Abstract
Autophagy degrades cytoplasmic components that are required for cell survival in response to starvation1. Autophagy has also been associated with cell death, but it is unclear what may distinguish autophagy during cell survival and death. Drosophila salivary glands undergo programmed cell death that requires autophagy genes2, and engulfment of salivary gland cells by phagocytes does not appear to occur3. Here we show that Draper (Drpr), the Drosophila orthologue of the C. elegans engulfment receptor CED-1, is required for autophagy during cell death. Null mutations in drpr, as well as salivary gland-specific knockdown of drpr, inhibits salivary gland degradation. drpr knockdown prevents the induction of autophagy in dying salivary glands, and Atg1 expression in drpr mutants suppresses the failure in salivary gland degradation. Surprisingly, drpr is cell-autonomously required for autophagy induction in dying salivary gland cells, while drpr knockdown does not prevent starvation-induced autophagy in the fatbody which is associated with survival. In addition, components of the conserved engulfment pathway are required for clearance of salivary glands. This is the first example of an engulfment factor that is autonomously required for self-clearance. Furthermore, Drpr is the first factor that distinguishes autophagy that is associated with cell death from cell survival.
Macroautophagy (autophagy) delivers cytoplasmic components to the lysosome for degradation in eukaryotic cells. Autophagy is an important cellular response to stress that is required for survival in response to starvation1, and has also been associated with cell death in several in vivo contexts2,4. It is not clear, however, how autophagy might function in cell death, and what might determine its function in cell death versus cell survival. Drosophila larval salivary gland cell death is induced by a rise in steroid 12 hours after puparium formation, and this tissue is completely degraded by 16 hours after puparium formation5. Both caspases and autophagy are induced by this rise in steroid, and function in an additive manner to kill and degrade salivary glands2. By contrast, autophagy is induced in response to starvation in the larval fatbody6, and this induction is associated with cell survival.
To identify genes that may regulate autophagy in cell-specific contexts, we queried genome-wide DNA microarray data from dying salivary glands. Interestingly, several factors that have been implicated in the engulfment of apoptotic cells are induced in dying salivary glands7 (Supplementary Table 1), while there are no detectable changes in these genes after larval starvation8. Although many engulfment factors are pleiotropic through their regulation of the cytoskeleton and vesicular transport, the identification of the engulfment receptor drpr9,10 is intriguing, as salivary gland destruction is thought to be largely independent of phagocytes.
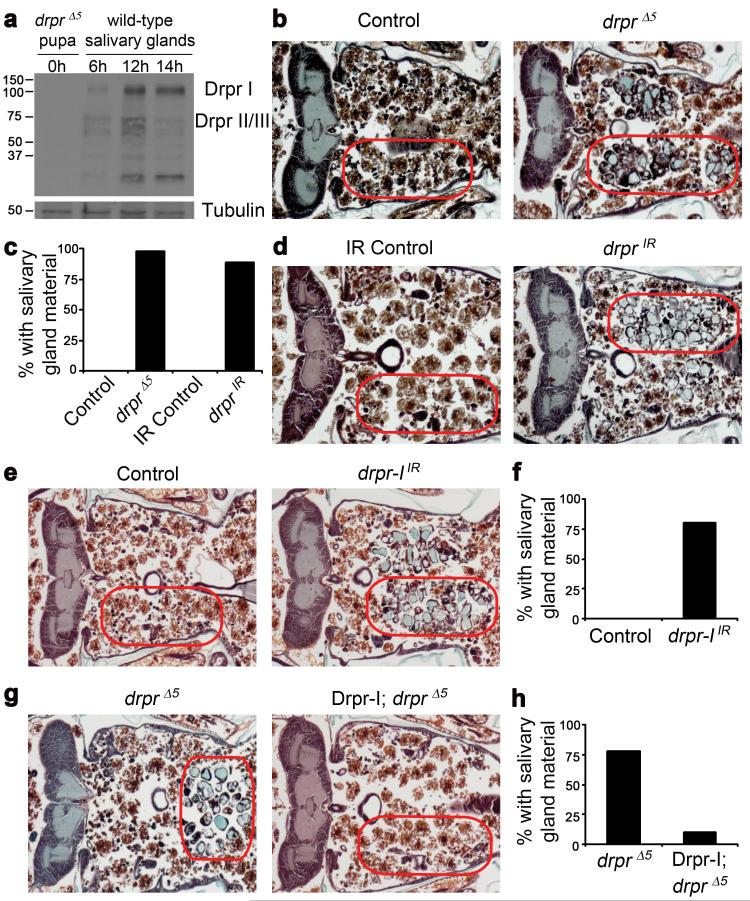
We analyzed whether Drpr is present in dying salivary glands. Whereas no Drpr protein is present in drprΔ5 null mutants, Drpr-I, II and/or III isoforms are present at low levels and localize to the luminal/apical region of salivary gland cells at 6 hours after puparium formation (Fig. 1a and Supplementary Fig. 1). Following the rise in steroid that triggers cell death 12 hours after puparium formation11, Drpr-I, II and/or III levels increase (Fig. 1a). Drpr-I protein levels remain high through 14 hours after puparium formation when a portion of Drpr changes from apical to cytoplasmic in localization, and this is coincident with a decrease in Drpr-II/III isoforms (Fig. 1a and Supplementary Fig. 1).
Figure 1. Draper is required for salivary gland cell degradation.
a, Protein extracts from drpr null (w; drprΔ5/drprΔ5) pupae at puparium formation (0h) and wild type (Canton-S) salivary glands 6h, 12h, and 14h after puparium formation, were analyzed by Western Blotting with anti-Drpr antibody. b, Control animals (+/w; +/drprΔ5), n=12, and drpr null mutants (w; drprΔ5/drprΔ5), n=47, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. c, quantification of data from b and d. d, Control animals (+/w; +/UAS-drprIR), n=11, and those with salivary gland-specific knockdown of drpr (fkh-GAL4/w; UAS-drprIR/+), n=19, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. e, Control animals (+/w; +/UAS-drpr-IIR), n=9, and those with salivary gland-specific knockdown of drpr-I (fkh-GAL4/w; UAS-drpr-IIR/+), n=20, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. f, quantification of data from e. g, drpr null animals (+/w; +/UAS-Drpr-I; drprΔ5/drprΔ5), n=9, and those with salivary gland-specific expression of Drpr-I (fkh-GAL4/w; UAS-Drpr-I/+; drprΔ5/drprΔ5), n=20, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. h, quantification of data from g.
The presence of Drpr protein in salivary glands suggested the possibility that Drpr could function directly within salivary gland cells to mediate their degradation. We tested whether drprΔ5 null mutants have a defect in salivary gland cell death. We found that 98% of homozygous drprΔ5 null mutants have persistent salivary gland material (Fig. 1b, c). By contrast, 0% of control drprΔ5/wild type heterozygous animals possess salivary gland material at 24 hours after puparium formation (Fig. 1b, c), and fatbody cells fill the void where salivary gland material has been cleared. As a known engulfment factor, Drpr could also function in phagocytic blood cells to mediate salivary gland clearance, and this could explain the defect in clearance observed in whole animal drprΔ5 null mutants. We tested this possibility by driving an upstream activating sequence (UAS)-regulated double-stranded inverse repeat construct designed to target drpr (drprIR) with the blood cell-specific hml-GAL4 driver and assaying for the persistence of salivary glands. We found that whereas hml-GAL4 is clearly expressed in blood cells but not salivary glands, drpr knockdown in blood cells does not lead to a defect in salivary gland clearance (Supplementary Fig. 2). By contrast, expression of drprIR in salivary glands with the salivary gland-specific fkh-GAL4 driver resulted in the persistence of salivary gland fragments in 89% of pupae compared to 0% of control animals lacking the fkh-GAL4 driver 24 hours after puparium formation (Fig. 1c, d). In addition, reduced function of any one of the engulfment pathway genes simu, crq, ced-6, src42a, ced-12, crk, and mbc also inhibited clearance of dying salivary glands (Supplementary Fig. 3). These data indicate that drpr, as well as other engulfment genes, function autonomously in salivary gland cells during degradation. Similar to animals with reduced function of either caspases or autophagy genes2, decreased function of drpr and other engulfment genes appears to inhibit clearance of dead fragmented salivary gland cells.
Drpr-I protein isoform levels remain elevated in extracts of dying salivary glands 14 hours after puparium formation (Fig. 1a). Therefore, we explored the role of Drpr-I in salivary gland cell death. Knockdown of drpr-I in salivary glands prevents tissue clearance in 80% of these animals compared to 0% of control animals lacking the fkh-GAL4 driver (Fig. 1e, f), suggesting that the defect in salivary gland cell degradation is due to decreased drpr-I function. In addition, salivary gland-specific expression of Drpr-I in drprΔ5 null mutant animals is sufficient to rescue the salivary gland persistence phenotype; 78% of Drpr-I drprΔ5 pupae that lack the GAL4 driver have persistent salivary gland material, while only 10% of drprΔ5 pupae expressing Drpr-I have persistent gland material (Fig. 1g, h). Taken together, these results indicate that drpr-I is required for salivary gland cell clearance.
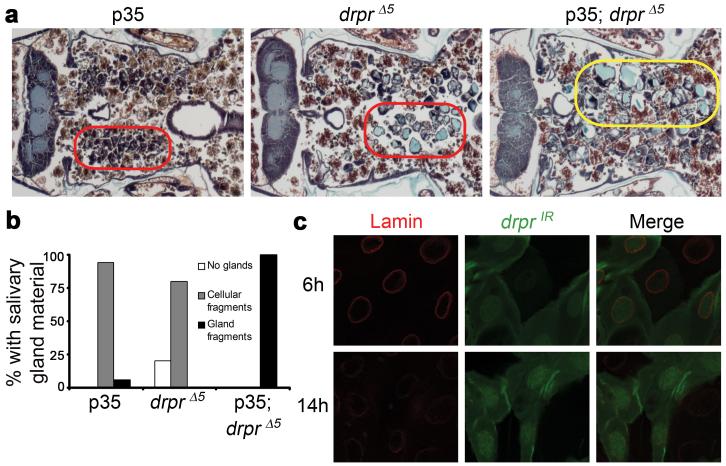
The expression of Drpr in dying cells prompted us to investigate whether Drpr might play a role in regulating an intracellular cell death process. Caspases and autophagy have been shown to function in an additive manner in the degradation of salivary glands, and inhibition of genes in either pathway leads to partial persistence of salivary glands2. Expression of the caspase inhibitor p35 alone leads to the persistence of condensed salivary gland cell fragments in 94% of pupae, and salivary gland fragments in 6% of pupae (Fig. 2a, b). Control homozygous drprΔ5 mutant pupae that contain the p35 transgene but lack the salivary gland GAL4 driver have persistent vacuolated salivary gland cell fragments in 80% of animals (Fig. 2a, b). However 100% of experimental drprΔ5 pupae that also express p35 in salivary glands contain larger amounts of persistent salivary gland material, including multi-cell gland fragments (Fig. 2a, b). Therefore, the salivary gland persistence phenotype in drprΔ5 is enhanced by the expression of p35, indicating that Drpr functions in an additive manner with caspases. To further test the relationship between Drpr and caspases, we tested whether drpr knockdown in salivary glands affects degradation of nuclear Lamin, a known caspase substrate in this tissue3. Clones of salivary gland cells that express drprIR and GFP possess strong Lamin staining 6 hours after puparium formation, and both control and drprIR-expressing cells contain decreased Lamin staining 14 hours after puparium formation (Fig. 2c), indicating that caspases are active in both control and drprIR-expressing cells 14 hours after puparium formation. Taken together, these results suggest that Drpr does not influence caspase activity, and functions downstream or parallel to caspases in dying salivary gland cells.
Figure 2. Draper functions downstream or in parallel to caspases during salivary gland cell death.
a, Animals with salivary gland-specific expression of p35 (fkh-GAL4/+; UAS-p35/+), n=18, drpr null animals (+/w; +/UAS-p35; drprΔ5/drprΔ5), n=10, and drpr null animals with salivary gland-specific expression of p35 (fkh-GAL4/w; UAS-p35/+; drprΔ5/drprΔ5), n=16, were analyzed by histology for the presence of salivary gland material 24h after puparium formation. Cell fragments are in red circles, and gland fragments are in the yellow circle. b, quantification of data from a. c, Salivary glands were dissected from animals expressing drprIR specifically in GFP-marked clone cells (hsflp/w; UAS-drprIR/+; act<FRT,cd2, FRT>Gal4, UAS-GFP/+) 6h and 14h after puparium formation. Salivary glands were stained with GFP antibody (green) to label cells expressing drprIR, and Lamin antibody (red).
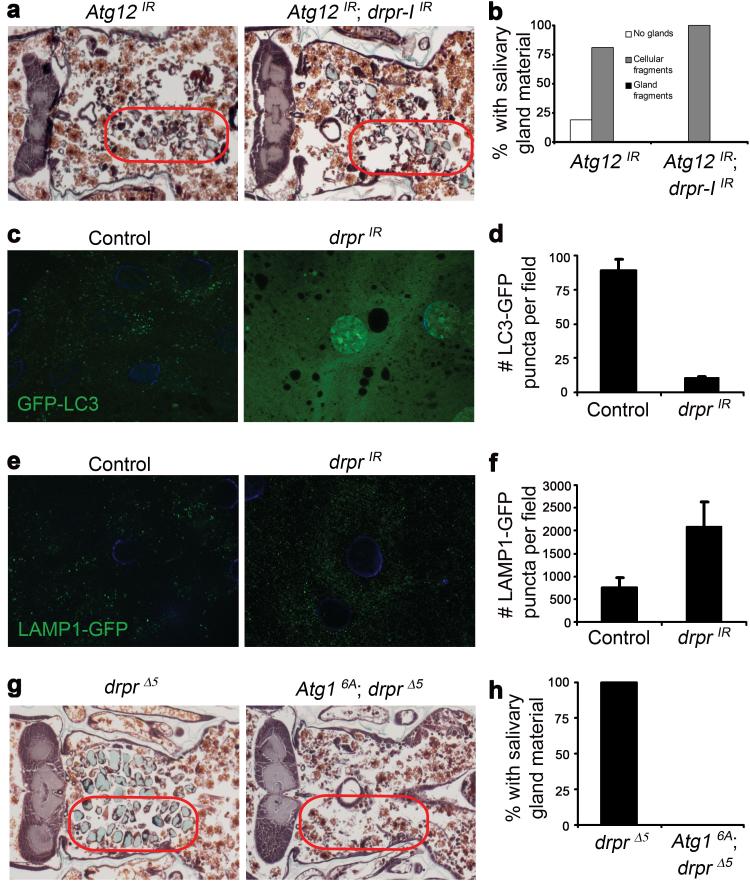
We next tested whether the salivary gland phenotype caused by expression of drprIR is enhanced by knockdown of Atg12, a gene required for autophagy. We found that 81% of pupae expressing Atg12IR in salivary glands have persistence of salivary gland cell fragments, whereas 100% of those expressing Atg12IR drpr-IIR have gland cell fragments (Fig 3a, b). These phenotypes are very similar to each other, and unlike expression of p35 in drprΔ5 (Fig. 2a), co-expression of Atg12IR and drpr-IIR does not lead to an increase in the amount of salivary gland material that persists, and multi-cell gland fragments were absent. This led us to hypothesize that Drpr could function in the same pathway as autophagy genes. To test this possibility, we knocked down drpr in salivary glands expressing the autophagy reporter GFP-LC3. Autophagy induction leads to the association of GFP-LC3 with autophagosomal membranes, which are visible as GFP puncta. We found that whereas autophagy is highly induced in control salivary glands 14 hours after puparium formation, those expressing drprIR contain few GFP-LC3 puncta at the same stage (Fig 3c, d). Autophagosomes are trafficked to and fuse with lysosomes, where autophagic content is degraded by lysosomal hydrolases. To test whether drpr knockdown affects lysosome numbers, we expressed drprIR in salivary glands and assayed for the number of Lysosome Associated Membrane Protein 1 (LAMP1)-GFP puncta. We found that salivary glands expressing drprIR contained more lysosomes than control salivary glands (Fig. 3e, f). Therefore, drpr is required for the induction of autophagy in salivary glands. The increase in lysosome numbers in drpr knockdown salivary gland cells is consistent with the failure in autophagy induction, given that lysosome numbers decrease when autophagy and autolysome formation are abundant12.
Figure 3. Draper is required for the induction of autophagy in dying salivary gland cells.
a, Animals with salivary gland-specific knockdown of Atg12 (fkh-GAL4/w; UAS-Atg12IR/+), n=21, and those with salivary gland-specific knockdown of both Atg12 and drpr-I (fkh-GAL4/w; UAS-Atg12IR/+; UAS-drpr-IIR/+), n=19, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. b, quantification of data from a. c, GFP-LC3 was expressed in salivary glands of control animals (+/w; UAS-GFP-LC3/+; fkh-GAL4/+) and those with salivary gland-specific knockdown of drpr (w; UAS-drprIR/UAS-GFP-LC3; fkh-GAL4/+). Salivary glands were dissected 6h and 14h after puparium formation, imaged for GFP-LC3, and LC3 puncta were quantified using Zeiss Automeasure software. d, quantification of data from c. Error bars represent s.e.m.; n ≥ 10; p < 0.0000001. e, LAMP-GFP was expressed in control animals (tub-LAMP-GFP/w; +/fkh-GAL4) and those with salivary gland-specific knockdown of drpr (tub-LAMP-GFP/w; UAS-drprIR/+; fkh-GAL4/+). Salivary glands were dissected 14h after puparium formation, imaged for LAMP-GFP, and LAMP puncta were quantified using Zeiss Automeasure software. f, quantification of data from e. Error bars represent s.e.m.; n ≥ 10; p < 0.05. g, drpr mutant animals (+/w; +/UAS-Atg16A; drprΔ5/drprΔ5), n=10, and those with salivary gland-specific expression of Atg16A (fkh-GAL4/w; UAS-Atg16A/+; drprΔ5/drprΔ5), n=16, were analyzed by histology for the presence of salivary gland material (red circles) 24h after puparium formation. h, quantification of data from g.
Expression of Atg1 is sufficient to induce ectopic autophagy and cell death in several Drosophila tissues, including the fatbody and salivary glands2,13. Therefore, we tested whether expression of Atg1 is sufficient to suppress the drprΔ5 null mutant defect in degradation of salivary glands. Whereas 100% of control Atg16A drprΔ5 animals that lack the GAL4 driver have persistent salivary gland material 24 hours after puparium formation, this phenotype is completely suppressed by the expression of Atg1 in drprΔ5 null mutant salivary glands (Fig. 3g, h). This suggests that Atg1 functions downstream of drpr.
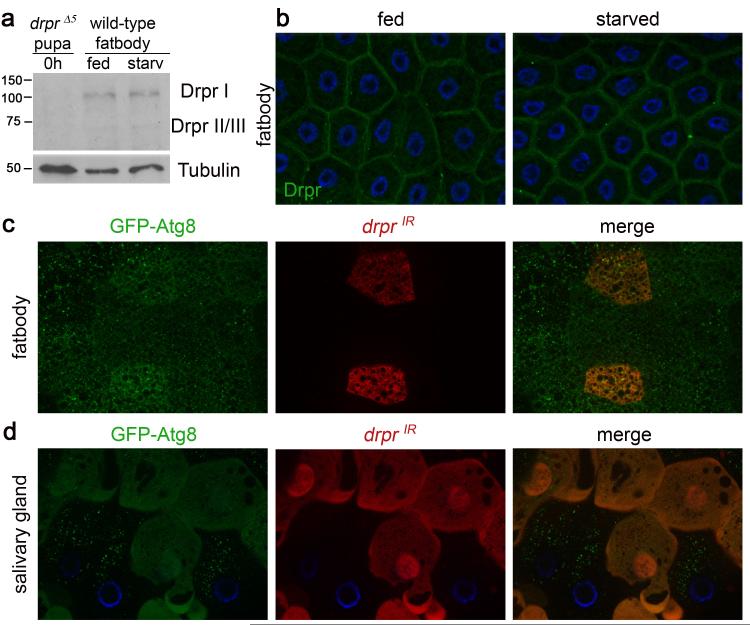
The question of whether autophagy plays dual roles in cell survival versus cell death has been controversial, and raises the possibility that different factors may regulate autophagy in different cell contexts. We analyzed whether Drpr protein is present in the larval fatbody, since drpr RNA was not detected in microarray analyses of starving larvae8. We found that Drpr is expressed in larval fatbody, but the levels and localization of Drpr do not change in response to starvation (Fig. 4a, b). Since drpr is required for autophagy during salivary gland cell death, we wondered whether drpr is required for starvation-induced autophagy in the Drosophila larval fatbody6. We starved third instar larvae expressing GFP-Atg8a (fly homologue of LC3 in mammals) in all cells and drprIR specifically in dsRed-marked cell clones, and assayed for the induction of autophagy. Surprisingly, we found that GFP-Atg8a puncta form in both drprIR-expressing cells, as well as neighboring control cells (Fig. 4c). In addition, loss of drprΔ5 function in mutant clones of cells failed to suppress autophagy in starved fatbody (Supplementary Fig. 4). Therefore, unlike in dying salivary glands, drpr is not required for starvation-induced autophagy in the Drosophila fatbody. One possibility is that drpr mediates a tissue-wide autophagic response, as we had not tested whether drpr functions in a cell autonomous manner in dying salivary glands. To test the cell-autonomous requirement of drpr for autophagy, salivary glands of animals expressing GFP-Atg8a in all cells, and drprIR specifically in dsRed-marked clone cells, were assayed for GFP-Atg8a puncta. Strikingly, we found that salivary gland cells expressing drprIR contain diffuse GFP-Atg8a, whereas neighboring control cells contain numerous GFP-atg8a puncta (Fig. 4d). Thus, drpr functions in a cell-autonomous manner upstream of autophagy in dying salivary glands, but not in the larval fatbody in response to starvation.
Figure 4. Drpr is cell-autonomously required for autophagy in dying salivary glands, but not in response to starvation in the fatbody.
a, Protein extracts from drpr null (w; drprΔ5/drprΔ5) pupae at puparium formation (0h) and from the fatbodies of wild type (Canton-S) third instar larvae were analyzed by Western Blotting with anti-Drpr antibody. Third instar larvae were either fed or starved 4h. b, Wild-type (Canton-S) third instar larvae were either fed or starved 4h, and fatbodies were dissected, stained with anti-Drpr antibody, and imaged for Drpr (green). Nuclei were stained with DAPI (blue). c, Third instar larvae expressing GFP-Atg8 in all cells, and drprIR specifically in dsRed-marked clone cells (hsflp/w; UAS-drprIR/+; hsGFPAtg8b, act<FRT,cd2, FRT>Gal4, UAS-dsRed/+), were starved 4h. Larval fatbodies were dissected and imaged for GFPAtg8 (green) and dsRed (red). d, Salivary glands of animals expressing GFP-Atg8 in all cells, and drprIR specifically in dsRed-marked clone cells (hsflp/w; UAS-drprIR/+; hsGFPAtg8b, act<FRT,cd2, FRT>Gal4, UAS-dsRed/+) were dissected 14h after puparium formation. Salivary glands were imaged for GFPAtg8 (green) and dsRed (red). Nuclei were stained with Höescht (blue).
Our findings indicate that Drpr is autonomously required for the clearance of cells that are not removed by phagocytosis. Although autophagy has been associated with engulfment in studies of TLR4- and CD46-mediated clearance of pathogens14, this is the first example of an “engulfment factor” regulating cell-autonomous clearance. Moreover, our work suggests that Drpr is required for the induction of autophagy in a cell death-specific context. Little is known about how autophagy induction leads to different outcomes in different contexts. It is possible that autophagy functions to deplete specific survival factors during cell death15. Alternatively, different levels of autophagy could also be induced during different contexts, and extensive autophagy and depletion of cell resources could kill a cell. How Drpr functions to regulate autophagy specifically in a cell death context remains to be determined. Given recent interest in manipulation of autophagy for therapy16, it is possible that factors such as Drpr could be used as biomarkers to distinguish autophagy leading to cell death versus cell survival.
METHODS SUMMARY
Genetic, histological, protein expression and localization, and reporter assays were performed as previously described2,3,17. Detailed genotypes of control and experimental individuals, as well as the detailed methods for each experiment, are described in Supplementary Information.
Supplementary Material
Acknowledgements
We thank A. Bergmann, U. Gaul, H. Kramer, E. Kurant, T.P. Neufeld, T.E. Rusten, H. Stenmark, the Bloomington Stock Center, the VDRC, and the Developmental Studies Hybridoma Bank for flies and antibodies, and R. Simin, T. Fortier and A. Sheehan for technical support. This work was supported by American Cancer Society Postdoctoral Fellowship PF-07-258-01-CSM to MAL, NIH grants NS053538 to MRF and GM079431 to EHB, and Merck and Co. Inc. to EHB. EHB is a member of the UMass DERC (DK32520).
Appendix
Methods
Drosophila strains
The Canton-S strain was used as the wild-type control. For loss of function studies, drprΔ5 9, simu2 18, ced-6J26 19, and Df(2R)w73-1 mutants were used. For RNAi studies, the following fly strains were used: pWiz-drprIR10, pWiz-drpr-IIR, UAS-Atg12-RNAIR20. The following Vienna Drosophila RNAi Center (VDRC) RNAi stocks were used: UAS-crqIR VDRC Transformant ID (TID) 45884, UAS-src42aIR VDRC TID 46019, UAS-ced-12IR TID 10455, UAS-crkIR TID 19081, UAS-mbcIR TID 16044. The sequences used for VDRC knock-down strains are available for each TID at http://stockcenter.vdrc.at/control/main. For ectopic expression studies, UAS-Drpr-I, UAS-p3521, and UAS-Atg16A 13were used. UAS-GFP-LC322, hs-GFP-Atg8a6 and tub-LAMP1-GFP22 were used as markers of autophagy and lysosomes.
Transgenic strains
To generate the UAS-Drpr-I strain, full length drpr-I cDNA (GH24127) was inserted into the XhoI site of the pUAST transformation vector23. The pWiz-drpr-IIR RNAi construct was made by amplifying a 1041 nucleotide sequence unique to Drpr-I (nucleotides 1331-2371 from GH 24127) and inserting this fragment into the NheI and XbaI sites of the pWIZ transformation vector24. The same sequence was then inserted in the XhoI and EcoRI sites in the 3′ to 5′ direction to generate the final pWiz-drpr-IIR RNAi construct. Constructs were sequenced and used to generate transgenic Drosophila (BestGene, Inc.).
Protein Extracts and Western Blotting
Protein extraction and Western Blotting were performed as described previously17. Primary antibodies used were rabbit anti-Drpr9 (1:1000), and mouse anti-Beta-Tubulin (1:50)(Developmental Studies Hybridoma Bank).
Histology
Histology was performed as described previously25.
Immunolabeling and Microscopy
Dissection, fixation, and antibody labeling of Drosophila tissues was performed as described previously3. Imaging was performed on a Zeiss Axiophot II microscope. Primary antibodies were used at the following concentrations: rabbit anti-Drpr9(1:500), mouse anti-Lamin DMO (1:10) (Developmental Studies Hybridoma Bank), mouse anti-Crumbs (1:10)(Developmental Studies Hybridoma Bank), and mouse anti-GFP (1:100)(Invitrogen Monoclonal). For LC3 and LAMP1 quantification, salivary glands were imaged without fixation on a Zeiss Axiophot II microscope and GFP puncta were quantified using Zeiss Automeasure software.
Induction of cell clones
To induce RNAi-expressing cell clones in Drosophila tissues, an overnight egg lay was obtained at 25°C, and following the egg lay, embryos were heat shocked at 37°C for 15 min. To induce expression of hsGFPAtg8, larvae were heat shocked at 37°C for 30 minutes, and then recovered at 25°C until the time of dissection. To induce drprΔ5 null mutant cell clones, y w hsFLP; UAS-pCherry-Atg8; UbiGFPnls, FRT2A virgins were crossed to CG-Gal4; UbiGFPnls, FRT2A, drprΔ5 males. An overnight egg lay was obtained at 25°C, and following the egg lay, embryos were heat shocked at 37°C for 1 hour.
Starvation of larvae
Third instar larvae were either allowed to remain in the food (fed) or removed from food and placed on moist petri dishes for 4 hours (starved).
- 18.Kurant E, Axelrod S, Leaman D, Gaul U. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell. 2008;133:498. doi: 10.1016/j.cell.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuraishi T, et al. Pretaporter, a Drosophila protein serving as a ligand for Draper in the phagocytosis of apoptotic cells. EMBO J. 2009;28:3868. doi: 10.1038/emboj.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 21.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 22.Rusten TE, et al. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev. Cell. 2004;7:179. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 24.Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods. 2003;30:322. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 25.Muro I, et al. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
Footnotes
Supplementary Information is linked to the online version of the paper atwww.nature.com/nature
References
- 1.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 2.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin DN, Baehrecke EH. Caspases function in autophagic cell death in Drosophila. Development. 2004;131:275. doi: 10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- 4.Hou YC, et al. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J. Cell Biol. 2008;182:1127. doi: 10.1083/jcb.200712091. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nezis IP, et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy. 2009;5:298. doi: 10.4161/auto.5.3.7454. [DOI] [PubMed] [Google Scholar]; Mohseni N, et al. Autophagy promotes caspase-dependent cell death during Drosophila development. Autophagy. 2009;5:329. doi: 10.4161/auto.5.3.7444. [DOI] [PubMed] [Google Scholar]; Denton D, et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr. Biol. 2009;19:1741. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C-Y, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 6.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 2004;7:167. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Lee C-Y, et al. Genome-wide analyses of steroid- and radiation-triggered programmed cell death in Drosophila. Curr. Biol. 2003;13:350. doi: 10.1016/s0960-9822(03)00085-x. [DOI] [PubMed] [Google Scholar]
- 8.Zinke I, et al. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman MR, et al. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald JM, et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C, Baehrecke EH, Thummel CS. Steroid regulated programmed cell death during Drosophila metamorphosis. Development. 1997;124:4673. doi: 10.1242/dev.124.22.4673. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, et al. Autophagy termination and lysosome reformation regulated by mTOR. Nature. 2010 doi: 10.1038/nature09076. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott RC, Juhász G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 2007;17:1. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joubert PE, et al. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]; Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 15.Yu L, et al. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. USA. 2006;103:4952. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009;15:5308. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta S, Baehrecke EH. Warts is required for PI3K-regulated growth arrest, autophagy, and autophagic cell death in Drosophila. Curr. Biol. 2008;18:1466. doi: 10.1016/j.cub.2008.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.