Abstract
Objective
To assess the effects of informational brochures and video decision aids about cancer screening on patient intention to engage in shared decision making and its predictors in a racially diverse sample.
Methods
Participants were recruited from 13 community-based primary care practices serving racially and ethnically diverse patients in predominately economically disadvantaged neighborhoods. Participants completed theory-based measures assessing attitudes, perceived social norms, self-efficacy and intentions for working with their physician to make a cancer screening decision after reviewing a brochure or video decision aid, but before seeing the physician. A post-questionnaire assessed screening decisions and participant knowledge.
Results
Participants who reviewed a video decision aid had higher knowledge and were more likely to want to be the primary decision-maker. They reported lower perceived social norms, self-efficacy and intentions to work with their physicians than participants who reviewed a brochure. Participants who decided against cancer screening reported lower intentions to work with their physician in making a decision and were less likely to report having spoken with their physician about screening.
Conclusion
Participants who opted against cancer screening after reviewing a brochure or decision aid were less likely to discuss their decision with their physician. The tendency toward autonomous decision-making was stronger among participants who reviewed a video decision aid.
1. Introduction
Despite a proliferation of research, most randomized trials of decision aids have been conducted in predominately Caucasian samples1;2. Little is known about using decision aids with racially diverse and socio-economically disadvantaged populations. A recent study with African Americans found that men who participated in a screening decision-making education session, in addition to reviewing an education pamphlet, were more likely to choose prostate cancer screening than men assigned to the control group, contrary to many other trials focused on prostate cancer screening decision-making3;4. Other researchers have begun developing decision aids specifically for low-literacy multi-ethnic populations, though evaluation of these tools remains preliminary5–8. In addition to the limited focus on ethnically and racially diverse patients, little is known about using decision aids in community-based primary care practices in the US9;10. Although some recent research in Canada and the UK has been conducted in community-based settings, most studies to date have been conducted in academically affiliated medical practices11–14.
The stated purpose of decision aids is not to replace a physician-patient consultation, but instead to serve as an adjunct by providing detailed information about clinical options and their likely outcomes, thereby facilitating shared decision-making15–17. However, what actually happens during a patient-physician encounter after a patient has reviewed a decision aid remains largely unexplored18. Little is known about subsequent patient and physician behavior as most trials have focused on endpoints such as patient knowledge and decision-making outcomes17. If the purpose of a decision aid is to facilitate shared decision-making, then it logically follows that studies of decision aids should examine the extent to which they increase patient behaviors necessary for shared decision-making. However, few, if any, studies to date, have built on existing theoretical models of behavior to better understand the effects of decision aids on patients’ engagement in these behaviors19;20.
The Integrative Model of Behavior incorporates the central empirical and theoretical components predicting behavior and behavioral change from several theories and includes three primary determinants of behavioral intention: 1) attitudes toward performing the behavior, 2) perceived social norms about performing the behavior, and 3) self-efficacy21–25. The methods for assessing these constructs are well described in the context of the Theory of Planned Behavior, one of the theories incorporated into the Integrative Model21;26. There are several plausible ways in which one could apply the Integrative Model to the effects of decision aids on patient behaviors. Decision aids may be a vehicle for influencing the salient beliefs underlying attitudes, perceived social norms and self efficacy. Many of these tools highlight the role of the patient in ensuring that the decision fits with his or her preferences. By explicitly encouraging participation they may impact salient beliefs that could result in more positive attitudes about taking an active role in decision-making. Many decision aids also include patient testimonials which previous studies suggest could impact decision-making 27;28. Testimonials may also impact salient beliefs underlying patients’ perceived social norms about making decisions together with their physicians. Finally, by increasing patient vicarious experience through testimonials, exposure to decision aids may reduce cognitive and perceived social barriers to participating in the process of decision-making with the physician, thereby increasing self-efficacy22.
The present study sought to compare whether diverse participants who viewed a video decision aid would have greater knowledge about prostate or colon cancer and would show more positive attitudes, stronger perceived social norms, greater self-efficacy and greater intentions to work with a physician in making a decision about cancer screening than participants who viewed a brief educational brochure.
2. Methods
2.1. Design
Our data were collected as part of an exploratory feasibility study on the implementation of decision aids in community-based primary care practices that serve racially and ethnically diverse patients in lower income neighborhoods29. Thirteen primary care practices were recruited throughout the Los Angeles area. Of these practices, 11 were solo-practitioner offices and 2 were community clinics that designated a single physician as the study participant. Each practice was asked to sequentially recruit 20 patient participants into two groups. The first 10 patients reviewed a brief informational brochure about prostate or colon cancer screening before seeing the physician (Phase I). Once 10 patients had been recruited, clinics were asked to recruit 10 more patients who reviewed a video decision aid about prostate or colon cancer screening before seeing the physician (Phase II). Our decision to use a sequential quasi-experimental design grew from a concern about maintaining the integrity of randomization while relying on clinic staff to recruit participants. The study protocol did not prescribe how many patients had to be recruited for prostate and colon cancer screening decision-making. Of the 13 participating practices, 9 were able to meet the patient recruitment goals for both phases. The remaining practices recruited patients into the brochure group but were unable to complete Phase I. In these practices no patients were recruited into Phase II.
2.2. Participants and procedure
Our protocol was reviewed and approved by the Institutional Review Board of the University of California, Los Angeles. Patients were eligible to participate in the study if they were age 50 or older (45 or older for African American men considering prostate cancer screening), did not have a personal history of prostate or colon cancer, and were eligible for screening based on current clinical guidelines30;31. Eligible patients were identified at the time of an office visit, rather than before a scheduled appointment. Contrary to our original plan, in most clinics (11 of 13) patients were recruited into the study by a project research assistant after a physician or clinic staff member identified the patient as eligible to participate in the study. Only in two clinics were the physician and designated staff members able to recruit participants independently without assistance from project staff. After reviewing the brochure or video decision aid but before seeing the physician, patients completed an initial questionnaire. Following the consultation with the physician, patients completed a second questionnaire.
2.3. Study brochures and video decision aids
The brochures about prostate and colon cancer screening used in the clinics were developed at the University of Texas and the Centers for Disease Control, respectively32. The brochures provided an overview of the cancer and related screening options and encouraged discussion with a physician. The video decision aids were developed by the Foundation for Informed Medical Decision Making and were each about 30 minutes long. The videos provided a detailed description of the cancer, the different screening options and outcomes associated with these, as well as testimonials from real patients and physicians discussing different points of view about screening. The brochure and decision aid for prostate cancer screening was available in English and Spanish, whereas materials for colon cancer screening were available in English only. To our knowledge, neither the brochures nor the decision aids were theory-based.
2.4. Measures
The initial questionnaire, completed after reviewing the brochure or video decision aid, but before seeing the physician, assessed patients’ role preferences in medical decision-making and the constructs of the Integrative Model. Role preferences were assessed with five questions that asked who should complete medical problem solving tasks and who should determine the utility of different options and make medical decisions33. The constructs of the Integrative Model were framed around “working with my doctor to make a decision about prostate/colon cancer screening, during a regular medical examination”. The specific questions were developed for this study, following previously published methods and prior work by the investigators26;34–37. Before responding to the specific items assessing these constructs, respondents read a brief paragraph that defined “working with my doctor” as consisting of “talking to your doctor about the choices for cancer screening; asking questions about what different choices mean for you; thinking about what is important to you in this decision and reaching an agreement with your doctor about what would be best for you”38. By defining the behavioral category of “working with my doctor”, we avoided having to increase respondent burden by assessing each behavior individually. Attitudes toward working with the physician were assessed with 7-point (with a mid-point of 0) semantic differential items of the following form: “Working with my doctor in making a decision about cancer screening would be”: “good” (+3) to “bad” (−3), “wise-foolish”, “necessary-unnecessary”, “beneficial-harmful”, “pleasant-unpleasant” and “enjoyable-unenjoyable”. Perceived social norms were assessed with items of the following form: “Most of the people who are important to me think I should work with my doctor in making a decision about cancer screening.” Responses were strongly agree (+3) to strongly disagree (−3) in seven steps. Self-efficacy refers to a person’s self-appraised ability to engage in a behavior or accomplish a particular outcome under a variety of different circumstances. For example: “I am confident that I could work with my doctor in making a cancer screening decision, even if my doctor doesn’t ask me to do so.” Responses were strongly agree (+3) to strongly disagree (−3). Internal consistency (Cronbach’s alpha) for attitudes, perceived social norms and self-efficacy were .71, .88 and .85, respectively. The second questionnaire, completed after seeing the physician, queried respondents’ cancer screening decisions (with “yes”, “no” and “unsure” options), knowledge about prostate or colon cancer, whether or not the respondent spoke with the physician about cancer screening and their demographics39.
2.5. Statistical analysis
The present sample has a multi-level data structure, with participants nested in clinics. However, owing to the exploratory nature of this study, the sample of clinics was limited and potentially inadequately powered for multi-level statistical models. We therefore report the results for our primary hypotheses using unadjusted analysis of variance methods and to explore the effects controlling for clinics, we also report these results using linear mixed models. Dichotomous categorical variables were analyzed with logistic regression. For each model we also explored the effect of controlling for clustering. Categorical variables with more than two levels were analyzed with Pearson chi-square. Because our study focused on two different cancer screening decisions, we explored between-groups variation in our primary dependent variables by including interaction terms. Data were analyzed with SPSS 14.0 and SAS 9.0 software packages.
3. Results
A total of 207 participants enrolled in the study between July 26, 2006 and November 27, 2007. The number of participants who reviewed an English language prostate or colon cancer brochure or a Spanish language prostate cancer brochure were 17, 62 and 28, respectively. The number of participants who reviewed an English language prostate or colon cancer decision aid or a Spanish language prostate decision aid were 21, 62 and 17, respectively. As shown in Table 1 there were no significant differences between the brochure and decision aid groups for any of the demographic variables. Also shown in Table 1 are the ranges for these demographic variables across the 13 participating primary practices. There was significant variability across the clinics making most of the comparisons statistically significant.
Table 1.
Demographics by group and ranges across clinics
| Brochure (n=107) | Decision Aid (n=100) | Comparison of Brochure and Decision Aid groups | Range across clinics | Comparison of clinics | |
|---|---|---|---|---|---|
| Age (Mean, Standard deviation) | 60.8 (9.6) | 61.7 (10.4) | p=.52 | 57.5 – 65.9 | p=.08 |
| Married | 48.4% | 49.5% | p=.89 | 11.1% – 81.8% | p=.000 |
| Male | 54.7% | 63.0% | p=.23 | 20.0% – 76.9% | p=.000 |
| Ethnicity | |||||
| African American | 37.6% | 35.8% | p=.84 | 0% – 100% | p=.000 |
| Latino | 43.5% | 38.9% | 0% – 100% | ||
| White | 9.4% | 10.5% | 0% – 34.8% | ||
| Asian | 7.1% | 10.5% | 0% – 52.6% | ||
| Other | 2.4% | 4.2% | 0% – 20.0% | ||
| Education | |||||
| 8th grade or less | 35.3% | 25.5% | p=.34 | 4.3% – 58.3% | p=.004 |
| High School graduate | 42.2% | 46.7% | 33.3% – 61.1% | ||
| College graduate | 20.5% | 22.3% | 5.0% – 43.5% | ||
| Graduate degree | 2.0% | 5.3% | 0% – 10.0% | ||
| Employed (part- or full-time) | 45.0% | 37.6% | p=.30 | 22.7% – 70.0% | p=.054 |
| Household income | |||||
| $15,000 or less | 39.5% | 28.6% | p=.80 | 0% – 90.5% | p=.000 |
| $15,001 – $25,000 | 24.2% | 26.0% | 0% – 76.2% | ||
| $25,001 – $35,000 | 9.9% | 11.7% | 9.5% – 69.6% | ||
| $35,001 or more | 26.4% | 33.7% | 0% – 29.4% | ||
| Uninsured | 22.0% | 18.5% | p=.55 | 0% – 90.5% | p=.000 |
Table 2 shows participants’ role preferences by group. The results suggest that participants who reviewed a video decision aid were more likely to desire being the primary or sole decision maker, with three of five questions showing statistically significant differences between the two groups, even after applying a Bonferroni adjustment for multiple comparisons. We explored whether there were any differences by language among participants who reviewed a prostate cancer brochure or decision aid, but did not find any significant effects (data not reported).
Table 2.
Role preferences by group
| Question | Physician or mostly physician | Shared | Patient or mostly patient | Statistic |
|---|---|---|---|---|
| Who should determine what the cancer screening options are? | ||||
| Brochure | 31.1% | 63.2% | 5.7% | χ2(2)=22.69, p=.000 |
| Decision aid | 34.3% | 37.4% | 28.3% | |
| Who should determine the risks and benefits of each screening option? | ||||
| Brochure | 50.5% | 40.2% | 9.3% | χ2(2)=4.11, p=.128 |
| Decision aid | 40.4% | 41.4% | 18.2% | |
| Who should determine how likely each of these risks and benefits are to happen? | ||||
| Brochure | 53.8% | 34.9% | 11.3% | χ2(2)=5.30, p=.071 |
| Decision aid | 45.9% | 30.6% | 23.5% | |
| Given this risks and benefits of these options, who should decide how acceptable those are to you? | ||||
| Brochure | 33.6% | 48.6% | 17.8% | χ2(2)=14.68, p=.001 |
| Decision aid | 28.3% | 30.3% | 41.4% | |
| Given all the information about risks and benefits of the possible screening options, who should decide which screening option should be selected? | ||||
| Brochure | 42.1% | 43.0% | 15.0% | χ2(2)=9.68, p=.008 |
| Decision aid | 31.3% | 35.4% | 33.3% | |
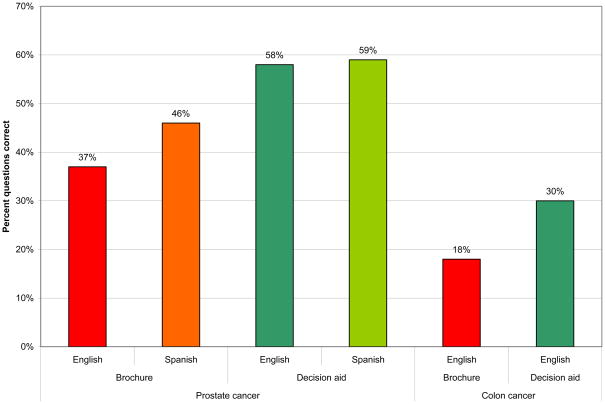
Our knowledge hypothesis was confirmed. As shown in Figure 1 participants who reviewed a decision aid had higher knowledge about prostate and colon cancer. The effects were significant using analysis of variance (Prostate cancer: F(1,82) = 9.36, p=.003; Colon cancer: F(1,120) = 11.22, p=.001) and using linear mixed models controlling for clustering in clinics (Prostate cancer: F(1, 79.73) = 11.23, p=.001; Colon cancer: F(1, 115.99) = 11.15, p=.001). The intra-class correlations were .03 and .09, for prostate and colon cancer knowledge, respectively. There were no significant differences by language among participants who reviewed a prostate cancer brochure or decision aid.
Figure 1.
Participant knowledge by group
Participants’ intentions to work with their doctor to make a cancer screening decision were significantly correlated with attitudes (ρ = .37, p<.001), perceived social norms (ρ = .51, p<.001) and self-efficacy (ρ = .53, p<.001). Together, these constructs accounted for 30.5% of the variance in behavioral intention (F(3,154)=22.07, p<.001, R2=.305). Table 3 shows the Integrative Model measures comparing brochure and decision aid groups, broken down by prostate and colon cancer screening. The table shows the statistics for univariate and multivariate main-effects and interaction effects, as well as the intra-class correlation, which indicates the degree of clustering by clinic. All models controlled for participants’ language. There were no significant differences in attitudes comparing the four groups. Contrary to our hypotheses, there were significant univariate and multivariate main effects indicating that participants who reviewed a brochure had stronger perceived social norms, greater self-efficacy and greater behavioral intentions about working with their physician to make a cancer screening decision. In addition, there were significant univariate interaction effects for each of these measures, suggesting that the prostate cancer decision aid produced the lowest scores. The multivariate interaction effect for perceived social norms was also significant, however, the multivariate interaction effects for self-efficacy and behavioral intentions were only marginally significant. The degree of clustering by clinic for these measures was limited. Table 4 shows the Integrative Model measures by language for participants who reviewed a prostate cancer brochure or decision aid. The main effects of brochure versus decision aid remained, but there were no significant effects for language or interaction effects.
Table 3.
Integrative Model measures
| Brochure | Decision aid | Tests | Univariate statistics | Multivariate statistics | |||
|---|---|---|---|---|---|---|---|
| Prostate cancer | Colon cancer | Prostate cancer | Colon cancer | ||||
| Attitudes | 2.24 (0.90) | 1.97 (0.94) | 1.89 (1.02) | 1.94 (0.88) | Brochure vs DA Prostate vs Colon Interaction Clustering by clinic |
F(1,192)=1.35, p=.247 F(1,192)=.119, p=.730 F(1,192)=.810, p=.369 |
F(1,185.84)=2.02, p=.157 F(1,188.57)=1.20, p=.275 F(1,185.28)=2.10, p=.149 ICC=.08 |
| Perceived social norms | 2.76 (0.67) | 2.40 (0.95) | 1.42 (1.73) | 1.99 (1.13) | Brochure vs DA Prostate vs Colon Interaction Clustering by clinic |
F(1,172)=23.21, p=.000 F(1,172)=.003, p=.955 F(1,172)=6.87, p=.01 |
F(1,166.86)=23.23, p=.001 F(1,151.64)=.074, p=.786 F(1,168.73)=6.41, p=.012 ICC=.03 |
| Self-efficacy | 2.64 (0.56) | 2.41 (0.84) | 1.82 (1.21) | 2.19 (1.07) | Brochure vs DA Prostate vs Colon Interaction Clustering by clinic |
F(1,166)=11.42, p=.001 F(1,166)=.002, p=.965 F(1,166)=3.94, p=.049 |
F(1,161.45)=11.19, p=.001 F(1,127.55)=.144, p=.705 F(1,162.93)=3.80, p=.053 ICC=.01 |
| Behavioral intention | 2.44 (1.02) | 2.19 (1.55) | 1.61 (1.70) | 2.12 (1.20) | Brochure vs DA Prostate vs Colon Interaction Clustering by clinic |
F(1,199)=5.38, p=.021 F(1,199)=.018, p=.895 F(1,199)=3.90, p=.05 |
F(1,194.01)=4.96, p=.027 F(1,176.49)=.29, p=.589 F(1,195.68)=3.35, p=.069 ICC=.02 |
Table 4.
Integrative Model measures by language - Prostate cancer only
| Brochure | Decision aid | |||
|---|---|---|---|---|
| English | Spanish | English | Spanish | |
| Attitudes | 2.25 (0.73) | 2.24 (1.00) | 1.59 (1.06) | 2.32 (0.82) |
| Perceived social norms** | 2.45 (0.98) | 2.92 (0.36) | 1.74 (1.35) | 1.04 (2.07) |
| Self-efficacy* | 2.44 (0.80) | 2.73 (0.38) | 2.02 (1.04) | 1.54 (1.40) |
| Behavioral intention* | 2.38 (0.78) | 2.48 (1.15) | 1.84 (1.26) | 1.29 (2.17) |
Main effect of Brochure vs Decision Aid:
p<.01,
p<.001
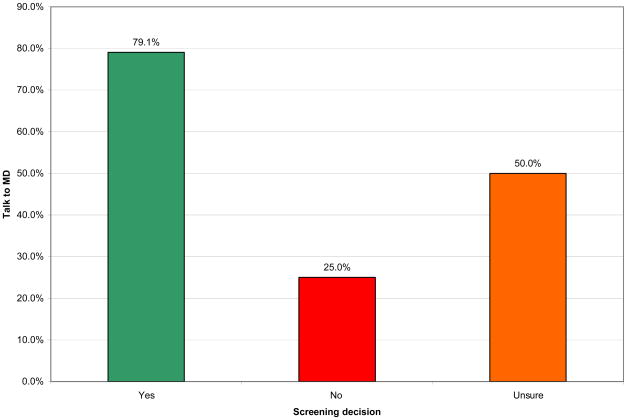
In the brochure group 57.1% of participants reported talking to their doctor about cancer screening compared to 45.8% in the decision aid group. The comparison was non-significant (χ2(1)=1.21, p=.272). In the brochure group 49.5% of participants reported choosing cancer screening, 41.7% decided not to screen and 8.7% reported still feeling unsure after seeing the physician. In the decision aid group 38.8% reported choosing cancer screening, 49.0% decided not to screen and 12.2% reported feeling unsure. The differences between the two groups were non-significant (χ2(2)=2.48, p=.289). Figure 2 shows the proportion of participants who reported talking to their physician about cancer screening by their screening decisions. There were significant differences (χ2(2)=27.53, p=.000), which remained after controlling for clustering (t(8)=3.49, p=.008), in how many participants spoke with their physicians depending on their screening decisions. There was no effect of language and no interaction effect. Participants who chose not to screen were least likely to talk to their physician about screening. There were also significant differences in participants’ intention to work with their physician to make a decision comparing different screening decisions (F(2,194)=6.58, p=.002). There was no effect of language and no interaction effect. These effects remained in multivariate models that controlled for patient clustering in clinics (F(2,180.82)=7.78, p=.001). Participants who chose not to screen (M=1.79, SD=1.69) or remained unsure (M=1.84, SD=1.28) had lower intentions to work with their physician to make a decision prior to entering the consultation than participants who chose screening (M=2.56, SD=0.89).
Figure 2.
Proportion of participants reporting talking to physician by screening decisions
4. Discussion & conclusion
4.1. Discussion
The present study addresses several limitations of the existing literature on decision aids and shared decision-making. First, most of our participants were drawn from racial and ethnic minority groups. Second, our study drew on a well-established behavioral theory and used reliable measures to assess the impact of decision aids on the constructs of this theory19;20. The present study is the first we are aware of that used theory-based measures to examine the effects of decision aids on participants’ attitudes, perceived social norms, self-efficacy and intentions related to working with a physician to make a decision. Our results raise important questions about the behavioral effects of decision aids on patients.
Consistent with other studies, we found that participants who reviewed a decision aid were more likely to want to be the primary or sole decision-maker17. Similarly, participants who reviewed a decision aid had significantly higher knowledge about prostate or colon cancer screening17. Contrary to our hypotheses, participants who reviewed a decision aid did not report more positive attitudes, stronger perceived social norms, greater self-efficacy and greater intentions to work with their physicians in making a cancer screening decision. Participants who reviewed a decision aid were less likely to talk to their physician about screening and less likely to desire screening; however, these differences were not significant. Overall, though, participants who chose not to screen for cancer were significantly less likely to talk to their physicians. Only a quarter of those who opted against screening reporting talking to their doctor about this. Intentions to work with the physician to make a decision, reported before the clinical consultation but after reviewing the brochure or decision aid, were significantly lower among those who subsequently reported choosing not to screen or those who remained unsure about screening. Taken together, these findings suggest that a substantial proportion of participants made screening decisions after reviewing the information provided and did not discuss these with their physicians. As our role preference findings suggest, this effect was somewhat more pronounced among participants who reviewed a decision aid.
The weaker perceived social norms reported by participants who reviewed a decision aid may reflect the way the programs frame the decisions as ones to be made by the patient, somewhat more so in the prostate cancer screening program. In the introduction of this program the narrator indicates that the decision “really depends on what the test means to you” and the program closes by stating that “you have to decide if screening is important to you”. Neither the physician testimonials, nor other parts of the video program, explicitly suggest that the decision should be made in direct consultation with the physician.
The lower levels of self-efficacy reported by participants who reviewed a decision aid are more counterintuitive. This may reflect the impact of learning about the uncertainties and complexities of making a cancer screening decision, especially in the case of prostate cancer screening, which was likely more pronounced among participants who viewed a 30-minute decision aid. Whether the same effect would be observed in a more educated patient sample should be the subject of future research.
It is important to note that our questionnaire did not assess the impact of the interventions on participants making a decision themselves. In future studies it will be important to measure the effects of decision aids on these constructs, as positive effects could explain part of the findings observed in the present data. Another important point is that the behavior reported by participants may be a reflection of the clinical decision they were considering. For some, preventive decisions such as cancer screening may have a low priority. Moreover, from the patients’ point of view, a decision not to receive a preventive screening doesn’t necessarily require the participation of a physician, though for medical-legal reasons it is important for physicians to document how a patient arrived at such a decision.
Despite these qualifications, our findings raise important questions about the behavioral impact of decision aids. According to the International Patient Decision Aid Standards Collaboration, decision aids are “designed to help people participate in decision making about health care options. They provide information on the options….[and] prepare patients to make informed value-based decisions with their practitioner.”40 Our results suggest the need for further research on how decision aids impact patient behaviors in the clinical encounter. More basic research to identify the salient beliefs associated with active participation in decision making by patients would be particularly helpful. Once identified, these could further inform theory-based measures to assess the effects of decision aids on encouraging patients to share decision-making with their physicians.
Our study has several other important limitations. First, there was no pure control group, leaving it unclear how our findings would compare to a group that did not receive an educational intervention prior to the consultation. Participant assignment to brochures or decision aids was not randomized. As noted, the decision to use a sequential design grew from concerns about ensuring the integrity of randomization in community-based primary care clinics. Although we cannot completely rule out a maturation effect, we believe it was unlikely, especially for the Integrative Model measures which were completed before seeing the physician. Our sample size was limited for a nested design, potentially limiting our statistical power. However, clustering within clinics was limited and most of the observed effects remained after accounting for clustering. The distributions of the Integrative Model measures exhibited significant positive skew. However, statistical effects did not vary when we used transformed variables or non-parametric tests (not reported). All of our measures were self-report measures and it is unclear to what degree these reflect what actually happened during the clinical consultations. Ideally, we would have had a direct measure of patients’ behavior in the clinical encounter to assess the validity of our measures. Another important consideration is that our Integrative Model measures assessed a behavioral category (working with a physician) that comprised several behaviors which were defined before participants answered questions. The practical benefit of doing this is that it limits respondent burden. However, it also reduces the precision of the resulting data, as participants’ interpretation of the behavioral category in question may have varied from the definition provided. This may explain why we only accounted for 31% of the variance in patients’ intention to work with their physicians. Alternately, the limited explanatory power of the Integrative Model measures may reflect that we did not frame our questions in a specific time context. Our ability to account for variance in participants’ intention may have been greater if we had asked about “working with a physician today”. Finally, we only measured patients’ screening decisions after the consultation with the physician, leaving the timing of the decision unclear.
4.2. Conclusion
In summary, our findings that the decision aids increased patients’ knowledge and desire to play an active role in decision-making are consistent with other studies in predominately Caucasian populations17. The theory-based measures to assess the behavioral effects of decision aids on patients suggest important areas for future inquiry. If our findings are replicated and confirmed in other populations this would have important implications for decision aid design. More basic research and use of theory-based measures in future decision aid evaluations will help clarify these issues.
Acknowledgments
This study was supported by a grant from the Foundation for Informed Medical Decision Making to Dr. Frosch and the UCLA Older Americans Independence Center (NIH/NIA Grant 5P30AG028748). Dr. Légaré holds the Tier 2 Canada Research Chair in Implementation of Shared Decision Making in Primary Care. Dr. Mangione received support from the UCLA Center for Health Improvement of Minority Elderly/Resource Centers for Minority Aging Research, NIH/NIA, under Grant 2P30AG021684. We thank Socorro Ochoa, Sandra Contreras, Naveen Dhawan and Irma Ocegueda for assistance in recruiting primary care practices and patient participants and Neil Steers for statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.O’Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff (Millwood) 2004;(Suppl Web Exclusive):VAR63–VAR72. doi: 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]
- 2.Briss P, Rimer B, Reilley B, Coates RC, Lee NC, Mullen P, Corso P, Hutchinson AB, Hiatt R, Kerner J, George P, White C, Gandhi N, Saraiya M, Breslow R, Isham G, Teutsch SM, Hinman AR, Lawrence R. Promoting informed decisions about cancer screening in communities and healthcare systems. Am J Prev Med. 2004;26:67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Evans R, Edwards A, Brett J, Bradburn M, Watson E, Austoker J, Elwyn G. Reduction in uptake of PSA tests following decision aids: systematic review of current aids and their evaluations. Patient Educ Couns. 2005;58:13–26. doi: 10.1016/j.pec.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Myers RE, Daskalakis C, Cocroft J, Kunkel EJ, Delmoor E, Liberatore M, Nydick RL, Brown ER, Gay RN, Powell T, Powell RL. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005;97:1143–1154. [PMC free article] [PubMed] [Google Scholar]
- 5.Jibaja-Weiss ML, Volk RJ, Granch TS, Nefe NE, Spann SJ, Aoki N, Robinson EK, Friedman LC, Beck JR. Entertainment education for informed breast cancer treatment decisions in low-literate women: development and initial evaluation of a patient decision aid. J Cancer Educ. 2006;21:133–139. doi: 10.1207/s15430154jce2103_8. [DOI] [PubMed] [Google Scholar]
- 6.Jibaja-Weiss ML, Volk RJ, Friedman LC, Granchi TS, Neff NE, Spann SJ, Robinson EK, Aoki N, Beck JR. Preliminary testing of a just-in-time, user-defined values clarification exercise to aid lower literate women in making informed breast cancer treatment decisions. Health Expect. 2006;9:218–231. doi: 10.1111/j.1369-7625.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jibaja-Weiss ML, Volk RJ. Utilizing computerized entertainment education in the development of decision AIDS for lower literate and naive computer users. J Health Commun. 2007;12:681–697. doi: 10.1080/10810730701624356. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence VA, Streiner D, Hazuda HP, Naylor R, Levine M, Gafni A. A cross-cultural consumer-based decision aid for screening mammography. Prev Med. 2000;30:200–208. doi: 10.1006/pmed.1999.0620. [DOI] [PubMed] [Google Scholar]
- 9.Holmes-Rovner M, Valade D, Orlowski C, Draus C, Nabozny-Valerio B, Keiser S. Implementing shared decision-making in routine practice: barriers and opportunities. Health Expect. 2000;3:182–191. doi: 10.1046/j.1369-6513.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, Sodano AG, King JS. Toward the ‘tipping point’: decision aids and informed patient choice. Health Aff (Millwood) 2007;26:716–725. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 11.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57:168–182. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Protheroe J, Bower P, Chew-Graham C, Peters TJ, Fahey T. Effectiveness of a computerized decision aid in primary care on decision making and quality of life in menorrhagia: results of the MENTIP randomized controlled trial. Med Decis Making. 2007;27:575–584. doi: 10.1177/0272989X07306785. [DOI] [PubMed] [Google Scholar]
- 13.Legare F, Tremblay S, O’Connor AM, Graham ID, Wells GA, Jacobsen MJ. Factors associated with the difference in score between women’s and doctors’ decisional conflict about hormone therapy: a multilevel regression analysis. Health Expect. 2003;6:208–221. doi: 10.1046/j.1369-6513.2003.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legare F, O’Connor AM, Graham ID, Wells GA, Jacobsen MJ, Elmslie T, Drake ER. The effect of decision aids on the agreement between women’s and physicians’ decisional conflict about hormone replacement therapy. Patient Educ Couns. 2003;50:211–221. doi: 10.1016/s0738-3991(02)00129-5. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136:127–35. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, Holmes-Rovner M, Barry M, Jones J. Decision aids for patients facing health treatment or screening decisions: systematic review. Brit Med J. 1999;319:731–4. doi: 10.1136/bmj.319.7212.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, Tait V, Tetroe J, Fiset V, Barry M, Jones J. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003;(2):CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 18.Guimond P, Bunn H, O’Connor AM, Jacobsen MJ, Tait VK, Drake ER, Graham ID, Stacey D, Elmslie T. Validation of a tool to assess health practitioners’ decision support and communication skills. Patient Educ Couns. 2003;50:235–245. doi: 10.1016/s0738-3991(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 19.Bowen DJ, Allen JD, Vu T, Johnson RE, Fryer-Edwards K, Hart A., Jr Theoretical foundations for interventions designed to promote informed decision making for cancer screening. Ann Behav Med. 2006;32:202–210. doi: 10.1207/s15324796abm3203_5. [DOI] [PubMed] [Google Scholar]
- 20.Mullen PD, Allen JD, Glanz K, Fernandez ME, Bowen DJ, Pruitt SL, Glenn BA, Pignone M. Measures used in studies of informed decision making about cancer screening: a systematic review. Ann Behav Med. 2006;32:188–201. doi: 10.1207/s15324796abm3203_4. [DOI] [PubMed] [Google Scholar]
- 21.Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1980. [Google Scholar]
- 22.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1986. [Google Scholar]
- 23.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- 24.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 25.Ajzen I. The Theory of Planned Behavior. Org Beh Hum Decis Proc. 1991;50:179–211. [Google Scholar]
- 26.Francis JJ, Eccles MP, Johnston M, Walker A, Grimshaw J, Foy R, Kaner EF, Smith L, Bonetti D. Constructing questionnaires based on the Theory of Planned Behavior - A manual for health services researchers. Newcastle upon Tyne, England: Centre for Health Services Research; 2004. [Google Scholar]
- 27.Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398–405. doi: 10.1177/0272989X05278931. [DOI] [PubMed] [Google Scholar]
- 28.Ubel PA, Jepson C, Baron J. The inclusion of patient testimonials in decision aids: effects on treatment choices. Med Decis Making. 2001;21:60–68. doi: 10.1177/0272989X0102100108. [DOI] [PubMed] [Google Scholar]
- 29.Frosch DL, Dhawan N, Contreras S, Ochoa S. Beyond the ivory tower: Integrating patient decision aids in community-based primary care. Paper presented at the Society for Medical Decision Making; Pittsburgh, PA. 2007. [Google Scholar]
- 30.US Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 31.US Preventive Services Task Force. Screening for Prostate Cancer: Recommendations and Rationale. Am Fam Phys. 2003;67:787–792. [PubMed] [Google Scholar]
- 32.Chan EC, Haynes MC, O’Donnell FT, Bachino C, Vernon SW. Cultural sensitivity and informed decision making about prostate cancer screening. J Community Health. 2003;28:393–405. doi: 10.1023/a:1026072022853. [DOI] [PubMed] [Google Scholar]
- 33.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–20. [PubMed] [Google Scholar]
- 34.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1485–1489. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- 35.Legare F, Godin G, Guilbert E, Laperriere L, Dodin S. Determinants of the intention to adopt hormone replacement therapy among premenopausal women. Maturitas. 2000;34:211–218. doi: 10.1016/s0378-5122(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 36.Legare F, Godin G, Ringa V, Dodin S, Turcot L, Norton J. Variation in the psychosocial determinants of the intention to prescribe hormone therapy prior to the release of the Women’s Health Initiative trial: a survey of general practitioners and gynaecologists in France and Quebec. BMC Med Inform Decis Mak. 2005;5:31. doi: 10.1186/1472-6947-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legare F, Graham ID, O’Connor AC, Aubin M, Baillargeon L, Leduc Y, Maziade J. Prediction of health professionals’ intention to screen for decisional conflict in clinical practice. Health Expect. 2007;10:364–379. doi: 10.1111/j.1369-7625.2007.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajzen I. Attitudes, personality and behavior. Maidenhead, UK: Open University Press; 1988. [Google Scholar]
- 39.Radosevich DM, Partin MR, Nugent S, Nelson D, Flood AB, Holtzman J, Dillon N, Haas M, Wilt TJ. Measuring patient knowledge of the risks and benefits of prostate cancer screening. Patient Educ Couns. 2004;54:143–152. doi: 10.1016/S0738-3991(03)00207-6. [DOI] [PubMed] [Google Scholar]
- 40.International Patient Decision Aid Standards Collaboration. [Accessed: 12-20-2007];2006 Available: http://ipdas.ohri.ca/what.html.