Abstract
Neuropsychiatric symptoms are well defined in behavioral variant frontotemporal dementia but are not as well studied in primary progressive aphasia. This study compared caregiver reported neuropsychiatric symptoms in these 2 forms of dementia at short and long disease duration. Patients with behavioral variant frontotemporal dementia had more symptoms than patients with primary progressive aphasia. However, when divided by duration of disease, patients with primary progressive aphasia with long duration had a similar number of symptoms to patients with behavioral variant frontotemporal dementia at either duration. Furthermore, this group of patients with primary progressive aphasia had more symptoms typical of behavioral variant frontotemporal dementia and less mood-related symptoms which were more common in patients with primary progressive aphasia with shorter duration. This study illustrates the emergence of neuropsychiatric symptoms as primary progressive aphasia progresses and highlights the increasing overlap with behavioral variant frontotemporal dementia because the disease affects areas outside of the language network.
Keywords: dementia, neuropsychiatric symptoms, primary progressive aphasia, frontotemporal dementia
Neuropsychiatric symptoms are common in the neurodegenerative dementias1 and are related to specific biochemical and anatomical changes that occur with these diseases.2 These symptoms include mood disturbances, psychoses, compulsions, anxiety, and agitation, symptoms that constitute a major source of morbidity and caregiver burden.3,4 Neuropsychiatric symptoms are more prevalent in certain types of dementia. Frontotemporal dementia (FTD) is an umbrella term for a group of dementia syndromes that present clinically without amnesia, especially in the early stages. FTD affects between 4% and 20% of patients presenting to dementia clinics5 and is found to be most prevalent in individuals below 60 years of age.6-8 FTD can be broadly divided into 2 clinical categories9: a behavioral variant frontotemporal dementia (bvFTD) and an aphasic variant known as primary progressive aphasia (PPA). Neuropsychiatric symptoms are the earliest manifestation in bvFTD and may occur in isolation or in addition to executive dysfunction. Different symptoms of aphasia occur early in PPA10-13 and remain most prominent over the course of illness. Although the postmortem neuropathological findings are varied in both PPA and bvFTD, they typically affect the prefrontal and anterolateral temporal areas of the brain and are therefore subsumed under the rubric of frontotemporal lobar degeneration (FTLD).
PPA refers to the class of clinical dementia syndromes in which aphasia is the earliest and most salient symptom, especially within the first 2 years of symptoms onset. Any type of aphasia can be manifested.12 PPA has recently been classified into subtypes based on distinctive clinical and neuroimaging features: labelled agrammatic or nonfluent, semantic dementia (SD), and logopenic.14-17 Prior to this, the Neary criteria had required that patients with SD fulfill criteria of both fluent, empty, spontaneous speech and an associative, multidomain agnosia. The inclusion of an agnosia during the first 2 years would have prevented such a patient from fulfilling Mesulam's criteria for PPA.
Early reports of patients with PPA indicated minimal or no neuropsychiatric symptoms.18 However, more recent studies describe the presence of neuropsychiatric symptoms, especially as the illness progresses.19,20 A longitudinal study compared the development of symptoms over time in bvFTD and PPA using the Frontal Behavioral Inventory (FBI).21 Over a period of 3 years, patients with PPA developed a similar number of psychiatric symptoms to those with bvFTD who had these symptoms from the outset22 To date, there have been no direct comparisons of neuropsychiatric symptoms between behavioral and language variants of FTD, specifically comparing groups based on duration of illness.
Several research studies have investigated the neuropsychiatric profiles of patients with bvFTD. One study retrospectively assessed the prevalence of symptoms listed in the most recent consensus criteria for the diagnosis of FTLD, which stipulated core and supportive features17 and found early disengagement and poor insight to be the most common features in a group of 53 patients with bvFTD.23 In contrast, social interpersonal conduct and emotional expression were frequently intact in the initial phases. Compulsive-like acts were also common, whereas psychotic symptoms were absent in all patients. Although duration of illness was not specified, at a 2-year follow-up point, the majority of patients had all the core features of bvFTD. Others have investigated particular symptoms in isolation; for example, a review concentrating on repetitive and compulsive behaviors found that such behaviors existed in 78% of reported cases with a pathologically confirmed diagnosis of frontal lobe degeneration.24
Comparisons of the prevalence of behavioral symptoms between patients with bvFTD and patients with Alzheimer disease (AD) highlight the different neuropsychiatric symptoms seen in these disorders. Caregiver reports of early personality change, unconcern, and inappropriate behavior reliably distinguished bvFTD from AD.25 Levy et al26 used the Neuropsychiatric Inventory (NPI)27; The patients with bvFTD had higher total scores and exhibited more apathy, disinhibition, euphoria, anxiety, and aberrant motor behaviors than patients with AD.26 Miller et al28 found that the behaviors that most reliably differentiated bvFTD and AD were loss of personal awareness, hyperorality, stereotyped and perseverative behaviors, progressive reduction in speech, and preserved spatial orientation.
In summary, apathy (lack of initiative, lack of interest, and lack of emotional concern)29; disinhibition (also described as loss of personal awareness, inappropriate behavior); compulsive or repetitive behavior; and sometimes, hyperorality (a symptom of Kluver-Bucy syndrome) appear to be common in bvFTD, whereas changes in mood appear to be rare.
There are a small number of studies comparing neuropsychiatric symptoms in patients with different forms of FTD. Using factor analysis, Bozeat et al30 used a newly created caregiver questionnaire to assess responses from caregivers of patients with frontal variant FTD (fvFTD), temporal variant FTD (used synonymously with SD by Bozeat), and AD. Stereotypic behavior, changes in eating preference, disinhibition, and reduced social awareness reliably distinguished the 2 FTD groups from AD. The patients with fvFTD and patients with SD were behaviorally very similar, although duration of disease was not reported, and both were distinct from AD. Depression was significantly more common in SD. In another study, emotional changes were again found to be more prevalent in the language variants than the behavioral variants.31
Depression, another common neuropsychiatric symptom in dementia, seems to be more frequent in PPA than in bvFTD. A patient suffering from deficits in language, with intact insight and judgment, would be expected to have a grief reaction to his/her loss. Patients with PPA have been shown to have intact insight32 and endorse more symptoms of depression than age-matched normal controls.33 The lack of depression in bvFTD may result from various mechanisms, perhaps most likely being due to patients’ loss of awareness into their disease process.
A few studies of neuropsychiatric symptoms in FTD do report disease duration; for example, Kertesz et al34 examined the prevalence and extent of behavioral symptoms in frontal lobe dementia ([FLD], a similar entity to bvFTD), vascular dementia, AD, PPA, and depressive disorder using the FBI. Duration of symptoms in patients with FLD was 28.8 months, whereas the mean duration in patients with PPA was 39.5 months. The patients with FLD had more symptoms on the FBI than any of the other groups, including PPA. Irritability was relatively common in the PPA group. Indifference, perseveration, and utilization behavior were fairly specific to FLD.34
Marczinski et al22 used the FBI to assess patient groups with either bvFTD or PPA. They found that symptoms in patients with bvFTD remained stable over a 3-year period, whereas similar symptoms (including apathy, poor judgment, and hyperorality) emerged in the patients with PPA over 3 years.35 Both the patient groups averaged 3 years of illness at the first time-point. Another study followed patients with bvFTD and patients with PPA and found that 20/27 patients initially presenting with PPA developed behavioral problems, and 11/17 patients with bvFTD developed a progressive logopenia, although it is unclear whether this was due to behavioral mutism or true progressive aphasia. Duration and severity of disease were not described in this study.22 The overlap of the 2 forms of FTD is highlighted by the results of these rare longitudinal studies, with patients presenting with the language variant developing behavioral symptoms over time and patients initially presenting with behavioral changes developing language deficits. Another longitudinal study showed that the type of behaviors in language variants of FTD was related to the nature of the language disorder.36 For example, patients with SD were found to have more apathy and changes in food preferences, whereas nonfluent patients had less behavioral symptoms but sometimes exhibited irritability. The increasing overlap of symptoms in patients with frontotemporal forms of dementia has been commented upon and viewed as a result of the spread of pathology among interconnected regions around the epicenters of prefrontal and perisylvian cortex.13
The present study was conducted to compare the prevalence and nature of neuropsychiatric symptoms in PPA and in bvFTD on a short version of the Neuropsychiatric Inventory,27 the Neuropsychiatric Inventory Questionnaire (NPI-Q37). It was predicted that patients with bvFTD would show more symptoms than those with PPA, irrespective of duration, but PPA patients with symptoms for more than 5 years would show more symptoms than those in earlier stages.
Method
Participants
Archival data were analyzed from patients with bvFTD (n = 28) and patients with PPA (n = 42) enrolled in the Northwestern Alzheimer's Disease Center (NADC) Clinical Core Subject Registry. Patients were diagnosed on the basis of the most recent consensus and clinical criteria12,17 as having any variant of PPA (logopenic, semantic, or agrammatic) or behavioral variant FTD. All patients underwent full diagnostic workup by a behavioral neurologist, which included structural, and in some cases, metabolic neuroimaging. They also completed a neuropsychological assessment, and their caregivers (most frequently a spouse but in some cases an adult child) completed the questionnaires. All patients in the core database whose caregivers had completed the NPI-Q37 and had the appropriate diagnosis were included in this retrospective study without exception. This included some patients who were followed via telephone interviews to complete the questionnaires with their caregivers because they were too impaired to be tested. Because the NPI-Q is a relatively newly published tool, many of the patients analyzed in the analysis did not have NPI-Q data at the initial visit but were given the measure at follow-up visits. In this case, data from the first follow-up visit where the patient underwent cognitive testing and the caregiver completed the NPI-Q were used in the analysis. Informed consent to participate in the registry and contribute to the database was obtained from all patients and caregivers, and the study protocol was approved by the Institutional Review Board at Northwestern University.
Initially, comparisons were made between the patients with bvFTD and patients with PPA. Then, each of these diagnostic groups was separated into short duration (ShD, symptoms for less than 5 years) and long duration (LD, symptoms for equal to or more than 5 years). Five years was chosen as a cutoff because it represented the mean duration of symptoms for the PPA group.
Measures
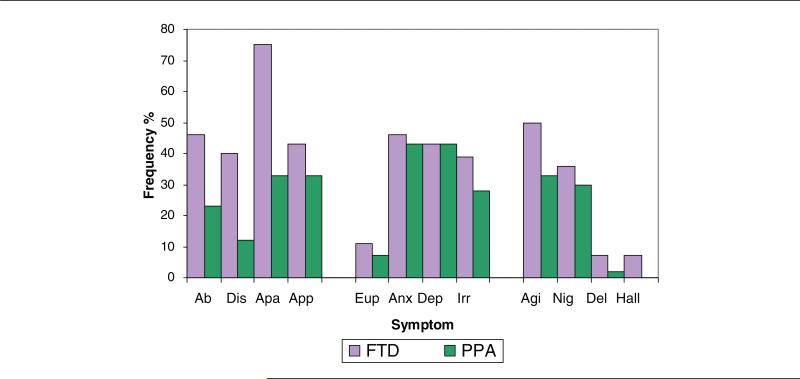
The NPI-Q37consists of a brief screening of neuropsychiatric symptoms and their severity in patients with dementia. The caregiver must rate each of 12 symptoms as present or absent in the previous month and, if present, its severity (Likert scale: 1, 2, or 3). They also must rate their own level of distress for each symptom; however, the distress index was not analyzed for this study. In this study, we counted the absolute number of symptoms endorsed. The 12 symptoms on the NPI-Q can be subcategorized qualitatively as behavioral/comportmental (aberrant motor behavior, disinhibition, apathy), appetite and eating behaviors; mood symptoms (euphoria, anxiety, depression, irritability); and disruptive/psychotic symptoms (agitation, nighttime behaviors, delusions, hallucinations; Figure 1). To compare the severity of symptoms between the groups, a symptom severity score (total severity score/total number of symptoms endorsed) was calculated.
Figure 1.
Percent frequency of patients with each symptom in each diagnostic group. Symptoms are divided into categories: frontal/comportmental, mood, and disruptive/psychotic. Ab indicates aberrant motor behavior; Dis = disinhibition; Apa = apathy; App = appetite and eating; Eup = euphoria; Anx = anxiety; Dep = depression; Irr = irritability; Agi = agitation; Nig = nighttime behaviors; Del = delusions; Hal = hallucinations.
All patients who had been seen in person at the time that the NPI-Q was first administered also underwent neuropsychological testing for characterization of their cognitive functioning in attention, language, memory, comportment, executive, and visuospatial functions. Scores from the Boston Naming Test (BNT38) were used as a measure of aphasia severity. For all patients, functional capacity was also measured with the activities of daily living questionnaire (ADLQ39). Where patients were followed by phone, the ADLQ and the Clinical Dementia Rating Scale (CDR) were completed in addition to the NPI-Q with the caregiver.
Disease Severity
Disease severity was measured using 2 scales, the Mini-Mental State Examination40 and the CDR41 derived by a clinician with information from a caregiver, generating a score that globally stages the level of impairment (0, no impairment; 0.5, very mild; 1, mild; 2, moderate; 3, severe dementia). The duration of illness was also noted.
Statistical Analysis
Demographic variables were compared between patients with bvFTD and patients with PPA, and number of symptoms in each group on the NPI-Q was compared using Student t tests. Comparison of the number of patients in each group exhibiting each symptom was carried out using chi-square tests. In addition, groups were split up by duration of illness, and analysis of variance with post hoc t tests compared the mean number of symptoms in each of the 4 groups so divided. Chi-square tests were run to compare the proportion of patients in each diagnostic group with each symptom. Symptom severity scores were then compared using Mann-Whitney U tests.
Results
Table 1 presents demographic variables, neuropsychological data, dementia severity scores, and NPI-Q scores for both the patient groups, irrespective of duration of illness. Patients with PPA were significantly older and had fewer functional impairments, according to scores on the ADLQ, than patients with bvFTD. Although the BNT score was lower in patients with PPA than in patients with bvFTD, the difference was not statistically significant.
Table 1.
Demographic and Severity Data for Entire Behavioral Variant of Frontotemporal Dementia and Primary Progressive Aphasia Groupa
| Demographic Variables | bvFTD (n = 28) | bvFTD (14M/14F) | PPA (n = 42) | PPA (19M/23F) | Significant Difference |
|---|---|---|---|---|---|
| Age | 28 | 62.14 (10.59) | 42 | 69.74 (5.84) | P < .05 |
| Education | 28 | 14.46 (2.60) | 42 | 15.57 (2.63) | |
| Duration | 28 | 3.94 (2.76) | 42 | 5.02 (3.73) | |
| MMSE | 24 | 18.04 (7.73) | 32 | 21.06 (8.24) | |
| CDR | 27 | 1.67 (.82) | 38 | 1.32 (.97) | |
| ADL | 27 | 38.67 (17.34) | 39 | 22.97 (22.04) | P < .05 |
| BNT | 20 | 37.25 (17.51) | 31 | 33.42 (20.54) |
NOTE: bvFTD = behavioral variant of frontotemporal dementia; PPA = primary progressive aphasia; MMSE = Mini-Mental Status Exam; CDR = Clinical Dementia Rating scale; ADL = activities of daily living questionnaire; BNT = Boston Naming test.
Significant differences are seen when means are compared using chi-square tests (P < .05); in some cases (bvFTD, n = 4; PPA, n = 10), patients were followed via telephone calls with caregivers hence the smaller number of patients for neuropsychological tests.
In all, 96% of patients with bvFTD and 91% of patients with PPA had at least 1 NPI-Q symptom. Figure 1 illustrates the frequencies of each symptom in each group. In all, 21% of patients with bvFTD had 2 or less symptoms, compared with 48% of patients with PPA; at the other extreme, 21% of patients with bvFTD had 7 or more symptoms, compared with 7% of patients with PPA.
There was a significant difference between the 2 groups in the total number of symptoms endorsed (t = 2.58; df = 68; P < .01;1-tailed). The bvFTD caregiver group had, on average, endorsed more symptoms (mean = 4.43; standard deviation = 2.55) compared with the PPA group (mean = 3.02; standard deviation = 1.99).
As can be seen from Figure 1, more patients in the bvFTD group were rated as apathetic (χ2 = 3.327; df = 1; P = .059) and disinhibited (χ2 = 3.145; df = 1; P = .072) than patients with PPA. Although these findings did not reach statistical significance, they suggested a trend. The PPA group did not exhibit any of the symptoms significantly more frequently than the bvFTD group. Mood symptoms (anxiety, irritability, depression, and euphoria) were similarly prevalent in patients with bvFTD and in patients with PPA. There were no significant differences in the aberrant motor behavior item. The bvFTD group had a significantly higher severity score than the PPA group (bvFTD mean severity score = 1.72, PPA mean severity score = 1.34; U = 410.50, N1 = 28, N2 = 42, P = .031, 2-tailed).
Patients were separated into groups based on duration of illness, as described above (Table 2). Number of symptoms in LD PPA patients did not significantly differ from patients with bvFTD regardless of their disease duration. Number of symptoms in ShD PPA patients differed from patients with bvFTD (ShD and LD bvFTD combined: t = 2.72, df = 52, P < .005, 1-tailed; ShD bvFTD patients: t = 2.72, df = 43, P < .005, 1-tailed). Thus, patients with PPA in the earlier stages of their disease had significantly fewer neuropsychiatric symptoms than patients with bvFTD at any stage.
Table 2.
Mean and Standard Deviation of Number of Symptoms in Each Category
| bvFTD |
||||
|---|---|---|---|---|
| PPA | ShD (n = 19) | LD (n = 9) | ShD (n = 26) | LD (n = 16) |
| Mean number of symptoms | 4.58 | 4.11 | 2.81 | 3.38 |
| Standard deviation | 2.65 | 2.42 | 1.72 | 2.39 |
NOTE: bvFTD = behavioral variant of frontotemporal dementia; PPA = primary progressive aphasia; ShD = short duration (<5 y); LD = long duration (>5 y).
Although cell size for individual symptoms in the groups, once broken down by duration, were somewhat small to reliably compare statistically, there were some indications that the LD PPA group showed more disinhibition (χ2 = 4.226; df = 1; P = .040) and trends toward more nighttime behaviors and less depression compared with the ShD PPA group (nighttime behaviors: χ2 = 2.658, df = 1, P = .103, 2-tailed; depression: χ2 = 3.365, df = 1, P = .067, 2-tailed). Overall, it appears that patients with PPA in the later stages of the disease showed more behavioral symptoms compared with patients with PPA in the early stages and were more similar (in terms of number of behavioral symptoms) to patients with bvFTD. The severity score did not differ between ShD or LD PPA groups or ShD or LD bvFTD groups.
Discussion
The present study compared caregiver-reported neuropsychiatric symptoms in bvFTD and PPA using the NPI-Q. Caregivers of both patients with bvFTD and patients with PPA reported neuropsychiatric symptoms, but these symptoms were more significant in patients with bvFTD. Patients with PPA in the earlier stages of the disease were reported to have fewer symptoms, whereas those with symptoms for over 5 years had more symptoms, similar to patients with bvFTD at any stage. Patient groups also differed in the quality of symptoms, with the PPA group showing more mood symptoms and the bvFTD group showing more symptoms of personality and behavioral change.
The symptoms in the bvFTD patients were consistent with those reported in other studies. Apathy/indifference was the most common symptom; this has been reported extensively by other researchers.25,31,30 Aberrant motor behavior (including repetitive or stereotyped behaviors), agitation, and irritability were also very common, reflecting earlier findings.43-45 Disinhibition was relatively common in this group of patients with bvFTD, although it seemed less common than reported in other studies31 and given its status as a “core diagnostic feature” in the most recent consensus criteria for FTLD.17
Caregivers of patients with PPA most commonly endorsed anxiety, apathy, and irritability. Depression was slightly more common in PPA group than in the bvFTD group, and disinhibition was more rare. However, mood symptoms were also common in the bvFTD group. A possible explanation for this is that the 2 groups may differ in the source of these mood symptoms. In PPA group, the symptoms may represent affective responses to their situation, whereas in bvFTD group, apathy may be interpreted as depression by the caregivers. However, this would require evidence that there are 2 different mechanisms for the same caregiver-perceived symptom in each group, which could not be addressed with the available data.
In the present study, symptom type also changed with duration of disease. Thus, patients with PPA early in the disease tended toward more mood symptoms such as depression, whereas disinhibition, a hallmark symptom of bvFTD, and nighttime behaviors were more common in LD PPA. This would suggest that, as PPA evolves, the initial selectivity of the disease for language regions expands to also affect brain regions that regulate behavior. This apparent tendency to develop additional symptoms over time indicates a problem inherent in existing studies which have compared patients in different diagnostic categories at mixed stages of disease.31,34,43 One potential limitation in the estimation of disease duration, of course, is that it is subjective. However, no other quantifiable means exist to measure duration, and thus subjective reporting to date is the most widely used.
Development of more neuropsychiatric symptoms over time in PPA, combined with the finding that these symptoms are more characteristic of bvFTD, can be explained by neurodegenerative changes increasingly involving adjacent or interconnected cortical and subcortical regions. This progression of neuropathological change has been observed in recent studies using magnetic resonance imaging (MRI) to measure cortical volumes in patients with FTD. Whitwell et al 44 analyzed volumetric MRI longitudinally finding that, in patients with SD, the atrophy, initially visible in the anterior temporal regions, involves the left inferior frontal regions over time. These frontal systems are believed to mediate motivation, socially responsive empathic behavior, and executive function.45 Mesulam et al13 have used the term PPA-plus syndrome to refer to the point at which PPA is accompanied by movement disorders or personality changes as a result of the extension of pathology to (in the case of behavior) more frontal regions.
The histopathology of bvFTD and PPA is diverse, but similar cellular-level changes are exhibited in comparable ratios in both forms. Although some researchers find that Pick bodies are more commonly seen at autopsy of patients with nonfluent aphasic presentations,46 research more persistently suggests that the histopathological variants of bvFTD and PPA as determined by current methods do not predict the clinical phenotype20,47. Therefore, that the location of pathology as opposed to the particular type of pathology that is predictive of clinical symptoms remains true.48 However, Cummings2 has found associations between specific molecular-level changes and behaviors For example, tauopathies in general are associated with compulsions, disinhibition, and apathy. Language deficits have been associated with ubiquitinopathy in some cases49 but with tauopathy in others.46 In a minority of cases of PPA (20%-30%), the neuropathology of Alzheimer disease has been reported.50 However, there is insufficient information on this subgroup to determine in what ways they differ from individuals with the typical clinical profile of amnestic dementia associated with AD and from individuals with any form of FTLD at postmortem examination. It would be of interest to examine whether specific histopathological features of individual cases of bvFTD or PPA are associated with distinct neuropsychiatric profiles.
Behavioral symptoms have only recently been discussed in the PPA literature, although the present and some previous studies have indicated that these symptoms are relatively prevalent. The lack of attention to neuropsychiatric symptoms in PPA compared to bvFTD may be explained by the quality of these symptoms. The patients PPA in the current study most commonly suffered from anxiety, irritability, and apathy. These symptoms may be considered reasonable emotional reactions to a progressive disease affecting relatively young people, preventing them from communicating. Medina et al33 have shown that, as a group, a large proportion of patients with PPA have scores in the depressed range on the Geriatric Depression Scale when compared with age-matched cognitively intact subjects. Neuropsychiatric profiles of patients with PPA and with bvFTD furthermore may appear more distinct due to the limiting effect of aphasia. For example, the expression of disinhibited behaviors may be suppressed by a lack of verbal expression, whereas patients with bvFTD are able to state tactless comments. Symptoms more common to bvFTD include disturbing, even bizarre behaviors, which were less likely to have been part of the individual's behavioral repertoire prior to the onset of the disorder. Although most people, at times, can be nervous, grumpy, or withdrawn, a flagrant lack of respect for social norms or compulsive repetition of activities tends to be limited to the mentally ill. Due to the disruptive nature of the symptoms, caregivers of patients with bvFTD may be more likely to report them.
There are treatment implications of these findings, both for the patient and for the caregiver. The study of neuropsychiatric symptoms in bvFTD has been informative in terms of diagnostic, prognostic, and treatment implications, including the promotion of a handful of drug studies.51-53 The lack of attention toward behavioral symptoms in PPA (with some exceptions22,50,54) is epitomized by the lack of drug studies and also by the relative lack of attention given to caregiver education, support, and respite care in PPA compared with bvFTD.55 A recent study assessing the specific psychosocial consequences of bvFTD outlined marital discord, criminal behavior, child endangerment, and driving violations as some of the products of behaviors related to the disorder, outlining the need for appropriate psychosocial education and support.4 Weintraub and Morhardt54 recently outlined a prescription for tailored care and education for patients with FTD and PPA and their caregivers. The present study indicates that it may be appropriate to educate families dealing with PPA that similar problems to those seen early in bvFTD are likely to emerge over time, and that they should address financial/legal affairs and health care proxy decisions while patients are still aware of their condition, and their symptoms are still restricted to language impairments. Programs implementing these suggestions have begun to emerge.56
This study had several limitations. The NPI-Q is a brief measure, asking just 1 question per symptom domain. Some domains are particularly complex, and the likelihood of that question capturing the nuances of a particular individual's presentation is small. The simple and brief nature of the NPI-Q also gives reduced information about the nature of symptoms. For example, we do not know whether the agitation is a secondary reaction to distressing and frustrating circumstances, or a more primary, less conscious form of agitation, similar to what is seen in later stages of Alzheimer disease.57-59 The more extensive NPI27 or a specialized instrument such as the FBI 21may offer additional information. Inevitably, there is some circularity in analyzing differences in behavior when diagnosis is based on clinical presentation including neuropsychiatric symptoms; however, the measure used was not the basis of the diagnostic discrimination. In addition, there is evidence to suggest that different clinical subtypes, each with distinct anatomic specificity, exist.14 Future research studying the evolution of neuropsychiatric symptoms in each of these subtypes may further elucidate the natural history of PPA as a syndrome.
Overall, this paper indicated that both patients with PPA and patients with bvFTD have neuropsychiatric symptoms. Initially, patients with PPA have fewer of these symptoms, and their symptoms tend to be mood related. Over time, the 2 groups become increasingly similar, both in terms of number and type of symptoms. This study provides further evidence for the relatedness of these 2 conditions and predicts the emergence of a more behavioral/comportmental profile over time in PPA, which has both clinical and practical applications.
Acknowledgment
This study was supported by Northwestern Alzheimer's Disease Core Center grant, P30 AG13854, from the National Institute on Aging to Northwestern University.
Referencess
- 1.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 2.Cummings J. Toward a molecular neuropsychiatry of neurodegenerative diseases. Ann Neurol. 2003;54:147–154. doi: 10.1002/ana.10616. [DOI] [PubMed] [Google Scholar]
- 3.Victoroff J, Mack WJ, Nielson KA. Psychiatric complications of dementia: impact on caregivers. Dement Geriatr Cogn Disord. 1998;9:50–55. doi: 10.1159/000017022. [DOI] [PubMed] [Google Scholar]
- 4.Passant U, Elfgren C, Englund E, Gustafson L. Psychiatric symptoms and their psychosocial consequences in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19(suppl 1):15S–18S. doi: 10.1097/01.wad.0000183084.22562.5a. [DOI] [PubMed] [Google Scholar]
- 5.Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- 6.Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126(pt 9):2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 7.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RJ, Rossor MN, Skelton-Robinson M, Garralda E. Young onset dementia: epidemiology, clinical symptoms, family burden, support and outcome London. Imperial College School of Medicine; United Kngdom: 1998. [Google Scholar]
- 9.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam MM. Primary progressive aphasia–differentiation from Alzheimer's disease. Ann Neurol. 1987;22:533–534. doi: 10.1002/ana.410220414. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 12.Mesulam MM. Primary progressive aphasia–a language-based dementia. N Engl J Med. 2003;349:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- 13.Mesulam MM, Grossman M, Hillis A, Kertesz A, Weintraub S. The core and halo of primary progressive aphasia and semantic dementia. Ann Neurol. 2003;54(suppl 5):11S–14S. doi: 10.1002/ana.10569. [DOI] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 16.Kertesz A, Munoz DG, Hillis A. Preferred terminology. Ann Neurol. 2003;54(suppl 5):3S–6S. doi: 10.1002/ana.10567. [DOI] [PubMed] [Google Scholar]
- 17.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 19.Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- 20.Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: the Pick complex. Dement Geriatr Cogn Disord. 1999;10(suppl 1):46–49. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 22.Marczinski CA, Davidson W, Kertesz A. A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cogn Behav Neurol. 2004;17:185–190. [PubMed] [Google Scholar]
- 23.Mendez MF, Perryman KM. Neuropsychiatric features of frontotemporal dementia: evaluation of consensus criteria and review. J Neuropsychiatry Clin Neurosci. 2002;14:424–429. doi: 10.1176/jnp.14.4.424. [DOI] [PubMed] [Google Scholar]
- 24.Ames D, Cummings JL, Wirshing WC, Quinn B, Mahler M. Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci. 1994;6:100–113. doi: 10.1176/jnp.6.2.100. [DOI] [PubMed] [Google Scholar]
- 25.Barber R, Snowden JS, Craufurd D. Frontotemporal dementia and Alzheimer's disease: retrospective differentiation using information from informants. J Neurol Neurosurg Psychiatr. 1995;59:61–70. doi: 10.1136/jnnp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Arch Neurol. 1996;53:687–690. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 28.Miller BL, Ikonte C, Ponton M, et al. A study of the Lund-Manchester research criteria for frontotemporal dementia: clinical and single-photon emission CT correlations. Neurology. 1997;48:937–942. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- 29.Robert PH, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17:1099–1105. doi: 10.1002/gps.755. [DOI] [PubMed] [Google Scholar]
- 30.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioral features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioral profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2005;76:1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina J, Weintraub S. Depression in primary progressive aphasia. J Geriatr Psychiatry Neurol. 2007;20:153–160. doi: 10.1177/0891988707303603. [DOI] [PubMed] [Google Scholar]
- 34.Kertesz A, Nadkarni N, Davidson W, Thomas AW. The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- 35.Kertesz A, Munoz DG. Primary progressive aphasia. Clin Neurosci. 1997;4:95–102. [PubMed] [Google Scholar]
- 36.Snowden JS, Neary D, Mann DM, Goulding PJ, Testa HJ. Progressive language disorder due to lobar atrophy. Ann Neurol. 1992;31:174–183. doi: 10.1002/ana.410310208. [DOI] [PubMed] [Google Scholar]
- 37.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Experimental Edition Aphasia Research Center, Boston University; Boston, MA: 1976. [Google Scholar]
- 39.Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The activities of daily living questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. 2004;18:223–230. [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 42.Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:367–378. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- 43.Nyatsanza S, Shetty T, Gregory C, Lough S, Dawson K, Hodges JR. A study of stereotypic behaviours in Alzheimer's disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74:1398–1402. doi: 10.1136/jnnp.74.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2004;17:307–310. doi: 10.1159/000077160. [DOI] [PubMed] [Google Scholar]
- 45.Cummings J. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 46.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 47.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005 Sep;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 48.Weintraub S, Mesulam M-M. Four neuropsychological profiles in dementia. In: Boller F, Spinnler H, editors. Handbook of Neuropsychology Volume 8. Elsevier; Amsterdam, Netherlands: 1993. pp. 253–282. [Google Scholar]
- 49.Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123(pt 2):267–276. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- 50.Kertesz A, Munoz D. Clinical and pathological characteristics of primary progressive aphasia and frontal dementia. J Neural Transm Suppl. 1996;47:133–141. doi: 10.1007/978-3-7091-6892-9_8. [DOI] [PubMed] [Google Scholar]
- 51.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49:13–19. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 52.Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry. 1997;58:212–216. [PubMed] [Google Scholar]
- 53.Pasquier F, Richard F, Lebert F. Natural history of frontotemporal dementia: comparison with Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:253–257. doi: 10.1159/000077148. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub S, Morhardt D. Treatment, education, and resources for non-Alzheimer's dementia: one size does not fit all. Alzheimer's Care Quarterly. 2005:6201–214. [Google Scholar]
- 55.Litvan I. Therapy and management of frontal lobe dementia patients. Neurology. 2001;56(suppl 4):41S–45S. doi: 10.1212/wnl.56.suppl_4.s41. [DOI] [PubMed] [Google Scholar]
- 56.Banks S, Rogalski E, Medina J, Skoglund A, Morhardt D. Organizing a series of edication and support conferences for caregivers of individuals with frontotemporal dementia. Alzheimer's Care Quarterly. 2006;7:243–250. [PMC free article] [PubMed] [Google Scholar]
- 57.Asada T, Kinoshita T, Kakuma T. Analysis of behavioral disturbances among community-dwelling elderly with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14:160–167. doi: 10.1097/00002093-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Devanand DP, Brockington CD, Moody BJ, et al. Behavioral syndromes in Alzheimer's disease. Int Psychogeriatr. 1992;4(suppl 2):161–184. [PubMed] [Google Scholar]
- 59.Teri L, Larson EB, Reifler BV. Behavioral disturbance in dementia of the Alzheimer's type. J Am Geriatr Soc. 1988;36:1–6. doi: 10.1111/j.1532-5415.1988.tb03426.x. [DOI] [PubMed] [Google Scholar]