Introduction
Cardiac health is dependent upon the hearts' ability to utilize different substrates to support overall oxidative metabolism in order to generate ATP. Indeed a loss in plasticity in substrate preference leading to an over dependence on the metabolism of an individual category of substrates is characteristic of a variety cardiac diseases such as diabetic heart disease where fatty acid metabolism predominates and dilated cardiomyopathy where glucose is the primary metabolic substrate. Because of the pleiotropic actions of myocardial substrate metabolism, the detrimental effects on cardiac health of a loss in plasticity in substrate preference can extend well beyond impairment in energy production. Examples include perturbations in various cell signaling pathways, alterations in cell growth and decreased cell survival. Despite the rapid growth in our understanding of the relationship between altered myocardial metabolism and cardiac disease many questions remain. For example, it is still unclear what are the key determinants of changes in myocardial substrate use and when these changes do occur to what extent are they adaptive or have the propensity to become maladaptive? Are these metabolic patterns of prognostic significance? Transgenic models targeting key aspects of myocardial substrate use are providing mechanistic insights into myocardial metabolic-functional relationships in various cardiac diseases. However, the relevance of the observed phenotypes to the corresponding human condition is frequently unclear. Finally, applied genomics have identified numerous gene variants intimately involved in the regulation of myocardial substrate use. Yet, identifying the clinically relevant genetic variants remains elusive. As a consequence, there is an ever growing demand for accurate non-invasive imaging approaches of myocardial substrate metabolism that provide linkage between the bench and the bedside leading to improved patient management paradigms. In this regard, radionuclide approaches has led the way. The most notable example that has achieved clinical applicability is the the detection of ischemic but viable myocardium with PET and 18F-fluorodexoglucose (FDG) for the management of patients with ischemic cardiomyopathy. In the subsequent discussion advances in metabolic imaging using radionuclide approaches and their potential future applications in the study of cardiovascular disease are discussed.
Methods to Image Myocardial Metabolism
Three methods are currently available to image myocardial metabolism noninvasively, magnetic resonance spectroscopy (MRS), single photon emission computed tomography (SPECT) and positron emission tomography (PET). A summary of each technique is listed below:
MRS
The advantages of MRS are the ability to measure multiple metabolic pathways simultaneously, the relative ease in performing serial measurements, and the lack of ionizing radiation. It can be combined with MRI permitting near simultaneous measurements of myocardial perfusion and mechanical function. A number of biologically important nuclei can be measured with MRS with phosphorous (31P) and hydrogen (1H), being the most common. The fundamental principle of MRS is that the chemical environment of nuclei induces local magnetic fields that shift their resonance frequency. Different metabolites exhibit a unique frequency shift resulting in a signal consisting of one or more discrete resonance frequencies. The Fourier transform of the acquired signal produces a spectrum with peaks at distinct frequencies. The MRS spectrum displays the signal intensity as a function of frequency measured in parts per million (ppm) relative to the frequency of a reference compound. The signal intensity at a given frequency is proportional to the amount of the respective metabolite and can be used to determine the absolute concentration of the metabolite using appropriate calibrating reference signal.1, 2
The disadvantages of MRS include low signal-to-noise, concomitant limited spatial resolution, intravoxel signal contamination and long acquisition times. Compared with nuclear imaging methods, MRS has a much lower sensitivity (detecting millimolar as opposed to nanomolar concentrations). As a consequence the initial success of imaging of cardiac metabolism using C-13 labeled agents in intact animals has not been translated to the study of humans.3 Cardiac applications for MRS become more limited as one moves from rodent to man as opposed to nuclear methods where the reverse occurs. This appears to be a function of both the higher field strength in the small bore systems and the use of radiofrequency coils that are in closer proximity to the entire heart used in small animal imaging. In contrast to rodent hearts where measurements of the entire left ventricular myocardium are obtained, measurements in human myocardium are typically limited to the anterior myocardium. Currently, only 31P and 1H have been widely used for in vivo clinical cardiac examinations focusing on myocardial energetics (31P) and lipid accumulation (1H).1, 3, 4
Single Photon Emission Computed Tomography (SPECT)
The advantages to SPECT for cardiac metabolic include; the inherent high sensitivity of the radionuclide method to measure metabolic processes, wide availability of the technology, with ECG-gating the ability to measure myocardial function simultaneously, and the long-physical half-life of SPECT radiotracers. The latter is an important attribute for it allows delivery of radiotracer to multiple sites which facilitates the performance of multi-center studies designed to answer clinically relevant questions about the potential utility of measurements of myocardial substrate metabolism in various cardiac diseases. Small animal SPECT and SPECT/CT systems are rapidly advancing, allowing the performance of myocardial metabolic studies in rodent models of cardiac disease. The major disadvantage of SPECT is the inability to quantify cellular metabolic processes primarily because of the technical limitations of SPECT (relatively poor temporal and spatial resolution and inaccurate correction for photon attenuation) and the complexity of the metabolism of the SPECT radiotracers relative to a metabolic process of interest.
Metabolic processes that can be measured by SPECT include (Table 1):
Table 1. Currently used Radiotracers of Metabolism.
| Radionuclide | Half-life | Compounda | Present use |
|---|---|---|---|
| SPECT | |||
| 123I | 13.3 hr | IPPA | Fatty acid metabolism |
| BMIPP | Fatty acid metabolism | ||
| PET | |||
| 15O | 2.04 min | 15O2 | Oxygen consumption |
| 11C | 20.4 min | Acetate | Oxygen consumption |
| Palmitate | Fatty acid metabolism | ||
| Glucose | Glucose metabolism | ||
| Lactate | Lactate metabolism | ||
| 18F | 110 min | Deoxyglucose | Glucose metabolism |
| FTHA | Fatty acid metabolism | ||
| FTP | Fatty acid metabolism | ||
| FCPHA | Fatty acid metabolism | ||
See text for names of compounds
Glucose Metabolism: No specific SPECT radiotracers are currently available to measure myocardial glucose metabolism. However, when combined with the appropriate detection scheme or collimator design, myocardial glucose metabolism can be measured with SPECT and FDG 5.
Fatty Acid Metabolism: One of the earliest and most promising SPECT radiotracers of fatty acid metabolism was 15-(p-iodophenyl)-pentadecanoic acid (IPPA).6-8 This radiotacer demonstrated rapid accumulation in the heart and exhibited clearance kinetics that followed a biexponential function characteristic for 11C-palmitate. Moreover, the clearance rates correlated directly with β-oxidation. In humans with coronary atherosclerosis, radtiotracer uptake and washout was reduced in regions subtended by occlude arteries consistent with ischemia 9. However, the poor temporal resolution of SPECT systems did not take advantage of the rapid turnover of IPPA. As a consequence quantification of myocardial fatty acid metabolism was not possible and image quality was reduced. This led to the development of branched-chain analogs of IPPA, such as 123I-beta-methyl-P-iodophenylpentadecanoic acid (BMIPP) (Table 1).8-11 Alkyl branching inhibits β-oxidation shunting radiolabel to the triglyceride pool thereby increasing radiotracer retention and improving image quality.
PET
One major advantage of PET is a detection scheme that permits quantification of radioactivity within the field of view. A second advantage is the use of radiopharmaceuticals labeled with the positron-emitting radionuclides. These radionuclides of the biologically ubiquitous elements oxygen (15O), carbon (11C), and nitrogen (13N), as well as fluorine (18F) substituting for hydrogen, can be incorporated into a wide variety of substrates or substrate analogues that participate in diverse biochemical pathways without altering the biochemical properties of the substrate of interest (Table 1). By combining the knowledge of the metabolic pathways of interest with kinetic models that faithfully describe the fate of the tracer in tissue, an accurate interpretation of the tracer kinetics as they relate to the metabolic process of interest can be achieved. The major disadvantages of PET are its complexity in both radiotracer design and image quantification schemes and expense. Metabolic processes that are typically measured with PET are:
-
Myocardial Oxygen Consumption (MVO2):
15O-oxygen: Because oxygen is the final electron acceptor in all pathways of aerobic myocardial metabolism, PET with 15O-oxygen has also been used to measure MVO2. The approach provides a measure of myocardial oxygen extraction which when combined with measurements of myocardial blood flow and arterial oxygen content measures MVO2 directly. Due to its short physical half-life, 15O-oxygen is readily applicable in studies requiring repetitive assessments, such as those with an acute pharmacologic intervention. Its major disadvantages are the need for a multiple-tracer study (to account for myocardial blood flow and blood volume) and fairly complex compartmental modeling to obtain the measurements. 12-14
11C-acetate: Currently, PET with 11C-acetate is the preferred method of measuring MVO2 noninvasively. Acetate is a two-carbon chain free fatty acid whose primary metabolic fate is rapidly conversion to acetyl–CoA and metabolism through the tricarboxylic acid cycle. Because of the close coupling between the tricarboxylic acid cycle and oxidative phosphorylation, myocardial turnover of 11C-acetate reflects overall oxidative metabolism or MVO2. Either exponential curve fitting or compartmental modeling is used to calculate MVO2. Modeling is typically preferable when cardiac output is low because marked dispersion of the input function and spill-over of activity from the lungs to the myocardium which decreases the accuracy of the curve-fitting method.15-19 However, it more complex that exponential curve-fitting and requires correction of blood radioactivity for 11CO2.
-
Carbohydrate metabolism:
FDG: Most studies of myocardial glucose metabolism with PET have used FDG. This radiotracer competes with glucose for facilitated transport into the sarcolemma then for hexokinase-mediated phosphorylation. In general, FDG-6-phosphate is trapped in the cytosol and the myocardial uptake of FDG is thought to reflect overall anaerobic and aerobic myocardial glycolytic flux.20-23 Overall image quality of FDG uptake is excellent but must be referenced to standardization of the substrate environment (e.g., glucose loading versus fasting). Myocardial glucose uptake can be assessed in either relative or in absolute terms (i.e., in nmol•g-1•min-1). For quantification, a mathematical correction for the kinetic differences between FDG and glucose called the lumped constant” must be used calculate rates of glucose. This value may vary depending upon the prevailing plasma substrate and hormonal conditions decreasing the accuracy of the measurement of myocardial glucose uptake.22, 24-26 Other disadvantages of FDG include the limited metabolic fate of FDG in tissue precluding determination of the metabolic fate (i.e., glycogen formation versus glycolysis) of the extracted tracer and glucose, and limitations on the performance of serial measurements of myocardial glucose uptake because of the relatively long physical half-life of fluorine-18.
Carbon-11 glucose: More recently, quantification of myocardial glucose metabolism has been performed with PET using glucose radiolabeled in the 1-carbon position with 11C (11C-glucose). An advantage of this approach is the lack of need for a lumped constant correction because 11C-glucose is chemically identical to unlabeled glucose and thus has the same metabolic fate as glucose. Other advantages include more accurate measurements of myocardial glucose uptake compared with FDG and the ability estimate the metabolic fate of extracted glucose (Figures 1 and 2).27-29 Disadvantages of this method include, a fairly complex synthesis of the tracer, the short physical half-life of 11C requires an on-site cyclotron, compartmental modeling that is more demanding with 11C-glucose than it is with FDG, and the need to correct the arterial input function for the production of 11CO2 and 11C-lactate.
11C-lactate Lactate metabolism in the heart is a key source of energy production, particularly during periods of increased cardiac work. Its impairment typifies various cardiac diseases such as diabetic heart disease.30 However, to date the ability to measure myocardial lactate has been limited by the lack the availability of an appropriate radiotracer and analysis scheme. Recently, a multi-compartmental model was developed for the assessment of myocardial lactate metabolism using PET and L-3-11C-lactate. PET derived extraction of lactate correlated well with lactate oxidation measured by arterial and coronary sinus sampling over a wide range of conditions, (Figure 3).31 When combined with either FDG or 11C-glucose it permits a more comprehensive measurement of myocardial carbohydrate metabolism.
-
Fatty acid metabolism:
11C-palmitate: The major advantage of 11C-palmitate is that its myocardial kinetics are identical to labeled palmitate. Exponential curve fitting typically identifies two clearance rates, an early rapid washout that reflects β-oxidation and a late slower washout reflecting incorporation into fatty acid intermediates and triglycerides. However, this method only provides semi-quantitative measurements of fatty acid metabolism and its accuracy is reduced under many pathologic conditions where radiotracer back-diffusion is present. Consequently, mathematical modeling techniques are typically used to circumvent these problems permitting the assessment of various aspects of myocardial fatty acid metabolism uptake such as uptake, oxidation and storage.32-34 However, this method does suffer from several disadvantages including, reduced image quality and specificity, a more complex analysis, and the need for an on-site cyclotron and radiopharmaceutical production capability.
Fatty acid analogues: Most of the PET tracers in this category have been designed to reflect myocardial β-oxidation. 14-(R,S)-18F-fluoro-6-thiaheptadecanoic acid (FTHA) was one of the first radiotracers developed using this approach. Myocardial uptake and retention tracked accordingly with changes in substrate delivery, blood flow and workload in animal models.35, 36 The effects of various diseases such as coronary artery disease and dilated cardiomyopathy on myocardial fatty acid metabolism have been evaluated with PET using this radiotracer.37, 38 However, uptake and retention of FTHA has been shown to be insensitive to the inhibition of β-oxidation by hypoxia reducing enthusiasm for this radiotracer to measure myocardial fatty acid metabolism. 39 To circumvent this problem 16-18F-fluoro-4-thia-palmitate (FTP) has been developed. This radiotracer retains the metabolic trapping function that is proportional to fatty acid oxidation under normal oxygenation and hypoxic conditions.39, 40 Similar to FDG, quantification of myocardial fatty acid metabolism with FTP requires the use of a lumped constant to correct for kinetic differences between the radiotracer and unlabeled palmitate. Furthermore the extent to which myocardial fatty acid uptake can be separated from oxidation based on the myocardial kinetics of FTP is unknown. Recently, a new F-18–labeled fatty acid radiotracer, trans-9(RS)-18F-fluoro-3,4(RS,RS) methyleneheptadecanoic acid (FCPHA), has been developed.41 This radiotracer is also trapped after undergoing several steps of β-oxidation. Preliminary results show that uptake of FCPHA into rat myocardium is approximately 1.5% injected dose per gram tissue at 5 minutes and is retained in tissue. However, the impact of alterations in plasma substrates, work load and blood flow on myocardial kinetics is unknown.
Figure 1.
Correlation in dog hearts between Fick-derived (x-axis) measurements of the rate of myocardial glucose utilization (rMGU) and PET-derived (y-axis) rMGU using (A)1-11C-glucose, (B) FDG before correcting PET values for the lumped constant (LC), (C) FDG after correcting PET values by the LC, and (D) FDG after correcting PET values by a variable LC (LCv) that accounts for varying substrate, hormonal and work environments. Correlation with Fick-derived values was significantly close when 1-11C-glucose (panel A), as opposed to 18F-FDG was used, regardless of whether an LC was used or the type of LC used (panels B-D). Reproduced with permission reference 27.
FIGURE 2.

Correlation between PET and arterial and coronary sinus (ART/CS) measurement of fractional glycolysis (A) and glucose oxidation (B). Reproduced with permission reference 29.
FIGURE 3.
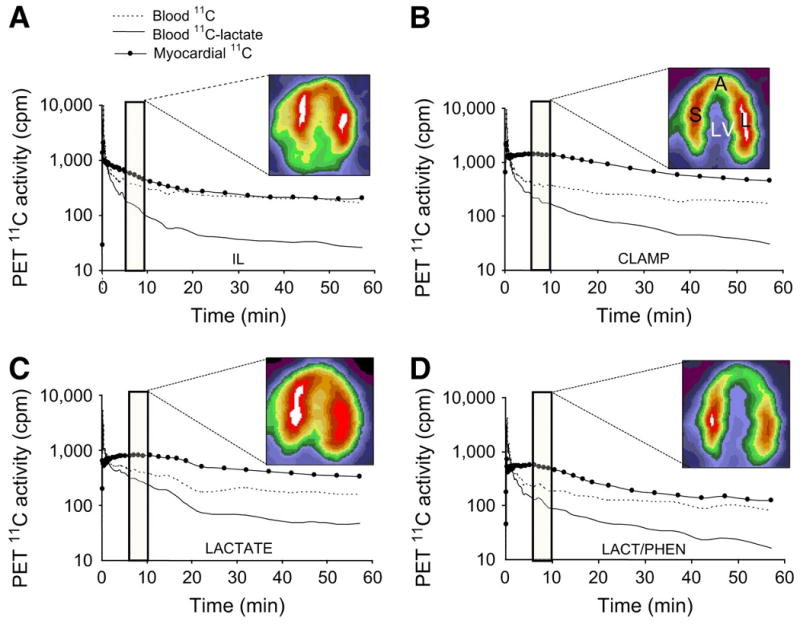
Representative PET time–activity curves of l-3-11C-lactate obtained from intralipid (IL), insulin clamp (CLAMP), lactate infusion (LACTATE), or lactate and phenylepherine (LAC/PHEN) studies and corresponding myocardial images obtained 5–10 min after tracer injection and depicting primarily early tracer uptake. Images are displayed on horizontal long axis. Blood 11C = 11C time–activity curves obtained from region of interest (ROI) placed on left atrium; blood 11C-lactate = blood 11C time–activity curves after removing 11CO2, 11C-neutral, and 11C-basic metabolites; myocardial 11C = 11C time–activity curves obtained from ROI placed on lateral wall. A = apical wall; S = septal wall; L = lateral wall; LV = left ventricle. Reproduced with permission reference 31.
Overview of Myocardial Metabolism - Plasticity in Substrate Use
The heart is an omnivore capable of switching between one substrate to another for energy production depending upon numerous factors such as the plasma substrate environment, neurohumoral milieu and level of cardiac work (Figure 4).42 Control of substrate switching can either represent an acute or chronic adaptation in response to either short or prolonged alterations in the physiological environment. The inter-regulation of short-term modulation is based on an effective interplay between various substrates with the metabolism of one substrate automatically inhibiting the pathway of another substrate via rapid enzymatic changes. Such is the case of the Randle Cycle where fatty acid oxidation inhibits glucose oxidation, glycolysis and ultimately glucose uptake via the coordinated inhibition of pyruvate dehydrogenase, phosphofructokinase and hexokinase.43
Figure 4.

Summary of myocardial substrate metabolism demonstrating the need for flexibility in myocardial substrate use tomaintain myocardial health. DCM, Dilated cardiomyopathy; IR, insulin resistance; DM, diabetes mellitus. Reproduced with permission Journal of Nuclear Cardiology 2005;12:345
In contrast, chronic metabolic adaptations reflect alterations in gene expression regulating various metabolic pathways. These changes can occur at the transcriptional and/or post-translational level through the coordinated up-regulation of enzymes and proteins in key metabolic pathways. One example is the hypoxia inducible transcription factor alpha (HIFα) which has several target genes that increase O2 delivery or survival during hypoxia such as genes involved in the up-regulation of glucose metabolism.44 Another example is the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα), which is a key regulator of myocardial fatty acid uptake, oxidation and storage.45 In diabetes mellitus, PPARα activity is increased leading to an up-regulation in genes controlling fatty acid uptake and oxidation.46 In contrast, pressure-overload hypertrophy PPARα activity is reduced leading to a down-regulation of genes controlling fat metabolism and in turn leading to an up-regulation of glucose use.47 Numerous detrimental effects ensue that extend beyond alterations in energy production and may include increases in oxygen free radical production, impaired energetics, increases in apoptosis, and the induction of left ventricular dysfunction.46, 48, 49 Discussed in the sections that follow is how metabolic imaging has helped characterize this loss of metabolic flexibility due to these chronic adaptations in various disease processes.
Aging and Gender
Marked changes in myocardial substrate metabolism occur with age. The fetal heart is typified by a preference for glucose primarily because of the relative hypoxia that occurs in utero. With birth and progression to adulthood fatty acids become the preferred energy source. In experimental models of aging the contribution of fatty acid oxidation to overall myocardial substrate metabolism declines with advancing age.50, 51 It appears the cause for the decrease in fatty acid oxidation is multi-factorial ranging from changes in mitochondrial lipid content, oxygen free radical injury, key enzyme systems, and a age-related decline in myocardial PPARα activity.52-54 Healthy older humans demonstrate a similar metabolic shift.55 However, despite the preference for glucose use, older individuals demonstrated a blunted increase in glucose utilization in response to dobutamine when compared to younger individuals. 56 Potential clinical ramifications of this blunted metabolic response include stress-related energy deprivation state in the aging heart or possibly increase the susceptibility of the heart to injury during periods of ischemia. However, these age related changes in substrate metabolism are not irreversible as the impairment in metabolic reserve can be ameliorated by endurance exercise training (Figure 5).57 Of interest, it appears the myocardial metabolic response to dobutamine following endurance exercise training is gender specific with men demonstrating an increase in myocardial glucose metabolism and a decline in fatty acid use whereas women exhibited increase in both glucose and fatty acid metabolism.
Figure 5.

Effect of endurance exercise training (EET) on myocardial glucose metabolism at rest and during intravenous dobutamine infusion both pre- and post-EET. MGUp, fractional myocardial glucose uptake; MGU, myocardial glucose utilization. Values are means ± SD. *P = 0.03 vs. Pre; †P = 0.04 vs. Pre. Reproduced with permission reference 5.
It is now apparent that gender is also a determinant of the pattern in the myocardial substrate metabolism. For example in female rats myocardial glucose metabolism was lower and fatty acid metabolism was higher compared with male rats.58, 59 These observations were confirmed in young healthy volunteers using PET with 11C-glucose and 11C-palmitate.60 Women exhibited lower levels of glucose metabolism compared with men. Although, no differences in myocardial fatty acid metabolism were noted, women also exhibited higher MVO2 compared with men as measured by PET with 11C-acetate. It appears these gender differences in substrate metabolism become more pronounced as one transitions to more pathologic conditions. For example in addition to the changes in glucose metabolism and MVO2, obese women exhibited higher fatty acid uptake and oxidation compared with obese men.61 The differences in myocardial metabolism could not be explained by differences in myocardial blood flow, insulin sensitivity, or hemodynamics. However, it should be noted that fatty acid release into the plasma is higher in women compared with men primarily due to a higher fat mass.62 Thus, differences in myocardial fatty acid delivery may be contributing to these metabolic differences. Sex hormone stimulation may also play a role since results of animal studies have shown that estrogen decreases glucose oxidation, gluconeogenesis, and glycogenolysis, and increases fatty acid oxidation in liver and skeletal muscle. 63 64 65 66 Estrogen also upregulates endogenous nitric oxide synthase which reduces GLUT-4 translocation to the cell surface, thereby inhibiting myocardial glucose uptake. 67 68 69 Indeed, in a small retrospective study PET-derived measurements of myocardial fatty acid metabolism were higher in post-menopausal women were higher compared with either post-menopausal women not receiving hormonal replacement therapy or age-matched men. 70 Although, requiring further study, these gender and age differences in metabolism may provide a partial explanation for the gender- and age-related outcome differences for various cardiovascular diseases where altered myocardial metabolism plays a role. Moreover, these observations highlight the need to account for age and gender differences when performing measurements of myocardial substrate metabolism.
Diabetes Mellitus
Cardiovascular disease is the leading cause of morbidity and mortality in patients with diabetes mellitus.71 Although numerous mechanisms such as increased prevalence of hyperlipidemia and hypertension, impaired fibrinolysis, abnormal myocardial endothelial function, and reduced sympathetic neuronal function are likely responsible, there is extensive evidence to suggest that abnormalities in myocardial substrate metabolism also contribute to the increased cardiac risk. 72, 73 As mentioned previously the overdependence on fatty acid metabolism and a decrease in glucose use typifies the metabolic phenotype in diabetes. This increase in fatty acid metabolism reflects in large part the effects of peripheral insulin resistance which increase plasma fatty acid levels resulting in increased fatty acid delivery to the myocardium. This results increased myocardial fatty acid uptake which activates key transcriptional pathways such as the PPARα/PGC-1 signaling network resulting in further myocardial fatty acid uptake and oxidation.74-76. Both insulin-mediated glucose transport and glucose transporter expression decline in diabetes mellitus. However, rates of myocardial glucose uptake are frequently normal due to the presence of hyperglycemia. The increase in myocardial fatty acid metabolism results in increased citrate levels which inhibit phosphofructokinase. Glucose oxidation is inhibited at the level of pyruvate dehydrogenase complex due to increased mitochondrial acetyl-CoA levels and the phosphorylation of pyruvate dehydrogenase kinase 4 by PPARα activation. Consequently, the maintenance of myocardial glucose uptake but a decrease in its downstream metabolism results in an accumulation of glucose metabolites. Potential detrimental effects associated with this shift in metabolism include: impaired mechanical function due to the inability to increase glucose metabolism in response to increase myocardial work, depletion of tricarboxylic acid cycle intermediates due to reduced anapleurosis, electrical instability and apoptosis, a greater sensitivity to myocardial ischemia and myocardial lipid and glucose metabolite accumulation leading to increased apoptosis.
Small animal radionuclide imaging has helped clarify the mechanisms responsible for the metabolic alterations that occur in diabetes mellitus. For example, mice with cardiac-restricted overexpression of PPARα (a metabolic phenotype that is similar to diabetic hearts).77 demonstrate an increase in fatty acid uptake and an abnormal suppression of glucose uptake using small animal PET with 11C-palmitate and FDG. Conversely, small animal PET measurements demonstrate an increase glucose uptake and reduced fatty acid uptake in mice with cardiac-restricted overexpression of PPARβ/σ.78 Together these observations demonstrate that PPARα and PPARβ/σ drive different metabolic regulatory programs in the heart and that imaging can help characterize genetic manipulations in mouse heart. However, the small size of the mouse heart permits only semi-quantitative measurements of tracer uptake highlighting the imaging challenges that still must be overcome before quantification of metabolic processes are possible in this species. In contrast, quantitative measures of myocardial substrate metabolism are now possible with small animal PET in rat heart. Rates of myocardial glucose uptake correlate directly and closely and GLUT 4 gene expression in the Zucker-Diabetic-Fat (ZDF) rat, a model of type-2 diabetes mellitus (Figure 6).79 Moreover, rates of myocardial glucose uptake and fatty acid uptake and oxidation measured with PET in the same disease model demonstrated the importance of increased fatty acid delivery to defining the metabolic phenotype in diabetes.80
Figure 6.
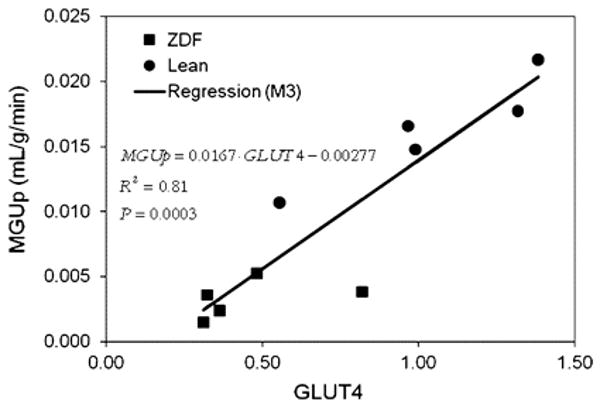
Regression model M3 was used to characterize dependence of MGUp on gene expression of GLUT-4 with R2 = 0.81, which is significant at P = 0.0003. Reproduced with permission reference 105.
Numerous studies have utilized PET with FDG to characterize myocardial glucose metabolism in patients with diabetes mellitus. These studies have been primarily limited to PET using FDG.81-83 In general, rates myocardial glucose uptake are reduced in patients with either type 1 or type 2 diabetes mellitus compared with non-diabetics except in under conditions of marked hyperglycemia or supraphysiological levels in plasma insulin (such as occurs with a hyperinsulinemic-euglycemic clamp. Increased myocardial fatty acid uptake measured by arterial-coronary sinus balance studies has been reported in humans with type-1 diabetes mellitus without coronary artery disease.84 Although, the impact of plasma levels of free fatty acids on the level of myocardial fatty acid uptake was not determined, a negative correlation between myocardial glucose uptake and plasma fatty acid levels was observed. Recently studies using PET and 11C-plamitate and 11C-glucose in patients with type-1 diabetes mellitus have helped verify these findings noninvasively. Patients with diabetes exhibited higher levels of fatty acid uptake and oxidation compared with non-diabetics primarily due to increased plasma fatty acid levels. In contrast, glucose uptake was reduced in these patients primarily due to decreased glucose transport mechanisms.33. Consistent to what has been observed in experimental models of diabetes mellitus, the metabolic fate of extracted glucose is impaired in diabetes with reduced rates of glycolysis and glucose oxidation which become more pronounced with increases in cardiac work induced by dobutamine (Figure 7).85 However, despite the overdependence on fatty acid metabolism the diabetic heart still is responsive to changes in plasma insulin and fatty acid levels but at a cost. For example, higher insulin levels are needed to achieve the same level of glucose uptake and glucose oxidation compared with non-diabetics consistent with myocardial insulin resistance. In response to higher plasma fatty acid levels myocardial fatty acid uptake is increased however limitations on the downstream oxidation rate results in a greater esterification rate.85, 86
Figure 7.

Measurements of overall myocardial glucose uptake (MGU), glycolysis, glucose oxidation and glycogen synthesis for normal volunteers (NV), type-1 diabetic patients studied under baseline metabolic conditions (DM1) and diabetic patients studied during hyperinsulinemic-euglycemic clamp (DM1-C) both at rest (open bars) and during dobutamine (closed bars). Reproduced with permission reference 79.
Therapeutic strategies are being designed to decrease fatty acid delivery to the in order to reduce the over dependence of the myocardium on fatty acid metabolism and perhaps improve energetics and function of the left ventricle. For example, the use of the insulin sensitizing agent troglitazone in ZDF rats reduces plasma fatty acid levels, decreases myocardial lipid accumulation, reduces apoptosis and improves left ventricular function.49 In PET with FDG studies in patients with type-2 diabetes mellitus, before and 26 weeks after treatment with rosiglitazone, demonstrated nearly a 40% increase in insulin-stimulated myocardial glucose uptake, implying reduced fatty acid uptake, which was attributed in large part to suppression in plasma fatty acid levels.87 Similar metabolic changes were not seen with the biguanide, metformin whose mechanism of action is designed to reduce hepatic glucose production. Of note, the improvement in insulin-stimulated glucose metabolism could not be predicted by changes in the plasma glucose or HgBA1C levels. These data suggest that metabolic imaging may provide unique insights into therapeutic myocardial metabolic modulation not attainable with more readily available clinical parameters are not predictive of a therapeutic response.
Obesity and Insulin Resistance
Given that obesity is a risk factor for type-2 diabetes mellitus it is not surprising that a significant increase in body mass index (BMI) induces marked increases in myocardial fatty acid metabolism. In either dietary-induced or transgenic models of obesity myocardial fatty acid uptake and oxidation are significantly increased.49, 88 This increase at least initially, reflects the increase in fatty acid delivery to the heart due to increased lipolysis from both visceral/abdominal and subcutaneous fat stores secondary to insulin resistance. As in diabetes mellitus the increase in cardiac fatty acid delivery initiates a cascade of events that increase myocardial fatty acid metabolism. Ultimately, fatty acid uptake may exceed oxidation leading to extracted fatty acids entering non-oxidative pathways resulting in lipid formation which can compromise cell survival and cardiac function.49
Using PET and 11C-acetate and 11C-palmitate it is now apparent that similar abnormalities in myocardial fatty acid metabolism occur in humans with obesity. For example, in otherwise healthy young obese women, myocardial fatty acid metabolism increases with an increase in BMI with the overdependence in fatty acid metabolism becoming more pronounced with worsening insulin resistance.89 There is little change in myocardial glucose metabolism. The increase in myocardial fatty acid use is paralleled by an increase in MVO2 and a decrease in energy transduction. These findings suggest that metabolic changes in obesity may play a role in the pathogenesis of cardiac dysfunction which is more pronounced in obese women.90 Of note, it appears there the myocardial metabolic response to obesity differs between women and men.61 In contrast to obese women, obese men exhibit a greater impairment in myocardial glucose metabolism per level plasma insulin, suggesting greater myocardial insulin resistance (Figure 8). In addition, obesity had less effect on myocardial fatty acid metabolism in men. In contrast, MVO2 is higher in the obese women compared with obese men. Thus it appears there appears to be a complex interplay between gender and obesity in influencing myocardial substrate metabolism.
Figure 8.

Shown are the levels for fractional myocardial glucose uptake and glucose utilization/plasma insulin, respectively, for the 4 groups. Obesity's and gender's significant interaction in the prediction of glucose uptake (P < .05) is shown graphically because both the magnitude and direction of the effect of obesity on myocardial glucose uptake differ in men and women. Obese men have lower uptake than nonobese men; obese women, however, have greater uptake than nonobese women. Obesity and gender also interacted in determining glucose utilization/plasma insulin (P < .01), as shown by the magnitude of the difference between the obese and nonobese men and that between the obese and nonobese women. Reproduced with permission reference 61.
Ischemia
With the induction of myocardial ischemia, fatty acid oxidation ceases and glucose becomes the primary substrate for both increased anaerobic glycolysis and for continued, albeit, diminished oxidative metabolism.91 This metabolic switch is prerequisite for continued a energy production and cell survival. When the ischemic insult is reversed, oxygen availability increases and oxidative metabolism resumes. It appears that these abnormalities in myocardial substrate metabolism may persist well after the resolution of ischemia, so called “ischemic memory”.92 Demonstration of either accelerated myocardial glucose metabolism or reduced fatty acid metabolism using FDG and BMIPP, respectively, has been used to document this phenomena. For example, increased myocardial FDG uptake has been shown in patients with unstable angina during pain-free episodes.12 Moreover, in patients with stable angina increased FDG uptake was demonstrated following exercise-induced ischemia-in the absence of either perfusion deficits or ECG abnormalities93. Similar observations have been made with SPECT using BMIPP. It is apparent that in patients with acute chest pain that abnormalities in myocardial BMIPP uptake may persist 24-36 hrs following the resolution of symptoms 94, 95 Moreover, this “metabolic fingerprint” appears superior to perfusion imaging for either identifying coronary artery disease as the cause of the chest pain or assigning prognosis.94 The persistence in the metabolic defect increases the flexibility of radiotracer administration for it allows for delivery of a unit dose after the patient has already been evaluated. This is in contrast to the use of perfusion radiotracers which frequently must be available on-site due to the narrow time window from the resolution of symptoms and normalization of the flow deficit. Based on these observations, BMIPP is currently undergoing Phase 3 evaluation for acute chest pain imaging. Recent studies also suggest that patterns of reduced fatty acid metabolism may be a marker of cardiac risk in certain high risk patient populations. For example in patients with end-stage renal disease, cardiac death was significantly associated with highly abnormal BMIPP uptake.96 Metabolic imaging with either FDG or BMIPP has also been used for direct ischemia detection during stress testing.5, 97, 98 The thought process being that abnormalities in vasodilator reserve with perfusion tracers will underestimate ischemia if oxygen demand and supply remain balanced. Results of initial studies where FDG were injected during exercise appears to support this contention with a greater detection rate for moderately severe coronary artery stenoses compared with perfusion imaging.5 Moreover, it appears defects in either glucose or fatty acid metabolism with exercise will persist for ∼24hrs (Figure 9).97, 98 Taken in sum, these observations suggest metabolic imaging may provide unique role in the management of patients with ischemic heart disease. However despite these promising results, numerous questions still remain as to the optimal imaging protocols, the impact of alterations in the plasma substrate environment on diagnostic accuracy, the usefulness in diabetic patients, whether added diagnostic and prognostic information is provided over perfusion imaging, and whether this information alters clinical management.
Figure 9.


Figure 9A: Left, Thallium-201 stress and reinjection (Reinj) images after treadmill exercise in the short-axis (SA) and vertical long-axis (VLA) SPECT tomograms (left). Thallium images demonstrate a severe reversible inferior defect (arrows), consistent with exercise stress-induced ischemia. Right, A similar defect is seen on the early BMIPP images in the same tomographic cuts (arrows), with BMIPP injected 22 hours after the stress-induced ischemia. The defect on the delayed BMIPP image is less prominent than on the early image. These image data suggest that BMIPP detects prolonged postischemic suppression of fatty acid metabolism for up to 22 hours after stress-induced ischemia. Reproduced with permission reference 95.
Figure 9B: Exercise and rest 99mTc-sestamibi perfusion and 18F-FDG images of 71-y-old man (patient 7) with exertional angina, in short-axis and vertical and horizontal long-axis slices. Reversible perfusion abnormality involving anterior and septal walls (white arrows) is shown. Intense 18F-FDG uptake in anterior and septal walls on exercise images is demonstrated. 18F-FDG uptake persists on rest images in anterior and septal walls, and lateral wall also showed intense 18F-FDG uptake (yellow arrows). This patient had 90% stenosis of left anterior descending and 80% stenosis of circumflex and right coronary artery. Reproduced with permission reference 96.
Hypertension/Left Ventricular Hypertrophy
There are numerous lines of evidence to support the notion of a relationship between between abnormalities in myocardial substrate metabolism and left ventricular hypertrophy. In experimental models of pressure-overload left ventricular hypertrophy myocardial fatty acid oxidation is decreased and there is an increase in glucose use.99, 100 Moreover, interventions that inhibit mitochondrial fatty acid β-oxidation result in cardiac hypertrophy.100 In humans, variants in genes regulating key aspects of myocardial fatty acid metabolism ranging from PPARα to various key β-oxidative enzymes are associated with left ventricular hypertrophy.101, 102
In a rat model of hypertrophy PET with FDG demonstrated myocardial glucose uptake tracked directly with increasing hypertrophy confirming this shift in substrate preference in-vivo.103. Similar results have been found in man. PET with 11C-palmitate in humans has shown the reduction in myocardial fatty acid oxidation is an independent predictor of left ventricular mass in hypertension.104 Combining measurements of left ventricular myocardial external work (either by echocardiography or MRI) with measurements of MVO2 performed by PET with 11C-acetate or 15O-oxygen, it is possible to estimate cardiac efficiency. Using this approach in patients with hypertension-induced left ventricular hypertrophy has shown that the decline in myocardial fatty acid metabolism is associated with a decline in efficiency, a condition that may increase the potential for the development of heart failure. 13, 105 Another potential application of PET is the phenotyping of patients with genetic variants related to myocyte growth. For example in patients with hypertrophic cardiomyopathy attributable to a known specific variant in the α-tropomyosin gene, it was observed that increased myocardial perfusion, fatty acid metabolism, and efficiency characterize patients with mild hypertrophy whereas these metabolic alterations decrease as hypertrophy becomes more advanced.106. Although, requiring further study in larger patient populations, this study suggests that metabolic imaging may identify relevant gene variants without waiting for more end-stage manifestations such as left ventricular remodeling and dysfunction to occur.
Nonischemic Dilated Cardiomyopathy
Perturbations in myocardial substrate metabolism have also been implicated in the pathogenesis of contractile dysfunction and heart failure. In experimental models of heart failure the progression from cardiac hypertrophy to ventricular dysfunction is paralleled by a decrease in the expression of genes encoding for enzymes regulating β-oxidation resulting in a shift in myocardial substrate metabolism to primarily glucose use, similar to that seen in the fetal heart.99, 107, 108 Paralleling these metabolic changes are the re-expression of fetal isoforms of a variety of contractile and calcium regulatory proteins program. The reactivation of the metabolic fetal gene program may have numerous detrimental consequences on myocardial contractile function ranging from energy deprivation to the inability to process fatty acids leading to accumulation of nonoxidized toxic fatty acid derivatives, resulting in lipotoxicity. Underscoring the importance of this metabolic shift in the pathogenesis of heart failure is the robust discovery and development of novel therapeutics which target specific aspects of cellular metabolism such as the partial fatty acid oxidation antagonists and the insulin sensitizer glucagon-like peptide-1.109
Both SPECT and PET techniques have documented in humans the metabolic shift associated with cardiomyopathy. For example, SPECT with BMIPP demonstrated reduced myocardial uptake and increased clearance radiotracer in patients with dilated cardiomyopathy compared with controls.110 Moreover, the magnitude of these metabolic abnormalities correlated with other measurements of heart failure severity such as left ventricular size and plasma b-natruietic peptide levels. It appears these abnormalities in BMIPP kinetics reflect the combined effects of reduced fatty acid uptake and oxidation as evidenced by PET with 11C-palmitate studies in a similar patient population. In this same study, myocardial glucose metabolsim was higher in the cardiomyopathic patients compared with controls confirming the metabolic shift.111 Similarly, PET has also been used provide mechanistic insights into the myocardial metabolic abnormalitie associated with heart failure. Rapid lowering of fatty acid delivery with acipimox results in reduced fatty acid uptake, MVO2, and cardiac work and no change cardiac efficiency in normal volunteers.106 However in patients with nonischemic dilated cardiomyopathy, there is a decrease in myocardial fatty acid uptake and cardiac work, no change in MVO2 and a decline in efficiency. Although limited by a small sample size, these results appear to reinforce to the central role of loss flexibility in myocardial substrate metabolism in the pathogenesis of heart failure with even minor changes in substrate delivery having detrimental consequences on cardiac energy transduction.
Metabolic imaging can also been used to study the effects of various treatments for cardiomyopathy. For example, treatment with the selective β-blocker metoprolol results in a reduction in oxidative metabolism and an improvement in cardiac efficiency as measured by PET in patients with heart failure.112 Cardiac efficiency has been shown to improve following either exercise training or cardiac resynchronization therapy, implicating improved myocardial energy transduction as a potential mechanism.113, 114 Moreover, normalization of the distribution of myocardial glucose metabolism may be partially responsible for the improved energetic following cardiac resynchronization therapy.115 Partial fatty acid oxidation inhibitors have been proposed for the treatment of heart failure based on the theory that decreasing myocardial fatty acid oxidation should increase the oxidation of glucose leading to a more favorable energetic state and improved left ventricular function. In support of this hypothesis is the finding that, the administration of trimetizidine to patients with dilated cardiomyopathy results in a significant improvement in left ventricular ejection fraction.106 However, the improvement in left ventricular function is paralleled by only a mild decrease in myocardial fatty acid oxidation. Thus it appears the improvement in left ventricular function reflects more than a shift in metabolism and is likely influenced by other factors such as improved whole-body insulin resistance and synergestic effects with β-blockade. In patients with dilated cardiomyopathy, it appears the fraction of myocardial glucose uptake, as measured by PET with FDG predicts the effectiveness of β-blocker therapy.116 Moreover, in patients with ischemic cardiomyopathy the extent of viable myocardium as measured with PET and FDG correlated with the hemodynamic response after cardiac resynchronization therapy suggesting a role for PET in discriminating responders from non-responders to this therapy.117 Thus, metabolic imaging can also be used to predict the response to specific therapies in heart failure patients.
Beyond the Myocardium - Vascular Imaging
Atherosclerosis is a inflammatory process characterized by cycles of intense activity and progression followed by intervals of stabilization. A common result of this process is coronary artery luminal stenosis that compromises myocardial blood flow and induces ischemia during stress. However, commonly the inciting event is acute plaque rupture with thrombosis leading to myocardial infarction and frequently, sudden cardiac death. Based on the premise that glucose metabolism is increased in activated macrophages which are a key component ofatherosclerotic plaque, FDG is being evaluated for the detection of “biologically active” atherosclerosis. Several groups have established that inflamed arterial vessels have increased uptake of FDG as measured by PET. The increased uptake has been noted in animal models of atherosclerosis, and verified in humans with atherosclerosis of the carotid artery and aorta.118-121 Moreover, a significant correlation between FDG uptake and CD68 cell staining has recently been established. It appears that vascular FDG imaging is fairly reproducible suggesting a role for monitoring of therapy.122 Indeed, a decrease in carotid artery FDG uptake is correlative with an increase in plasma high-density lipoprotein levels following statin therapy or life style modifications (Figure 10).120, 123 However many questions remain about this technique such as the site of localization of radiotracer (e.g., plaque or smooth muscle), the suitability of the method for evaluating the coronary arteries, and whether the information provides more refined risk stratification compared with other more widely applicable methods or if it alters therapy.
Figure 10.

Serial FDG PET/CT images of 62-y-old male patient. (A) Initial study demonstrates focal site of increased FDG uptake in right common carotid artery. Lesion–to–blood-pool peak SUV ratio was 1.2. (B) On 1-y follow-up study, carotid artery lesion was no longer visible, and peak SUV ratio was reduced to 0.9. Reproduced with permission reference 120.
Future Directions
Despite the success of metabolic imaging in furthering our understanding of disease mechanism and in identifying and evaluating potential novel therapeutics for various forms of cardiovascular disease, advances are needed in several areas. There needs to be continued improvement in instrumentation design for imaging of humans and small animals. For example, advances in PET detector design and post-detector electronics will further increase counting statistics which would improve the ability to perform more complex compartmental modeling permitting more complete characterization of the metabolism of a given substrate. New designs in SPECT technology may permit dynamic data acquisitions permitting quantitative or semi-quantitative measurements of substrate metabolism. For small animal imaging tracer quantification is still difficult but should be facilitated with the new hybrid systems where accurate attenuation correction can be performed. There is a fundamental need for the development of new radiotracers that permit characterization of key metabolic pathways such as uptake, storage or oxidation that are linked to disease manifestations. Moreover, new radiotracers are needed to provide insights into the linkage between substrate metabolism and cell growth, cell survival and energy transduction. Ultimately, for metabolic imaging to achieve clinical relevance beyond viability detection will require the performance of appropriately powered clinical trials designed to answer key questions about the utility of metabolic imaging for diagnosis, risk stratification and monitoring of therapy in specific patient populations. To perform such studies will necessitate the development of radiotracers radiolabeled with either F-18 or I-123 and readily exportable image analysis schemes. However once these challenges are met, metabolic imaging will be positioned as an indispensible tool for the management of the cardiac patient.
Acknowledgments
This work was supported in part by PO1-HL13851, R01-HL73120 and RO1-HL69100
References
- 1.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida T. The rate of phosphocreatine hydrolysis and resynthesis in exercising muscle in humans using 31P-MRS. J Physiol Anthropol Appl Human Sci. 2002;21:247–255. doi: 10.2114/jpa.21.247. [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski ED. Cardiac carbon 13 magnetic resonance spectroscopy: on the horizon or over the rainbow? J Nucl Cardiol. 2002;9:419–428. doi: 10.1067/mnc.2002.125811. [DOI] [PubMed] [Google Scholar]
- 4.Bottomley PA, Weiss RG. Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet. 1998;351:714–718. doi: 10.1016/S0140-6736(97)06402-7. [DOI] [PubMed] [Google Scholar]
- 5.He ZX, Shi RF, Wu YJ, Tian YQ, Liu XJ, Wang SW, Shen R, Qin XW, Gao RL, Narula J, Jain D. Direct imaging of exercise-induced myocardial ischemia with fluorine-18-labeled deoxyglucose and Tc-99m-sestamibi in coronary artery disease. Circulation. 2003;108:1208–1213. doi: 10.1161/01.CIR.0000088784.25089.D9. [DOI] [PubMed] [Google Scholar]
- 6.DeGrado TR, Holden JE, Ng CK, Raffel DM, Gatley SJ. Quantitative analysis of myocardial kinetics of 15-p-[iodine-125] iodophenylpentadecanoic acid. J Nucl Med. 1989;30:1211–1218. [PubMed] [Google Scholar]
- 7.Dormehl IC, Hugo N, Rossouw D, White A, Feinendegen LE. Planar myocardial imaging in the baboon model with iodine-123-15-(iodophenyl)pentadecanoic acid (IPPA) and iodine-123-15-(P-iodophenyl)-3-R,S-methylpentadecanoic acid (BMIPP), using time-activity curves for evaluation of metabolism. Nucl Med Biol. 1995;22:837–847. doi: 10.1016/0969-8051(95)00015-p. [DOI] [PubMed] [Google Scholar]
- 8.Eckelman WC, Babich JW. Synthesis and validation of fatty acid analogs radiolabeled by nonisotopic substitution. J Nucl Cardiol. 2007;14:S100–109. doi: 10.1016/j.nuclcard.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Reske SN, Sauer W, Machulla HJ, Knust J, Winkler C. Metabolism of 15 (p 123I iodophenyl-)pentadecanoic acid in heart muscle and noncardiac tissues. Eur J Nucl Med. 1985;10:228–234. doi: 10.1007/BF00254466. [DOI] [PubMed] [Google Scholar]
- 10.Ambrose KR, Owen BA, Goodman MM, Knapp FF., Jr Evaluation of the metabolism in rat hearts of two new radioiodinated 3-methyl-branched fatty acid myocardial imaging agents. Eur J Nucl Med. 1987;12:486–491. doi: 10.1007/BF00620471. [DOI] [PubMed] [Google Scholar]
- 11.Goodman MM, Kirsch G, Knapp FF., Jr Synthesis and evaluation of radioiodinated terminal p-iodophenyl-substituted alpha- and beta-methyl-branched fatty acids. J Med Chem. 1984;27:390–397. doi: 10.1021/jm00369a027. [DOI] [PubMed] [Google Scholar]
- 12.Iida H, Rhodes CG, Araujo LI, Yamamoto Y, de Silva R, Maseri A, Jones T. Noninvasive quantification of regional myocardial metabolic rate for oxygen by use of 15O2 inhalation and positron emission tomography. Theory, error analysis, and application in humans. Circulation. 1996;94:792–807. doi: 10.1161/01.cir.94.4.792. [DOI] [PubMed] [Google Scholar]
- 13.Laine H, Katoh C, Luotolahti M, Yki-Jarvinen H, Kantola I, Jula A, Takala TO, Ruotsalainen U, Iida H, Haaparanta M, Nuutila P, Knuuti J. Myocardial oxygen consumption is unchanged but efficiency is reduced in patients with essential hypertension and left ventricular hypertrophy. Circulation. 1999;100:2425–2430. doi: 10.1161/01.cir.100.24.2425. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, de Silva R, Rhodes CG, Iida H, Lammertsma AA, Jones T, Maseri A. Noninvasive quantification of regional myocardial metabolic rate of oxygen by 15O2 inhalation and positron emission tomography. Experimental validation. Circulation. 1996;94:808–816. doi: 10.1161/01.cir.94.4.808. [DOI] [PubMed] [Google Scholar]
- 15.Armbrecht JJ, Buxton DB, Schelbert HR. Validation of [1-11C]acetate as a tracer for noninvasive assessment of oxidative metabolism with positron emission tomography in normal, ischemic, postischemic, and hyperemic canine myocardium. Circulation. 1990;81:1594–1605. doi: 10.1161/01.cir.81.5.1594. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Marshall DR, Sobel BE, Bergmann SR. Delineation of myocardial oxygen utilization with carbon-11-labeled acetate. Circulation. 1987;76:687–696. doi: 10.1161/01.cir.76.3.687. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Myears DW, Bergmann SR. Noninvasive assessment of canine myocardial oxidative metabolism with carbon-11 acetate and positron emission tomography. J Am Coll Cardiol. 1988;12:1054–1063. doi: 10.1016/0735-1097(88)90476-7. [DOI] [PubMed] [Google Scholar]
- 18.Buck A, Wolpers HG, Hutchins GD, Savas V, Mangner TJ, Nguyen N, Schwaiger M. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32:1950–1957. [PubMed] [Google Scholar]
- 19.Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, Kofoed KF, Weismueller S, Czernin J, Phelps ME, Schelbert HR. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39:272–280. [PubMed] [Google Scholar]
- 20.Choi Y, Hawkins RA, Huang SC, Gambhir SS, Brunken RC, Phelps ME, Schelbert HR. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-d-glucose studies. J Nucl Med. 1991;32:733–738. [PubMed] [Google Scholar]
- 21.Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, Rochette L. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem. 2006;283:147–152. doi: 10.1007/s11010-006-2518-9. [DOI] [PubMed] [Google Scholar]
- 22.Iozzo P, Chareonthaitawee P, Di Terlizzi M, Betteridge DJ, Ferrannini E, Camici PG. Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab. 2002;282:E1163–1171. doi: 10.1152/ajpendo.00386.2001. [DOI] [PubMed] [Google Scholar]
- 23.Krivokapich J, Huang SC, Selin CE, Phelps ME. Fluorodeoxyglucose rate constants, lumped constant, and glucose metabolic rate in rabbit heart. Am J Physiol. 1987;252:H777–787. doi: 10.1152/ajpheart.1987.252.4.H777. [DOI] [PubMed] [Google Scholar]
- 24.Botker HE, Bottcher M, Schmitz O, Gee A, Hansen SB, Cold GE, Nielsen TT, Gjedde A. Glucose uptake and lumped constant variability in normal human hearts determined with [18F]fluorodeoxyglucose. J Nucl Cardiol. 1997;4:125–132. doi: 10.1016/s1071-3581(97)90061-1. [DOI] [PubMed] [Google Scholar]
- 25.Hariharan R, Bray M, Ganim R, Doenst T, Goodwin GW, Taegtmeyer H. Fundamental limitations of [18F]2-deoxy-2-fluoro-D-glucose for assessing myocardial glucose uptake. Circulation. 1995;91:2435–2444. doi: 10.1161/01.cir.91.9.2435. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Nishimura T, Imahashi KI, Yamaguchi H, Hori M, Kusuoka H. Lumped constant for deoxyglucose is decreased when myocardial glucose uptake is enhanced. Am J Physiol. 1999;276:H129–133. doi: 10.1152/ajpheart.1999.276.1.H129. [DOI] [PubMed] [Google Scholar]
- 27.Herrero P, Sharp TL, Dence C, Haraden BM, Gropler RJ. Comparison of 1-(11)C-glucose and (18)F-FDG for quantifying myocardial glucose use with PET. J Nucl Med. 2002;43:1530–1541. [PubMed] [Google Scholar]
- 28.Herrero P, Weinheimer CJ, Dence C, Oellerich WF, Gropler RJ. Quantification of myocardial glucose utilization by PET and 1-carbon-11-glucose. J Nucl Cardiol. 2002;9:5–14. doi: 10.1067/mnc.2001.120635. [DOI] [PubMed] [Google Scholar]
- 29.Herrero P, Kisrieva-Ware Z, Dence CS, Patterson B, Coggan AR, Han DH, Ishii Y, Eisenbeis P, Gropler RJ. PET measurements of myocardial glucose metabolism with 1-11C-glucose and kinetic modeling. J Nucl Med. 2007;48:955–964. doi: 10.2967/jnumed.106.037598. [DOI] [PubMed] [Google Scholar]
- 30.Chatham JC, Gao ZP, Forder JR. Impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol. 1999;277:E342–351. doi: 10.1152/ajpendo.1999.277.2.E342. [DOI] [PubMed] [Google Scholar]
- 31.Herrero P, Dence CS, Coggan AR, Kisrieva-Ware Z, Eisenbeis P, Gropler RJ. L-3-11C-lactate as a PET tracer of myocardial lactate metabolism: a feasibility study. J Nucl Med. 2007;48:2046–2055. doi: 10.2967/jnumed.107.044503. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. [PubMed] [Google Scholar]
- 33.Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;4:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Kisrieva-Ware Z, Coggan AR, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial triglyceride oxidation with PET and 11C-palmitate. J Nucl Cardiol. doi: 10.1007/s12350-009-9051-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeGrado TR. Synthesis of 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA) J Label Comp Radiopharm. 1991;29:989–995. [Google Scholar]
- 36.DeGrado TR, Coenen HH, Stocklin G. 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA): evaluation in mouse of a new probe of myocardial utilization of long chain fatty acids. J Nucl Med. 1991;32:1888–1896. [PubMed] [Google Scholar]
- 37.Schulz G, von Dahl J, Kaiser HJ, Koch KC, Sabri O, Banneitz L, Cremerius U, Buell U. Imaging of beta-oxidation by static PET with 14(R,S)-[18F]-fluoro-6-thiaheptadecanoic acid (FTHA) in patients with advanced coronary heart disease: a comparison with 18FDG-PET and 99Tcm-MIBI SPET. Nucl Med Commun. 1996;17:1057–1064. doi: 10.1097/00006231-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 39.DeGrado TR, Wang S, Holden JE, Nickles RJ, Taylor M, Stone CK. Synthesis and preliminary evaluation of (18)F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nucl Med Biol. 2000;27:221–231. doi: 10.1016/s0969-8051(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 40.DeGrado TR, Kitapci MT, Wang S, Ying J, Lopaschuk GD. Validation of 18F-fluoro-4-thia-palmitate as a PET probe for myocardial fatty acid oxidation: effects of hypoxia and composition of exogenous fatty acids. J Nucl Med. 2006;47:173–181. [PubMed] [Google Scholar]
- 41.Shoup TM, Elmaleh DR, Bonab AA, Fischman AJ. Evaluation of trans-9-18F-fluoro-3,4-Methyleneheptadecanoic acid as a PET tracer for myocardial fatty acid imaging. J Nucl Med. 2005;46:297–304. [PubMed] [Google Scholar]
- 42.Bing RJ. The metabolism of the heart. Harvey Lect. 1955;50:27–70. [PubMed] [Google Scholar]
- 43.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 45.Kelly DP. PPARs of the heart: three is a crowd. Circ Res. 2003;92:482–484. doi: 10.1161/01.RES.0000064382.46274.95. [DOI] [PubMed] [Google Scholar]
- 46.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 48.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 49.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Erreish GM, Neely JR, Whitmer JT, Whitman V, Sanadi DR. Fatty acid oxidation by isolated perfused working hearts of aged rats. Am J Physiol. 1977;232:E258–262. doi: 10.1152/ajpendo.1977.232.3.E258. [DOI] [PubMed] [Google Scholar]
- 51.McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc Res. 1993;27:2222–2228. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- 52.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I. Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol. 2002;283:H1750–1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 53.Odiet JA, Boerrigter ME, Wei JY. Carnitine palmitoyl transferase-I activity in the aging mouse heart. Mech Ageing Dev. 1995;79:127–136. doi: 10.1016/0047-6374(94)01552-w. [DOI] [PubMed] [Google Scholar]
- 54.Paradies G, Ruggiero FM, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-L-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim Biophys Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- 55.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 56.Soto PF, Herrero P, Kates AM, Dence CS, Ehsani AA, Davila-Roman VG, Schechtman KB, Gropler RJ. Impact of aging on myocardial metabolic response to dobutamine. Am J Physiol Heart Circ Physiol. 2003;285:2158–2164. doi: 10.1152/ajpheart.00086.2003. [DOI] [PubMed] [Google Scholar]
- 57.Soto PF, Herrero P, Schechtman KB, Waggoner AD, Baumstark JM, Ehsani AA, Gropler RJ. Exercise training impacts the myocardial metabolism of older individuals in a gender-specific manner. Am J Physiol Heart Circ Physiol. 2008;295:H842–850. doi: 10.1152/ajpheart.91426.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desrois M, Sidell RJ, Gauguier D, Davey CL, Radda GK, Clarke K. Gender differences in hypertrophy, insulin resistance and ischemic injury in the aging type 2 diabetic rat heart. J Mol Cell Cardiol. 2004;37:547–555. doi: 10.1016/j.yjmcc.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Dyck JR, Lopaschuk GD. Glucose metabolism, H+ production and Na+/H+-exchanger mRNA levels in ischemic hearts from diabetic rats. Mol Cell Biochem. 1998;180:85–93. [PubMed] [Google Scholar]
- 60.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–581. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peterson LR, Soto PM, Herrero P, Mohammed S, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to Obesity. J Am Coll Cardiol Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135:681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 63.Petitpas I, Grune T, Bhattacharya AA, Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol. 2001;314:955–960. doi: 10.1006/jmbi.2000.5208. 4. [DOI] [PubMed] [Google Scholar]
- 64.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 65.Matute ML, Kalkhoff RK. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology. 1973;92:762–768. doi: 10.1210/endo-92-3-762. [DOI] [PubMed] [Google Scholar]
- 66.Kendrick ZV, Ellis GS. Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. J Appl Physiol. 1991;71:1694–1699. doi: 10.1152/jappl.1991.71.5.1694. [DOI] [PubMed] [Google Scholar]
- 67.Lei B, Matsuo K, Labinskyy V, Sharma N, Chandler MP, Ahn A, Hintze TH, Stanley WC, Recchia FA. Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo. Proc Natl Acad Sci U S A. 2005;102:6966–6971. doi: 10.1073/pnas.0500768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Massie BM, Schaefer S, Garcia J, McKirnan MD, Schwartz GG, Wisneski JA, Weiner MW, White FC. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91:1814–1823. doi: 10.1161/01.cir.91.6.1814. [DOI] [PubMed] [Google Scholar]
- 69.Recchia FA, McConnell PI, Loke KE, Xu X, Ochoa M, Hintze TH. Nitric oxide controls cardiac substrate utilization in the conscious dog. Cardiovasc Res. 1999;44:325–332. doi: 10.1016/s0008-6363(99)00245-x. [DOI] [PubMed] [Google Scholar]
- 70.Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, Gropler RJ. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005;12:574–581. doi: 10.1016/j.nuclcard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 72.Stanley WC, Lopaschuck GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34(1):25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 73.Rodrigues B, Cam MC, McNeill JH. Myocardial substrate metabolism: implications for diabetic cardiomyopathy. J Mol Cell Cardiol. 1995;27:169–179. doi: 10.1016/s0022-2828(08)80016-8. [DOI] [PubMed] [Google Scholar]
- 74.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 75.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 76.Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–411. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 77.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoghi KI, Gropler RJ, Sharp T, Herrero P, Fettig N, Su Y, Mitra MS, Kovacs A, Finck BN, Welch MJ. Time course of alterations in myocardial glucose utilization in the Zucker diabetic fatty rat with correlation to gene expression of glucose transporters: a small-animal PET investigation. J Nucl Med. 2008;49:1320–1327. doi: 10.2967/jnumed.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Welch MJ, Lewis JS, Kim J, Sharp TL, Dence CS, Gropler RJ, Herrero P. Assessment of myocardial metabolism in diabetic rats using small-animal PET: a feasibility study. J Nucl Med. 2006;47:689–697. [PubMed] [Google Scholar]
- 81.Monti LD, Landoni C, Setola E, Galluccio E, Lucotti P, Sandoli EP, Origgi A, Lucignani G, Piatti P, Fazio F. Myocardial insulin resistance associated with chronic hypertriglyceridemia and increased FFA levels in Type 2 diabetic patients. Am J Physiol Heart Circ Physiol. 2004;287:H1225–1231. doi: 10.1152/ajpheart.00629.2003. [DOI] [PubMed] [Google Scholar]
- 82.Monti LD, Lucignani G, Landoni C, Moresco RM, Piatti P, Stefani I, Pozza G, Fazio F. Myocardial glucose uptake evaluated by positron emission tomography and fluorodeoxyglucose during hyperglycemic clamp in IDDM patients. Role of free fatty acid and insulin levels. Diabetes. 1995;44:537–542. doi: 10.2337/diab.44.5.537. [DOI] [PubMed] [Google Scholar]
- 83.vom Dahl J, Herman WH, Hicks RJ, Ortiz-Alonso FJ, Lee KS, Allman KC, Wolfe ER, Jr, Kalff V, Schwaiger M. Myocardial glucose uptake in patients with insulin-dependent diabetes mellitus assessed quantitatively by dynamic positron emission tomography. Circulation. 1993;88:395–404. doi: 10.1161/01.cir.88.2.395. [DOI] [PubMed] [Google Scholar]
- 84.Avogaro A, Nosadini R, Doria A, Fioretto P, Velussi M, Vigorito C, Sacca L, Toffolo G, Cobelli C, Trevisan R, et al. Myocardial metabolism in insulin-deficient diabetic humans without coronary artery disease. Am J Physiol. 1990;258:E606–618. doi: 10.1152/ajpendo.1990.258.4.E606. [DOI] [PubMed] [Google Scholar]
- 85.Herrero P, McGill JB, Lesniak D, Matthew S, Dence C, Scott S, Kisrieve-Ware Z, Gropler RJ. Pet dection of the impact of dobutamine on myocardial glucose metabolism in women with type 1 diabetes mellitus. J Nucl Cardiol. 2008;15:598–604. doi: 10.1007/BF03007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peterson LR, Herrero P, McGill J, Schechtman KB, Kisrieva-Ware Z, Lesniak D, Gropler RJ. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 87.Hallsten K, Virtanen KA, Lonnqvist F, Janatuinen T, Turiceanu M, Ronnemaa T, Viikari J, Lehtimaki T, Knuuti J, Nuutila P. Enhancement of insulin-stimulated myocardial glucose uptake in patients with Type 2 diabetes treated with rosiglitazone. Diabet Med. 2004;21:1280–1287. doi: 10.1111/j.1464-5491.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 88.Commerford SR, Pagliassotti MJ, Melby CL, Wei Y, Gayles EC, Hill JO. Fat oxidation, lipolysis, and free fatty acid cycling in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2000;279:E875–885. doi: 10.1152/ajpendo.2000.279.4.E875. [DOI] [PubMed] [Google Scholar]
- 89.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 90.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 91.Lopaschuk G. Regulation of carbohydrate metabolism in ischemia and reperfusion. Am Heart J. 2000;139:S115–119. doi: 10.1067/mhj.2000.103919. [DOI] [PubMed] [Google Scholar]
- 92.McNulty PH, Jagasia D, Cline GW, Ng CK, Whiting JM, Garg P, Shulman GI, Soufer R. Persistent changes in myocardial glucose metabolism in vivo during reperfusion of a limited-duration coronary occlusion. Circulation. 2000;101:917–922. doi: 10.1161/01.cir.101.8.917. [DOI] [PubMed] [Google Scholar]
- 93.Camici P, Araujo LI, Spinks T, Lammertsma AA, Kaski JC, Shea MJ, Selwyn AP, Jones T, Maseri A. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation. 1986;74:81–88. doi: 10.1161/01.cir.74.1.81. [DOI] [PubMed] [Google Scholar]
- 94.Kawai Y, Tsukamoto E, Nozaki Y, Morita K, Sakurai M, Tamaki N. Significance of reduced uptake of iodinated fatty acid analogue for the evaluation of patients with acute chest pain. J Am Coll Cardiol. 2001;38:1888–1894. doi: 10.1016/s0735-1097(01)01634-5. [DOI] [PubMed] [Google Scholar]
- 95.Tamaki N. Role of BMIPP imaging for risk stratification in patients with coronary artery disease. J Nucl Cardiol. 2005;12:148–150. doi: 10.1016/j.nuclcard.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Nishimura M, Tsukamoto K, Hasebe N, Tamaki N, Kikuchi K, Ono T. Prediction of cardiac death in hemodialysis patients by myocardial fatty acid imaging. J Am Coll Cardiol. 2008;51:139–145. doi: 10.1016/j.jacc.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 97.Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram MY, Goodbody AE, Babich JW, Udelson JE. Metabolic imaging with beta-methyl-p-[(123)I]-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation. 2005;112:2169–2174. doi: 10.1161/CIRCULATIONAHA.104.530428. [DOI] [PubMed] [Google Scholar]
- 98.Dou KF, Yang MF, Yang YJ, Jain D, He ZX. Myocardial 18F-FDG Uptake After Exercise-Induced Myocardial Ischemia in Patients with Coronary Artery Disease. J Nucl Med. 2008;49:1986–1991. doi: 10.2967/jnumed.108.052936. [DOI] [PubMed] [Google Scholar]
- 99.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 100.Rupp H, Jacob R. Metabolically-modulated growth and phenotype of the rat heart. Eur Heart J. 1992;13 D:56–61. doi: 10.1093/eurheartj/13.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- 101.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 102.Jamshidi Y, Montgomery HE, Hense HW, Myerson SG, Torra IP, Staels B, World MJ, Doering A, Erdmann J, Hengstenberg C, Humphries SE, Schunkert H, Flavell DM. Peroxisome proliferator--activated receptor alpha gene regulates left ventricular growth in response to exercise and hypertension. Circulation. 2002;105:950–955. doi: 10.1161/hc0802.104535. [DOI] [PubMed] [Google Scholar]
- 103.Handa N, Magata Y, Mukai T, Nishina T, Konishi J, Komeda M. Quantitative FDG-uptake by positron emission tomography in progressive hypertrophy of rat hearts in vivo. Ann Nucl Med. 2007;21:569–576. doi: 10.1007/s12149-007-0067-2. [DOI] [PubMed] [Google Scholar]
- 104.de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Davila-Roman VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension. 2003;41:83–87. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 105.de las Fuentes L, Soto PF, Cupps BP, Pasque MK, Herrero P, Gropler RJ, Waggoner AD, Davila-Roman VG. Hypertensive left ventricular hypertrophy is associated with abnormal myocardial fatty acid metabolism and myocardial efficiency. J Nucl Cardiol. 2006;13:369–377. doi: 10.1016/j.nuclcard.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 106.Rigazio S, Lehto HR, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab. 2008;295:E413–419. doi: 10.1152/ajpendo.00744.2007. [DOI] [PubMed] [Google Scholar]
- 107.Buttrick PM, Kaplan M, Leinwand LA, Scheuer J. Alterations in gene expression in the rat heart after chronic pathological and physiological loads. J Mol Cell Cardiol. 1994;26:61–67. doi: 10.1006/jmcc.1994.1008. [DOI] [PubMed] [Google Scholar]
- 108.Sack MN, Kelly DP. The energy substrate switch during development of heart failure: gene regulatory mechanisms (Review) Int J Mol Med. 1998;1:17–24. doi: 10.3892/ijmm.1.1.17. [DOI] [PubMed] [Google Scholar]
- 109.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. doi: 10.1161/01.CIR.0000139340.88769.D5. [DOI] [PubMed] [Google Scholar]
- 110.Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol. 2003;42:1587–1593. doi: 10.1016/j.jacc.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 112.Beanlands RS, Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, Fallen E. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: A double-blind, placebo-controlled, positron-emission tomography study. Circulation. 2000;102:2070–2075. doi: 10.1161/01.cir.102.17.2070. [DOI] [PubMed] [Google Scholar]
- 113.Stolen KQ, Kemppainen J, Ukkonen H, Kalliokoski KK, Luotolahti M, Lehikoinen P, Hamalainen H, Salo T, Airaksinen KE, Nuutila P, Knuuti J. Exercise training improves biventricular oxidative metabolism and left ventricular efficiency in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:460–467. doi: 10.1016/s0735-1097(02)02772-9. [DOI] [PubMed] [Google Scholar]
- 114.Sundell J, Engblom E, Koistinen J, Ylitalo A, Naum A, Stolen KQ, Kalliokoski R, Nekolla SG, Airaksinen KE, Bax JJ, Knuuti J. The effects of cardiac resynchronization therapy on left ventricular function, myocardial energetics, and metabolic reserve in patients with dilated cardiomyopathy and heart failure. J Am Coll Cardiol. 2004;43:1027–1033. doi: 10.1016/j.jacc.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 115.Nowak B, Sinha AM, Schaefer WM, Koch KC, Kaiser HJ, Hanrath P, Buell U, Stellbrink C. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2003;41:1523–1528. doi: 10.1016/s0735-1097(03)00257-2. [DOI] [PubMed] [Google Scholar]
- 116.Hasegawa S, Kusuoka H, Maruyama K, Nishimura T, Hori M, Hatazawa J. Myocardial positron emission computed tomographic images obtained with fluorine-18 fluoro-2-deoxyglucose predict the response of idiopathic dilated cardiomyopathy patients to beta-blockers. J Am Coll Cardiol. 2004;43:224–233. doi: 10.1016/j.jacc.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 117.van Campen CM, Visser FC, van der Weerdt AP, Knaapen P, Comans EF, Lammertsma AA, de Cock CC, Visser CA. FDG PET as a predictor of response to resynchronisation therapy in patients with ischaemic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2007;34:309–315. doi: 10.1007/s00259-006-0235-y. [DOI] [PubMed] [Google Scholar]
- 118.Magata Y, Kawaguchi T, Ukon M, Yamamura N, Uehara T, Ogawa K, Arano Y, Temma T, Mukai T, Tadamura E, Saji H. A Tc-99m-labeled long chain fatty acid derivative for myocardial imaging. Bioconjug Chem. 2004;15:389–393. doi: 10.1021/bc034190p. [DOI] [PubMed] [Google Scholar]
- 119.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 120.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 121.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 122.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 123.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]



