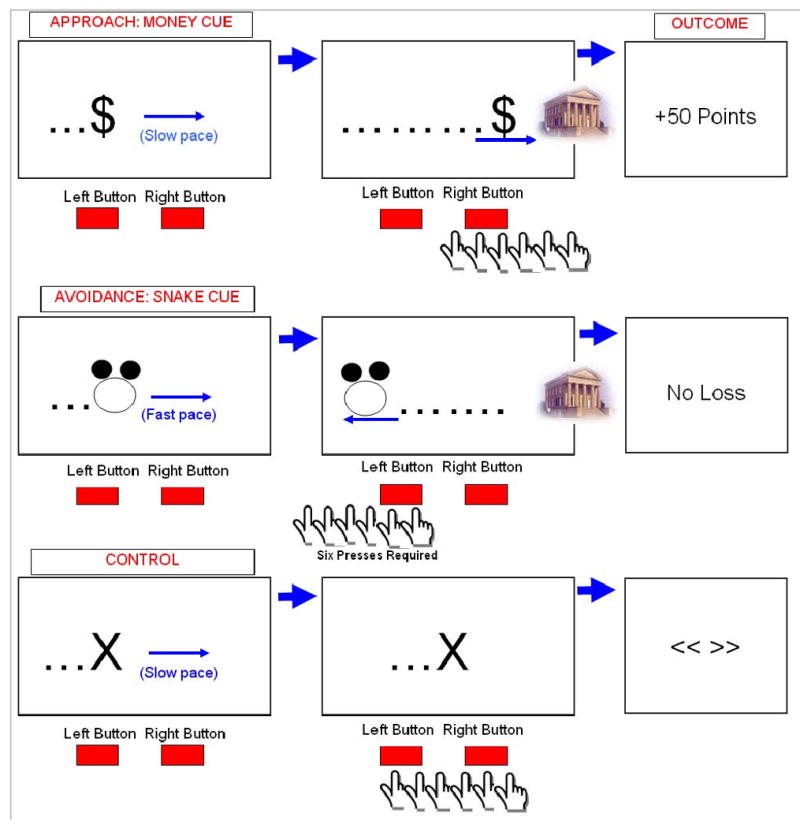
The schematic reveals the general structure of approach, avoidance and control trials---see Methods for timing parameters. One cue was presented per trial and presentation order was randomized over two imaging sessions. Prior to neuroimaging, subjects learned to use two available response button to physically move cues towards (right) or away (left) from a hypothetical off-screen ‘bank’ containing their points. During presentation of a slow paced Approach cue (described as a ‘Money’ cue) all subjects advanced the cue towards the bank by repeatedly pressing a target button in order to earn points. During a fast paced Avoidance cue (described as a point-eating ‘Snake’ cue) all subjects moved the cue away from the bank by repeatedly pressing a target button in order to prevent point loss. During a slow paced control cue subjects were instructed that moving the cue in either direction was inconsequential, with correct responding producing an arbitrary symbol.
Image Acquisition
During two 7 min 12 sec functional runs, thirty 3.2mm slices were acquired parallel to the AC-PC line using a reverse-weighted echoplaner (EPI) pulse sequence (3T Seimman's Allegra scanner, T2*-weighted images depicting BOLD contrast; TR=1500ms, TE=25ms, FOV=20cm, flip=73°) yielding 288 frames per run. Stimuli were displayed in black on a white background via a back-projection screen. Responses were recorded using a Psychology Software Tools™ glove. In addition, high resolution T1-weighted MPRAGE images (1mm, axial) were collected for use in cross-registration. Eprime controlled stimulus presentation and recorded behavioral responses. Responses were made using a 5-button glove-shaped response box in which subjects rested one finger on each button.
Image Analysis
fMRI data preparation was conducted via locally developed NeuroImaging Software (NIS) and AFNI (Cox, 1996). Following motion correction using the 6 parameter AFNI 3dVolReg algorithm, linear trends within runs were removed to eliminate effects of scanner drift. Outliers outside the Tukey Hinges +/- 1.5IQR were rescaled to that threshold. fMRI data were temporally smoothed (five-point middle-peaked filter), and cross-registered to the Montreal Neurological Institute (MNI) brain using the 32 parameter non-linear AIR algorithm (Woods et al., 1993), and spatially smoothed (6mm FWHM). Data analysis was performed using SPM 2 (http://www.fil.ion.ucl.ac.uk/spm). A canonical hemodynamic response function was used as a covariate in a general linear model and a parameter estimate, which equates to percent change in the global mean BOLD signal, was generated for each voxel that corresponded to the onsets of each cue and subsequent outcome using Dirach delta function. Parameter estimates derived from the mean least squares fit of the model to the data reflect the strength of covariance between the data and the canonical response function for our events of interest. Separate contrast images for each cue and outcome were generated and subjected to one-sample t-tests at a second-level group analysis in which t values were calculated for each voxel, treating intersubject variability as a random effect. The t values were transformed to unit normal Z distribution to create statistical parametric maps. Activation was identified using the thresholds p < .001, uncorrected for multiple comparisons and an empirically determined extent threshold (k) of 40 contiguous voxels, which corresponds to a corrected cluster threshold of p <. 05 for our regions of interest (amygdala, insula, striatum, thalamus and anterior cingulate). Relationships between rates of avoiding and approaching and cue elicited activation were highlighted with regression analyses using the thresholds p < .01, uncorrected, and (k) of 153 contiguous voxels, which corresponds to a corrected cluster threshold of p <. 05 for our regions of interest. Each extent threshold was empirically derived using a mask restricted to our regions of interest which was subjected to Monte-Carlo simulations, accounting for the spatial correlation of obtained maps, via AFNI's (Cox 1996) AlphaSim routine. The location of voxels with significant activation was summarized by their local maxima separated by at least 8 mm, and by converting the maxima coordinates from MNI to Talairach coordinate space using recommended transformations (Lancaster et al, 2007). These coordinates were finally assigned neuroanatomic labels using the Talairach brain atlas (Talairach & Tournoux, 1998) and the Talairach Daemon database (http://www.talairach.org/daemon.html). Resulting statistical parametric maps were then overlaid onto a reference brain using MRIcron software (http://www.sph.sc.edu/comd/rorden/mricron/).
Results
Behavioral
Accuracy
The positive and negative reinforcement contingencies used within the avoidance paradigm maintained stable and accurate performances during neuroimaging. Table 1 provides descriptive information for each condition. Response accuracy for Avoidance was 100% for 14/15 subjects, for Approach was 100% for 14/15 subjects and for Control was greater than 88% for 14/15 subjects. These findings highlight that point gain served as an effective positive reinforcer for approach behavior and the absence of point loss served as an effective negative reinforcer for avoidance behavior. Accuracy for Avoidance, Approach and Control responding was not significantly correlated (p >.05) with age.
Table 1.
Group mean and standard deviation by condition.
| Avoid M(SD) | Approach M(SD) | Control M(SD) | |
|---|---|---|---|
| Percent Correct Responses | 97 (11.47) | 99.6 (1.43) | 98.8 (3.2) |
| Responses per Second | 2.73 (.67) | 2.79 (.66) | 2.52 (.49) |
Response Rates
Each condition employed a fixed-ratio six (FR6) response requirement which enabled us to calculate condition-specific rates of responding per second, also shown in Table 1. Across conditions, the mean number of responses per second emitted ranged between 2.52 and 2.79. A three-way analysis of variance (ANOVA) showed a main effect of condition (F(2,13) = 7.004, p = .009; η2 = .51) and ANOVA post-hoc contrasts showed no significant rate differences between Avoidance and Control (F(1,14) = 3.45, p = .08; η2 = .20) or Avoidance and Approach (F(1,14) = .22, p = .65; η2 = .02), but Approach rates were significantly faster than Control rates (F(1,14) = 13.94, p < .002; η2 = .50). Response rates for Avoidance, Approach and Control were not significantly correlated (p >.05) with age.
Neuroimaging
Brain Activation
A central issue addressed in the present investigation was whether brain regions emphasized in the EFF model show activation to avoidance cues in healthy youths. Results showed activation to the Avoidance cue was increased relative to the Control cue in “upstream process” regions of the EFF model including the right amygdala and thalamus (Figure 2, Table 2). Also noted was activation in the insula which has been observed in studies on aversion, but is not part of the EFF model. Figure 2 plots of individual subject contrast values for avoidance (i.e., avoidance parameter estimate – control parameter estimate) in the right amygdala and insula reveals relatively consistent activation across subjects and marked between-subject variability. Increased activity during Avoidance was also noted in the “downstream process” of the EFF model including the caudate and putamen (Table 2). No significant increases or decreases in activation were observed between imaging sessions. Activation to the Approach cue was limited to inferior frontal, anterior cingulate, cuneus and middle occipital regions (Figure 2, Table 2). No differences in activation were observed between Avoidance and Approach cues. Lastly, no significant correlations were found between age and activation in our regions of interest, suggesting our findings were not influenced by age-related differences in gross cognitive, emotional or physical abilities. These findings suggest some of the brain mechanisms supporting nonhuman instrumental avoidance may generalize to account for avoidance in childhood. Perhaps most importantly, the amygdala/insula activation observed suggests threat related responses can be maintained even when aversive events are consistently avoided, which may account for the persistence of avoidance-coping in childhood anxiety.
Figure 2. Regional activation to Avoidance and Approach cues.
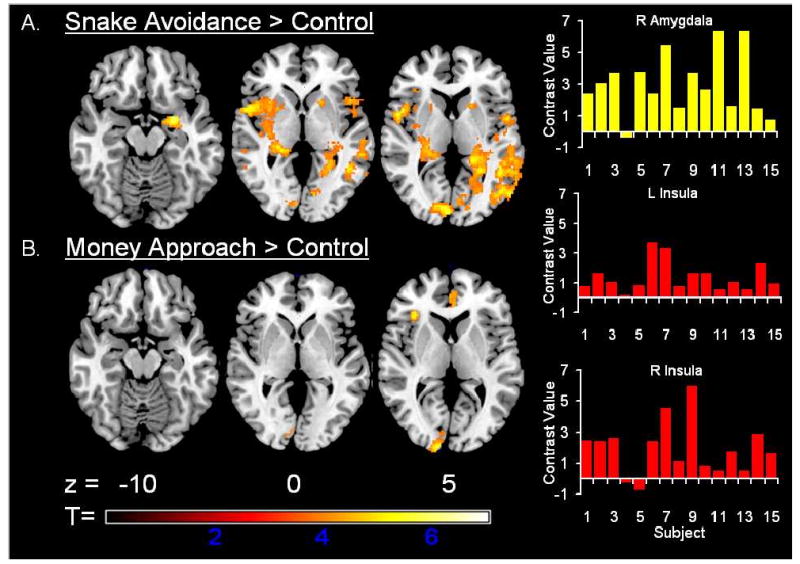
Panel A highlights activation to the avoidance cue in regions hypothesized to support nonhuman instrumental avoidance (amygdala, insula, thalamus and striatum). Plots show individual subject contrast values (avoidance parameter estimate – control parameter estimate) for avoidance in the right amygdala and insula. Panel B highlights limited activation to the cue that prompted approach behavior.
Table 2.
Activation associated with Avoidance and Approach cues.
| Contrast | Region | X | Y | Z | Voxel Cluster | Voxel T | |
|---|---|---|---|---|---|---|---|
| Avoidance Snake > Control | |||||||
| Left | Middle Temporal Gyrus | -53 | -29 | -7 | 103 | 7.16 | |
| Lingual Gyrus | -9 | -83 | 4 | 599 | 6.95 | ||
| Superior Parietal Lobule | -15 | -61 | 54 | 927 | 6.52 | ||
| Cuneus | -7 | -92 | 4 | (599) | 6.10 | ||
| Precentral Gyrus | -49 | 4 | 9 | 1586 | 6.08 | ||
| Inferior Parietal Lobule | -26 | -44 | 57 | (927) | 5.95 | ||
| Insula | -34 | 10 | -1 | (1586) | 5.48 | ||
| Insula | -34 | 24 | 13 | 58 | 5.36 | ||
| Middle Temporal Gyrus | -51 | -51 | -7 | 57 | 5.35 | ||
| Thalamus | -24 | -30 | 2 | (1586) | 4,82 | ||
| Superior Frontal Gyrus | -22 | 24 | 53 | 110 | 4.64 | ||
| Middle Frontal Gyrus | -27 | 24 | 48 | (110) | 4.50 | ||
| Posterior Cingulate | -7 | -68 | 12 | (599) | 4.20 | ||
| Putamen | -23 | 8 | 2 | (1586) | 4.14 | ||
| Caudate Head | -14 | 18 | 6 | (1586) | 4.07 | ||
| Right | Insula | 42 | 12 | 1 | 2847 | 6.66 | |
| Middle Occipital Gyrus | 39 | -85 | 4 | (2847) | 6.02 | ||
| Amygdala | 25 | -5 | -12 | (2847) | 5.96 | ||
| Superior Pareital Lobule | 22 | -56 | 59 | 266 | 5.69 | ||
| Precuneus | 15 | -52 | 55 | (266) | 5.65 | ||
| Cuneus | 12 | -75 | 6 | 241 | 5.53 | ||
| Lingual Gyrus | 4 | -75 | -1 | (241) | 5.22 | ||
| Precentral Gyrus | 45 | -5 | 53 | 116 | 5.19 | ||
| Cuneus | 21 | -93 | 8 | 65 | 4.84 | ||
| Precentral Gyrus | 35 | -9 | 55 | (116) | 4.83 | ||
| Cuneus | 24 | -76 | 34 | (185) | 4.53 | ||
| Putamen | 18 | 14 | 2 | 41 | 4.99 | ||
| Thalamus | 20 | -28 | 10 | (2847) | 3.79 | ||
| Approach Money > Control | |||||||
| Left | Inferior Frontal Gyrus | -32 | 24 | 10 | 68 | 6.21 | |
| Cuneus | -12 | -95 | -1 | 149 | 5.53 | ||
| Lingual Gyrus | -9 | -82 | 2 | (149) | 4.15 | ||
| Right | Middle Occipital Gyrus | 34 | -88 | 4 | 178 | 5.43 | |
| Cuneus | 21 | -91 | 10 | (178) | 5.25 | ||
| Anterior Cingulate | 3 | 43 | 12 | 93 | 4.44 | ||
( )'s regions within the same cluster
Correlations of brain activation and response rate
A secondary aim examined the relationship between rates of approach and avoidance and activation in regions emphasized in the EFF model. Analyses revealed that amygdala activation to the Avoidance cue showed a significant positive correlation with mean avoidance response rates (Figure 3, Table 3). In contrast, insula, anterior cingulate and BA 24 activation to the Avoidance cue showed a significant negative correlation with mean avoidance response rates (Figure 3, Table 3). Similar relations were not observed between Approach cue activation and response rates. However, Table 4 provides complete information on regions that evidenced significant negative correlations with approach rates. These findings suggest rates of avoidance behavior may provide an index of regional responsiveness to threats in children.
Figure 3. Correlations between activation to avoidance cue and avoidance response rates.
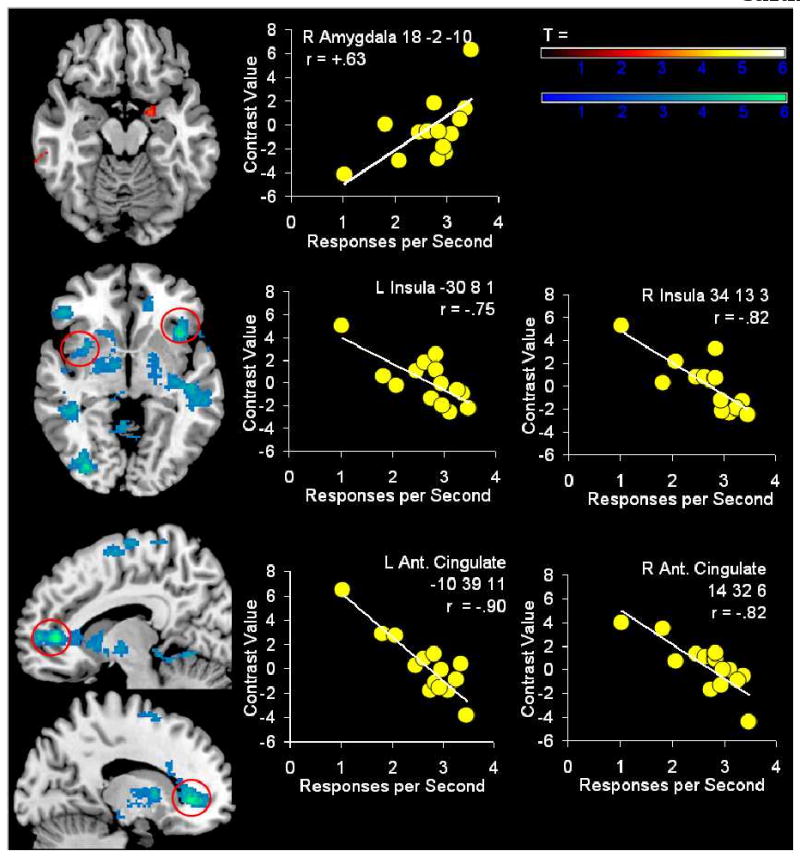
The top plot highlights a positive correlation in the amygdala between avoidance cue activation and avoidance response rates. The remaining plots highlight negative correlations in the insula and anterior cingulate between avoidance cue activation and avoidance response rates. Similar relations were not observed for the approach cue.
Table 3.
Regions correlated with rates of Avoidance responding (p<.01, extent = 153 voxels).
| Region | X | Y | Z | Voxel Cluster | Voxel T | Voxel r | ||
|---|---|---|---|---|---|---|---|---|
| Positive Correlation | ||||||||
| Right | Amygdala | 18 | -2 | -10 | 22 | 2.96* | .63 | |
| Negative Correlation | ||||||||
| Left | Anterior Cingulate | -10 | 39 | 11 | 5247 | 7.41 | -.90 | |
| Precentral Gyrus | -17 | -20 | 62 | 574 | 7.28 | -.90 | ||
| Medial Frontal Gyrus | -14 | 50 | 14 | (5247) | 6.69 | -.88 | ||
| Fusiform Gyrus | -42 | -45 | -12 | 1552 | 5.63 | -.84 | ||
| Middle Occipital Gyrus | -29 | -80 | -7 | 278 | 5.56 | -.84 | ||
| Middle Temporal Gyrus | -47 | -27 | -10 | (1552) | 5.35 | -.83 | ||
| Superior Temporal Gyrus | -51 | 9 | -8 | 180 | 5.09 | -.82 | ||
| Lingual Gyrus | -33 | -72 | -8 | (278) | 4.99 | -.81 | ||
| Postcentral Gyrus | -44 | -24 | 57 | 541 | 4.67 | -.79 | ||
| Inferior Frontal Gyrus | -45 | 30 | 3 | 207 | 4.40 | -.77 | ||
| Insula | -30 | 8 | 1 | (207) | 4.07 | -.75 | ||
| Postcentral Gyrus | -35 | -35 | 60 | (541) | 4.37 | -.77 | ||
| Middle Temporal Gyrus | -27 | -73 | 1 | (278) | 3.80 | -.73 | ||
| Medial Frontal Gyrus | -11 | -9 | 63 | (574) | 3.74 | -.72 | ||
| BA 24 | -3 | 33 | 11 | (5247) | 3.54 | -.70 | ||
| Inferior Frontal Gyrus | -40 | 24 | 8 | (207) | 3.12 | -.65 | ||
| Right | Anterior Cingulate | 14 | 32 | 6 | (5247) | 5.23 | -.82 | |
| Insula | 34 | 13 | 3 | (5247) | 5.10 | -.82 | ||
| Insula | 42 | -20 | -4 | 629 | 4.57 | -.79 | ||
| Putamen | 32 | -18 | -2 | (629) | 4.34 | -.77 | ||
| Medial Frontal Gyrus | 7 | -11 | 59 | 210 | 4.28 | -.76 | ||
| Middle Temporal Gyrus | 43 | -28 | -3 | (629) | 4.26 | -.76 | ||
| Precentral Gyrus | 30 | -26 | 60 | 387 | 4.20 | -.76 | ||
| Medial Frontal Gyrus | 15 | -1 | 58 | (210) | 3.59 | -.71 | ||
| Precentral Gyrus | 28 | -17 | 63 | (387) | 3.53 | -.70 | ||
| BA 24 | 3 | 31 | 13 | (5247) | 3.29 | -.67 | ||
| Postcentral Gyrus | 20 | -32 | 63 | (387) | 3.14 | -.66 | ||
( )'s regions listed as secondary local maxima.
significant at p < .05, extent 20 voxels
Table 4.
Regions correlated with rates of Approach responding (p<.01, extent = 153 voxels).
| Region | X | Y | Z | Voxel Cluster | Voxel T | Voxel r | ||
|---|---|---|---|---|---|---|---|---|
| Positive Correlation: None | --- | --- | --- | --- | --- | --- | ||
| Negative Correlation | ||||||||
| Left | Precuneus | -13 | -55 | 48 | 381 | 4.95 | -.81 | |
| Medial Frontal Gyrus | -2 | 9 | 45 | 165 | 4.52 | -.78 | ||
| Postcentral Gyrus | -35 | -34 | 51 | 668 | 4.06 | -.75 | ||
| Cingulate Gyrus | -1 | -25 | 29 | 363 | 3.99 | -.74 | ||
| Superior Frontal Gyrus | -7 | 13 | 50 | (165) | 3.44 | -.69 | ||
| Right | Cingulate Gyrus | 4 | -20 | 33 | (363) | 3.96 | -.74 | |
| Superior Parietal Lobule | 28 | -51 | 43 | 341 | 3.73 | -.72 | ||
| Precentral Gyrus | 56 | -15 | 40 | 158 | 3.70 | -.72 | ||
| Inferior Parietal Lobule | 35 | -51 | 40 | 347 | 3.64 | -.71 | ||
( )'s regions listed as secondary local maxima.
Relationships between brain regions
We examined bivariate relationships between beta-weights for functionally defined regions across individuals. This analysis did not examine functional relations between regions within any individual. Table 5 shows the zero order correlation of beta-weights for each brain region with beta weights for each other region (lower half) that responded to Avoidance cue presentations compared to the Control cue. These relationships were also examined after controlling for avoidance response rates (upper half). Table 5 shows amygdala and insula activity were inversely related, with increases in amygdala activation correlated with decreases in bilateral insula activation (r =∼-.57). The table also shows a negative correlation between amygdala and right anterior cingulate activity (r =-.5 to -.8, depending on how regions were defined) that was only partially mediated by avoidance responses (residual r's =-.3 to -.7), consistent with inhibition of amygdala function by the rostral cingulate. Insula activity was moderately correlated with anterior cingulate activity (r =.4-.7) but this relationship was not significant after conservatively controlling for type I error across all examined regions. Homologues were significantly positively related for all examined structures (e.g., strong correlation existed between left and right insula) supporting internal consistency among regional activation.
Table 5.
Pearson correlations (unshaded) and partial correlations controlling for avoidance (shaded).
| Avoid Respons e Rate | L Amygdala | L Insula | R Insula | L Ant Cing | R Ant Cing | L BA 24 | R BA 24 | ||
|---|---|---|---|---|---|---|---|---|---|
| L Amygdala | Coefficient | .634* | ----- | -0.2 | -0.142 | -0.534* | -0.653* | -0.308 | -0.427 |
| P | 0.011 | ----- | 0.492 | 0.629 | 0.049 | 0.011 | 0.284 | 0.128 | |
| L Insula | Coefficient | -0.748* | -.577* | ----- | 0.751* | 0.247 | 0.348 | -0.024 | -0.13 |
| P | 0.001 | 0.024 | ----- | 0.002 | 0.394 | 0.222 | 0.936 | 0.659 | |
| R Insula | Coefficient | -.817** | -.581* | .899** | ----- | 0.053 | 0.123 | -0.223 | -0.113 |
| P | >.001 | 0.023 | 0 | ----- | 0.858 | 0.676 | 0.444 | 0.701 | |
| L Ant Cing | Coefficient | -.899** | -0.751* | .745* | .748* | ----- | 0.611* | 0.612* | 0.474 |
| P | >.001 | 0.001 | 0.001 | 0.001 | ----- | 0.02 | 0.02 | 0.087 | |
| R Ant Cing | Coefficient | -.824** | -.808** | .747* | .713* | .892** | ----- | 0.708* | 0.744* |
| P | >.001 | >.001 | 0.001 | 0.003 | >.001 | ----- | 0.005 | 0.002 | |
| L BA 24 | Coefficient | -.700* | -.614* | 0.513 | 0.48 | .821** | .864** | ----- | 0.793* |
| P | 0.004 | 0.015 | 0.05 | 0.07 | >.001 | >.001 | ----- | 0.001 | |
| R BA 24 | Coefficient | -.674* | -.671* | 0.441 | 0.502 | .759* | .867** | .890** | ----- |
| P | 0.006 | 0.006 | 0.1 | 0.057 | 0.001 | >.001 | >.001 | ----- |
significant at the 0.05 level (2-tailed).
significant at the corrected 0.0018 level (2-tailed).
BA = Brodman Area; Ant Cing = Anterior Cingulate; Avoid Response Rate = Avoidance Responses per Second
Discussion
This investigation focused on highlighting the neural mechanisms supporting active instrumental avoidance that involves selecting, controlling and modifying threatening external situations. Our primary aim was to evaluate the generality of nonhuman neurophysiological findings on avoidance to healthy youths. Based on the EFF model (Cain & LeDoux, 2008), nonhuman lesion studies on avoidance (Winocur & Mills, 1969; Allen et al., 1972; Allen & Davison, 1973; Grossman et al., 1975; Poremba & Gabriel, 1995, 1997, 1999) and human neuroimaging studies on avoidance and aversion (Jensen et al., 2003; Simmons et al, 2004: Nitschke et al, 2006), our analyses focused on the amygdala, insula, striatum, thalamus and anterior cingulate. A secondary aim was to examine relations between individual levels of avoidance and approach behavior (i.e., rates of responding to avoidance and approach cues) and associated brain activation and examine interrelations among functionally determined regions of interest. In general, findings suggest avoidance appears to be supported by an integrated multi-structure brain system and nonhuman neurophysiological research findings on instrumental avoidance, some of which are emphasized in the EFF model, may generalize to neural systems associated with avoidance in childhood. Perhaps most importantly, the amygdala/insula activation observed suggests threat related responses can be maintained even when aversive events are consistently avoided, which may account for the persistence of avoidance-coping strategies in childhood anxiety.
One set of results consistent with the EFF model was increased activation in the amygdala, thalamus, and striatum to a cue that prompted avoidance responding relative to a control cue that prompted arbitrary responding. Results highlighting activation in the amygdala is consistent with the EFF model's emphasis on the role of the amygdala in supporting the Pavlovian relationship between the avoidance cue as a conditioned threat through its association with potential money loss. Whether the amygdala activation we observed reflects a negative emotional state remains unclear, but the significant increase in activation observed and subsequent avoidance is consistent with the proposal that the amygdala responded to a salient event that derived its aversive motivational properties through prior association with money loss (Sander et al., 2003). Our findings revealing amygdala recruitment in avoidance establishes significant links to clinical research that has highlighted the prevalence and function of avoidance and safety behaviors in psychopathology and findings from a range of neuroimaging studies highlighting amygdala hyperactivity in mood disorders characterized by avoidance behavior, such as post-traumatic stress disorder, social anxiety disorder, and specific phobia (Etkin & Wager, 2007). Results highlighting activation in the striatum (i.e, caudate and putamen) is also consistent with the EFF model's emphasis on the role of these regions in supporting the instrumental relationship between responding and control of aversive events. Collectively, the results of the present investigation highlight some of the basic brain mechanisms of human avoidance that is jointly supported by Pavlovian and instrumental learning processes emphasized in theories of avoidance.
One finding that diverged from EFF model predictions was insula activation to the Avoidance cue. This region has shown activity in human neuroimaging studies on instrumental avoidance (Jensen et al., 2003) and aversion in which cues predict forthcoming aversive images (Simmons et al., 2004, 2006). Although the EFF model has chosen to emphasize amygdala, a recent review of human neuroimaging studies on fear-conditioning found that the amygdala, the anterior cingulate and insular cortex were crucial structures in the acquisition of aversive delay conditioning, independent of general design characteristics (Sehlmeyer et al., 2009). Such findings are consistent with discriminated avoidance, and arguably real-life situations, where threats or conditioned threats are present for some extended time period. Delay conditioning characteristics are consistent with two-factor accounts of avoidance because responding is purportedly negatively reinforced by terminating the cued threat, which needs to be present for an extended time period to allow for responding. Our finding of insula activation to avoidance cues is also consistent with a range of neuroimaging studies highlighting insula hyperactivity in mood disorders characterized by avoidance behavior (Etkin & Wager, 2007). Indeed, it has been hypothesized that insula dysfunction may increase anxious affect, worrisome thoughts and other avoidance behaviors by producing an increased prediction signal of a prospective aversive body state (Paulus & Stein 2006). The present results potentially suggest an expansion of the EFF model to humans to include the insula.
Another set of findings that diverged from EFF model predictions was that threat-related activation was not attenuated after avoidance was learned, that is, when aversive outcomes were consistently avoided during neuroimaging. Our findings highlighting sustained amygdala and insula activation is consistent with basic studies showing increased self reports of fear and heightened physiological responses during avoidance (Rose et al., 1995; Solomon et al., 1980; Eifert & Heffner, 2003) and clinical reports suggesting avoidance may maintain or enhance negative thoughts and emotions (Craske et al., 1989). These findings illustrate that amygdala and insula activation can be maintained independently of repeated contacts with an aversive event, which is consistent with clinical presentations of chronic avoidance-coping behaviors. One explanation for the sustained amygdala and insula activation we observed after initial avoidance learning is that it reflects a continued strengthening of neural responses even though avoidance behavior was stable; an effect that has been reported with instrumental behavior (Schlund & Ortu, 2009). Further investigations are needed to determine whether regional activation changes with extended avoidance training and whether activation patterns are modulated by gender differences in sensitivity to classical and operant learning processes reported in nonhumans (e.g., Dalla & Shors, 2009).
Our results also provide some insights into the working relationships among the brain regions recruited during avoidance in healthy youths. The observed inverse relationships between rostral cingulate and amygdala reactivity to threat could reflect regulatory control of the amygdala by the rostral cingulate (as proposed by Davidson, 2000). Potentially this aspect of regulatory control over limbic responsivity is compromised in avoidance pathologies, such as childhood anxiety, such that individuals with high levels of anxiety might not display the observed inverse relationship of these structures. High levels of avoidance might then be associated with high levels of activity in both structures, indicating a lack of inhibition of the amygdala by the rostral cingulate. Anterior insula activity has been proposed to reflect bodily response monitoring (Craig, 2008). The observed relationship of rostral cingulate to insula activity might occur if awareness of bodily sensation is associated with volitional recruitment of regulatory resources. This explanation is consistent with the observed similar declines in both insula and rostral cingulate reactivity to cue response rates. That is, with slower response rates, there could be decreased proprioceptive and other associated bodily cues leading to decreased recruitment of the cingulate regulatory control. Alternately, increased proprioceptive focus (e.g., focusing on breathing or body sensations during the task) could be the opposite of more cognitive/emotional dread- or worry-like reactivity that yields avoidance behavior.
In addition to replicating some prior neuroimaging findings highlighting insula, striatal and anterior cingulate recruitment during human instrumental avoidance (e.g., Jensen et al., 2003; Mobbs et al., 2009), results favor extending what is known about the neural systems supporting nonhuman avoidance to humans. However, further investigations are needed to evaluate whether the EFF model can be applied to understand other forms of avoidance, such as passive avoidance, and escape. Presumably, the basic learning processes are the same across different forms of avoidance and escape. But a critical difference between active and passive forms of avoidance is that in the former responding involves actively controlling aversive events through termination or postponement, whereas in the latter it is not. How such differences in outcome control (i.e, response contingency) affects the different neural systems emphasized in the EFF model remains unclear. It seems plausible to suggest active and passive avoidance, as well as escape responding, would similarly recruit the “upstream process' involving the sensory cortex, thalamus and amygdala, but only active avoidance and escape would additionally recruit the “downstream process” involving the nucleus accumbens, ventral pallidum and downstream motor systems.
The threat-related activation to avoidance cues observed in the amygdala and insula in youths also provides the necessary first steps towards examining potential changes in avoidance neurocircuitry during development and pertubations that place individuals at risk for neuropsychiatric disorders. Given the two-factor theoretical framework of avoidance which emphasizes both classical and instrumental learning process, we are unaware of any behavioral or neurophysiological research findings that suggest instrumental learning processes, specifically negative reinforcement processes responsible for maintaining avoidance, are majorly influenced by development. However, there is some neurophysiological evidence indicating development differences in fear-conditioning, particularly across children ages 2-11 years (Block et al., 1970; Gao et al., 2010). One implication of increased susceptibility to fear-conditioning with age is that the emergence of instrumental avoidance behavior, which occurs subsequent to fear-conditioning, may also show developmental differences. Consequently, learned avoidance as a primary coping strategy may be less prevalent in young children but more prevalent near adolescence. Adolescent studies on emotional reactivity and the amygdala also hint that pubertal maturation may contribute to developmental differences in learned avoidance. Some have proposed that adolescent development may be characterized by low reactivity in neural systems underlying the processing of threat-related stimuli and the regulation of behavior and emotion (Ernst et al., 2006) while others suggest high reactivity (Avenevoli et al., 2003; Quevedo et al 2009). A third perspective suggests pubertal change may be associated with relatively normal or heightened reactivity that would typically engage avoidance neural systems but may in fact be overshadowed by greater reactivity in approach neural systems (Dahl & Spear, 2004; Forbes et al., In Press). One implication of abnormal amygdala and insula reactivity during adolescent development is that it may generate a bias away from normally adaptive levels of avoidance behavior. Whether the effects noted during adolescence are attenuated or continue into adulthood remains to be investigated.
Finally, our results demonstrate the efficacy of a novel fMRI discriminated instrumental avoidance paradigm for children that uses loss of money-backed points as an aversive stimulus. Behavioral measures showed that the paradigm rapidly established and maintained contingency appropriate behavior during neuroimaging when point gain served as a positive reinforcer for approach behavior and avoided point loss served as a negative reinforcer for avoidance behavior. These findings suggest money loss may serve as an ethical non-invasive aversive stimulus in developmental affective neuroscience research on avoidance. Our use of point gain and loss also likely minimized confounds in brain activation that might otherwise be present in tasks that use different appetitive and aversive stimuli within the same context. The procedure is also easily modified within the constraints imposed by the neuroimaging environment to assess various predictions of avoidance theories, such as whether the duration of a threatening cue is correlated with the duration of amygdala and insula activation, as well as questions regarding whether the amygdala and insula contribute to maintaining established forms of avoidance in humans. Moreover, the paradigm seems appropriate for assessing brain mechanisms supporting chronic avoidance in sensitive clinical populations, even those with significant cognitive dysfunction. At a broader level, our avoidance paradigm also addresses concerns that fear conditioning procedures using cue-danger (e.g., light/CS-shock/US) associations do not model the anxiety characterizing many emotional disorders (Barlow, 2000; Grillon et al., 1998) and concerns that many investigations fail to provide convincing demonstrations of human avoidance (Grillon et al., 2006).
The approach described in the present investigation may offer developmental affective neuroscience a conceptual and methodological framework for investigating relations between threat, avoidance and negative emotion during development. Results of the present investigation suggest nonhuman neurophysiological research findings on avoidance may inform investigation of avoidance-related neuropathology in childhood anxiety. In healthy youths, results showed avoidance did not attenuate brain mechanisms associated with basic threat response, e.g., in the amygdala and insula, suggesting that threat related responses can be maintained despite consistent avoidance of an aversive event. This finding may account for the persistence of avoidance-coping in childhood anxiety.
Acknowledgments
We would like to thank the authors of SPM and AFNI software for making it freely available and Danny Pine, M.D. for helpful comments on a previous draft of this manuscript. This paper was made possible with the support of NIH grant funding MH41712 (R.D.), P50 MH080215 (N.R.) and K02 MH082998 (G.S.)
Footnotes
Author contributions: Design: M.S., G.S. and M.C. Data collection: J.S., C.L., G.S., R.D. and N.R. Analysis: M.S. and G.S. Writing: M.S., G.S., M.C., C.L., J.S., E. F., R.D. and N.R
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JD, Davison CS. Effects of caudate lesions on signaled and nonsignaled Sidman avoidance in the rat. Behav Biol. 1973;8(2):239–250. doi: 10.1016/s0091-6773(73)80023-9. [DOI] [PubMed] [Google Scholar]
- Allen JD, Mitcham JC, Byrd JL. Effects of caudate lesions on the acquisition and retention of Sidman avoidance in the rat. Psychon Sci. 1972;27:157–160. [Google Scholar]
- Avenevoli S, Merikangas KR, Stolar M, Grillon C. Pubertal development, startle modulation, and changes in affective expression. Paper presented at the Society for Research in Child Development Biennial Meeting; Tampa, FL. 2003. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55(11):1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Baron A, Perone M. Explaining avoidance: two factors are still better than one. J Exp Anal Behav. 2001;75(3):357–378. doi: 10.1901/jeab.2001.75-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett PM, Rapee RM, Dadds MM, Ryan SM. Family enhancement of cognitive style in anxious and aggressive children. J Abnorm Child Psychol. 1996;24(2):187–203. doi: 10.1007/BF01441484. [DOI] [PubMed] [Google Scholar]
- Block JD, Sersen EA, Wortis J. Cardiac classical conditioning and reversal in the mongoloid, encephalopathic, and normal child. Child Dev. 1970;41:771–785. [Google Scholar]
- Blume AW. Negative Reinforcement and Substance Abuse: Using a Behavioral Conceptualization to Enhance Treatment. The Behavior Analyst Today. 2001;2(2):86–90. [Google Scholar]
- Bolles RC. The avoidance learning problem. In: Bower GH, editor. The psychology of learning and motivation. Vol. 6. Academic Press; New York: 1972. pp. 97–145. [Google Scholar]
- Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2003;23(3):339–376. doi: 10.1016/s0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Brown SA, Williams K, Collins A. Past and recent deliberate self-harm: emotion and coping strategy differences. J Clin Psychol. 2007;63(9):791–803. doi: 10.1002/jclp.20380. [DOI] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. Emotional processing and motivation: In search of brain mechanisms. In: Elliot AJ, editor. Handbook of approach and avoidance motivation. Psychology Press; New York: 2008. pp. 17–34. [Google Scholar]
- Chorpita BF, Albano AM, Barlow DH. Cognitive processing in children: Relation to anxiety and family influences. J Clin Child Adolesc Psychol. 1996;25(2):170–176. [Google Scholar]
- Cioffi D, Holloway J. Delayed costs of suppressed pain. J Pers Soc Psychol. 1993;64(2):274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- Clark DA. A cognitive approach to panic. Behav Res Ther. 1986;24(4):461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers in Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craske MG, Street L, Barlow DH. Instructions to focus upon or distract from internal cues during exposure treatment of agoraphobic avoidance. Behav Res Ther. 1989;27(6):663–672. doi: 10.1016/0005-7967(89)90150-2. [DOI] [PubMed] [Google Scholar]
- Creswell C, Schniering CA, Rapee RM. Threat interpretation in anxious children and their mothers: Comparison with nonclinical children and the effects of treatment. Behav Res Ther. 2005;43(10):1375–1381. doi: 10.1016/j.brat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. Vol. 1021. New York: New York Academy of Sciences; 2004. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Labouliere CD, Phelps EA. Fear of losing money? Aversive conditioning with secondary reinforcers. Soc Cogn Affect Neurosci. 2006;1(3):250–259. doi: 10.1093/scan/nsl025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eclancher F, Karli P. Effects of infant and adult amygdaloid lesions upon acquisition of two-way active avoidance by the adult rat: Influence of rearing conditions. Physiol Behav. 1980;24(5):887–893. doi: 10.1016/0031-9384(80)90146-8. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Heffner M. The effects of acceptance versus control contexts on avoidance of panic-related symptoms. J Behav Ther Exper Psychiatry. 2003;34(3-4):293–312. doi: 10.1016/j.jbtep.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. doi: 10.1080/87565641.2010.550178. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent Symptom Inventory - 4: Norms Manual. Checkmate Plus; New York: 1998a. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventory - 4: Screening Manual. Checkmate Plus; New York: 1998b. [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev Sci. 2010;13(1):201–212. doi: 10.1111/j.1467-7687.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol Psychiatry. 2006;60(7):752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44(10):1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grossman SP, Grossman L, Walsh L. Functional organization of the rat amygdala with respect to avoidance behavior. J Comp Physiol Psychol. 1975;88(2):829–850. doi: 10.1037/h0076396. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Method and theory in the study of avoidance. Psychol Rev. 1969;76(1):49–69. doi: 10.1037/h0026786. [DOI] [PubMed] [Google Scholar]
- Hogg S, Moser PC, Sanger DJ. Mild traumatic lesion of the right parietal cortex of the rat: selective behavioural deficits in the absence of neurological impairment. Behav Brain Res. 1998;93(1-2):143–155. doi: 10.1016/s0166-4328(97)00146-0. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kamin LJ, Brimer CJ, Black AH. Conditioned suppression as a monitor of fear of the CS in the course of avoidance training. J Comp Physiol Psychol. 1963;56(3):497–501. doi: 10.1037/h0047966. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. The effects of termination of the CS and avoidance of the US on avoidance learning. J Comp Physiol Psychol. 1956;49(4):420–424. doi: 10.1037/h0088011. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501–1508. doi: 10.1176/appi.ajp.160.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, Saunders JC, Weidemann G, Mitchell CJ. Evidence for expectancy as a mediator of avoidance and anxiety in a laboratory model of human avoidance learning. Quarterly J Exp Psychol. 2008;61(8):1199–1216. doi: 10.1080/17470210701503229. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanov EA. Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav Brain Res. 2006;173(2):229–236. doi: 10.1016/j.bbr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Miller NE. Studies of fear as an acquirable drive: I. Fear as motivation and fear-reduction as reinforcement in the learning of new responses. J Exp Psychol. 1948;38(1):89–101. doi: 10.1037/h0058455. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29(39):12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH, Lamoreaux RR. Fear as an intervening variable in avoidance conditioning. J Comp Psychol. 1946;39(1):29–50. doi: 10.1037/h0060150. [DOI] [PubMed] [Google Scholar]
- Mowrer OH. On the dual nature of learning: a reinterpretation of “conditioning” and “problem solving”. Harvard Educational Review. 1947;17:102–148. [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29(1):106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. The amygdala is necessary for the initial acquisition but not for maintenance of discriminative avoidance behavior in rabbits. Soc Neurosci Abstr. 1995;21:1930. [Google Scholar]
- Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J Neurosci. 1997;17(13):5237–5244. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits. J Neurosci. 1999;19(21):9635–9641. doi: 10.1523/JNEUROSCI.19-21-09635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev Psychopathol. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Kennedy S, Ingram M, Edwards S, Sweeney L. Prevention and early intervention of anxiety disorders in inhibited preschool children. J Consult Clin Psychol. 2005;73:488–497. doi: 10.1037/0022-006X.73.3.488. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Rose P, McGlynn FD, Lazarte A. Control and attention influence snake phobics' arousal and fear during laboratory confrontations with a caged snake. J of Anxiety Disorders. 1995;9(4):293–302. [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. The central amygdala is involved in conditioning but not in retention of active and passive shock avoidance in male rats. Behav Neural Biol. 1993;59(2):143–149. doi: 10.1016/0163-1047(93)90873-g. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Ortu D. Experience-dependent changes in human brain activation during contingency learning. Neurosci. 2009 doi: 10.1016/j.neuroscience.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Avoidance conditioning with brief shock and no exteroceptive warning signal. Science. 1953;118(3058):157–158. doi: 10.1126/science.118.3058.157. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15(14):2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S, Holmes DS, McCaul KD. Behavior control over aversive events: does control that requires effort reduce anxiety and physiological arousal? J Pers Soc Psychol. 1980;39(4):729–736. doi: 10.1037//0022-3514.39.4.729. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportional system: An approach to cerebral imaging. Thieme; Germany: 1998. [Google Scholar]
- Winocur G, Mills JA. Effects of caudate lesions on avoidance behavior in rats. J Comp Physio Psychol. 1969;68(4):552–557. doi: 10.1037/h0027645. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17(4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]


