Abstract
Sleep loss leads to profound performance decrements. Yet many individuals believe they adapt to chronic sleep loss or that recovery requires only a single extended sleep episode. To evaluate this, we designed a protocol whereby the usual sleep:wake ratio was reduced from 1:2 to 1:3.3, while the durations of both sleep and wake episodes were increased to ten hours and 32.85 hours respectively. These sleep and wake episodes were distributed across all circadian phases, enabling measurement of the effects of acute and chronic sleep loss at different times of the circadian day and night. Despite recurrent acute and substantial chronic sleep loss, ten hour sleep opportunities consistently restored vigilance performance for several hours of wakefulness. However, chronic sleep loss increased the rate of deterioration in performance across wakefulness, particularly during the circadian “night”. Thus, extended wake during the circadian night reveals the cumulative detrimental effects of chronic sleep loss on performance, with potential adverse health and safety consequences.
Introduction
The capacity to sustain alertness and attention is essential for survival, yet it is a finite resource that progressively declines over consecutive hours awake. Homeostatic physiologic processes that occur during sleep replenish this capacity, but how much sleep is required for satisfactory alertness and performance continues to be debated. There are two types of sleep loss: acute sleep loss consisting of one continuous extended wake episode and chronic sleep loss consisting of insufficient sleep over multiple days. A parsimonious account of sleep homeostasis is that the degree of impairment reflects the accumulation of excess wakefulness independent of whether the accumulation occurs acutely or chronically (1). However, recent animal data suggest that acute and chronic sleep loss have distinct homeostatic mechanisms. Understanding fundamental properties of the sleep homeostatic regulation of alertness and performance in humans has both public health relevance for occupational policy and therapeutic implications for the discovery of novel wake-promoting therapies.
A significant experimental limitation in studying the homeostatic regulation of performance based on the prior sleep-wake history is the confounding influence of circadian rhythms. Endogenous circadian rhythms are coordinated by pacemaker activity of the suprachiasmatic nucleus (SCN) of the hypothalamus (2). The circadian system promotes wakefulness and performance during the circadian day; this alerting signal dissipates during the circadian night at which time the circadian pacemaker promotes sleep (3). However, acute sleep loss can directly influence neural activity in the SCN (4), and the amplitude of the circadian oscillation in performance increases with longer consecutive hours awake (5-8). As a result of these non-linear interactions, the circadian influence on performance during acute sleep loss is small early in a waking bout, but after many consecutive hours awake, performance and alertness become increasingly dependent on the circadian phase. In previous chronic sleep loss protocols, waking performance was only assessed at restricted combinations of homeostatic pressure and circadian phase. In order to determine fundamental properties of sleep homeostasis, it is necessary to account for the confounding influence of circadian phase by distributing the sleep-wake schedule across the full circadian cycle.
We designed a protocol to separate the influences of chronic sleep loss, long consecutive hours awake, and circadian timing on a task of sustained attention. The protocol utilized a forced desynchrony (FD) paradigm, which allows the circadian pacemaker to cycle at its endogenous period (∼24.2 h in humans, (9)) independent from the experimental sleep-wake schedule. The FD protocol lasted 21 calendar days, consisting of 12 sleep-wake cycles of 42.85 hours, each with 32.85 hours of scheduled wakefulness and 10 hours of scheduled sleep. This wake duration is similar to that of a medical resident working “on call” or others who work through the night and into the next day. The reduced sleep-to-wake ratio of 1:3.3 during FD, which is comparable to 5.6 hours sleep every 24 hours, imposed chronic sleep loss across the three weeks on the FD protocol in addition to the acute sleep loss from the long consecutive hours awake within each 42.85 hour FD cycle. The FD protocol began under entrained conditions with a normal phase relationship between the circadian and scheduled sleep-wake cycles. The differential length between the 42.85-hour sleep-wake cycle and the near-24 hour circadian cycle caused uncoupling of the two cycles, allowing measurement of performance at different combinations of length of time awake and specific circadian phases. We compared this current chronic sleep loss protocol to data from a separate 42.85-hour FD protocol with no intentional sleep loss as a control (Fig. 1, (8)). In that study, scheduled sleep episodes were 14.28 hours long and scheduled wake episodes were 28.57 hours long (sleep:wake ratio 1:2, comparable to 8 hours of sleep per every 24 hours).
Figure 1. Double raster plot demonstrating the timing of sleep in the chronic sleep loss and control protocols.
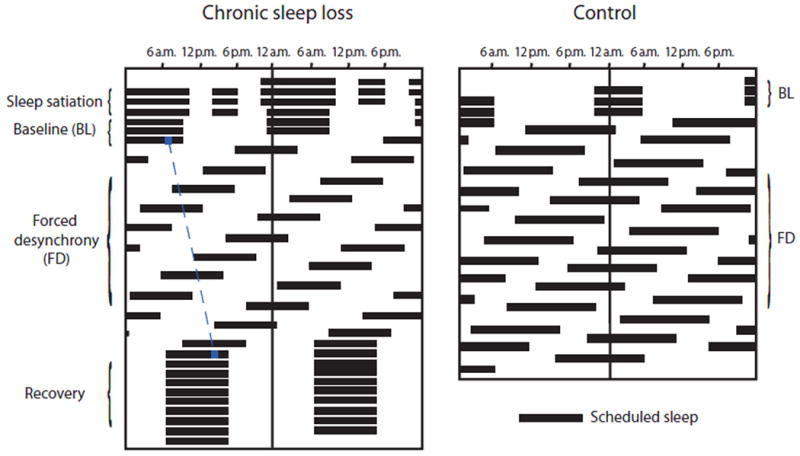
In a raster plot, time in hours is plotted on the horizontal axis and days on the vertical axis; in a double raster plot, two days are included on each horizontal line with the second day also plotted on the left side of the next row. Black horizontal bars indicate scheduled sleep episodes. In the chronic sleep loss protocol, participants were realigned to their habitual phase relationship between the sleep-wake and circadian cycles during the recovery (last 10 days); this was adjusted for each participant using their free-running period as determined during the FD portion of the experiment. For example, the blue dotted line represents the drift of a circadian phase marker for a subject with an endogenous circadian period of ∼24.3 hours.
Although the sleep:wake ratio in the chronic sleep loss protocol was reduced compared to the control protocol, the 10-hour sleep episodes were longer than each participants' habitual sleep duration. We tested the hypothesis that acute homeostatic regulation of performance over hours was separable from chronic homeostatic regulation over days to weeks, in contrast to a single sleep homeostatic mechanism. Within a framework of a single homeostatic mechanism, sleep pressure would rise during wakefulness and incompletely dissipate during sleep within each sleep-wake cycle, secondary to the reduced sleep:wake ratio (10) and would thus accumulate over weeks on the chronic sleep loss protocol. This framework predicts that performance early in each wake episode would become progressively worse across the three weeks. In contrast, in a framework of multiple homeostatic processes, the sleep achieved within each 10-hour sleep opportunity could be enough to completely dissipate the acute homeostatic sleep pressure and restore performance early in the wake episode to baseline levels, but a separate chronic homeostatic process accumulating during the 21-day experiment could be predicted to increase the rate of performance deterioration over consecutive hours awake (11).
Results
The Psychomotor Vigilance Task (PVT), a test of sustained attention, was the primary performance measure. Under real-world conditions of chronic sleep restriction, poor PVT performance has been associated with impairments to levels similar to that of driving while intoxicated in other performance measures that may have more obvious ecological validity, such as simulated driving (12). The PVT was administered every four hours starting two hours after waking to nine individuals in the chronic sleep loss group (sleep:wake ratio 1:3.3) and every two hours to eight individuals in the control group (sleep:wake ratio 1:2). The primary analyses focused on the median reaction time (RT), a measure that is less sensitive to the effects of acute sleep loss compared to other PVT measures (for example the mean RT) (13) and therefore a conservative estimate of the potential impairment induced by chronic sleep loss. To distinguish between the hypotheses of a single versus multiple homeostatic processes, we analyzed the median RT as a function of consecutive hours awake averaged across all circadian phases (Fig. 2A). When comparing the first PVT results at 2 hours awake, performance was near baseline levels across all three weeks of chronic sleep loss (two baseline days: median RT = 256msec; 1st week: median RT =256msec; 2nd week: median RT = 278msec; and 3rd week: median RT = 291msec), and not significantly different from the control data with a 1:2 sleep:wake ratio (1st week: 270msec; 2nd week: 305msec; 3rd week: 309msec; p=0.42). In contrast, when comparing the last performance test of each wake episode, which was administered at 30 consecutive hours awake, there was a dramatic increase in median RT between the first and second weeks on the chronic sleep loss protocol (1st week: median RT = 667ms; 2nd week: median RT = 1,954msec; 3rd week: median RT = 2,013msec). When the chronic sleep loss data were fit with a linear model, there was no significant difference across weeks of the protocol in the y-intercept, which reflects the theoretical effect of acute homeostatic sleep pressure at awakening, ignoring the impact of sleep inertia (10). This is not consistent with the single homeostatic process predictions. However, there was a marked increase in slope after the first week, reflecting slower reaction times with increasing hours awake (1st week: slope = 24 msec/hour awake; 2nd week: slope = 69 msec/hour awake; 3rd week: slope = 65 msec/hour awake). The differences in slope between the 1st and 2nd weeks were significant (p<0.0001). Other PVT measures showed this pattern as well, including the mean RT, 5th percentile RT, and 95th percentile RT (see Supplementary Material). This analysis indicates that the 10-hour sleep opportunities in the chronic sleep loss protocol were sufficient to dissipate an acute homeostatic process to baseline levels in this task even during the 3rd week, but a separate chronic sleep homeostatic process accelerated the performance deterioration over consecutive hours awake.
Figure 2. Separating acute and chronic sleep homeostatic processes.
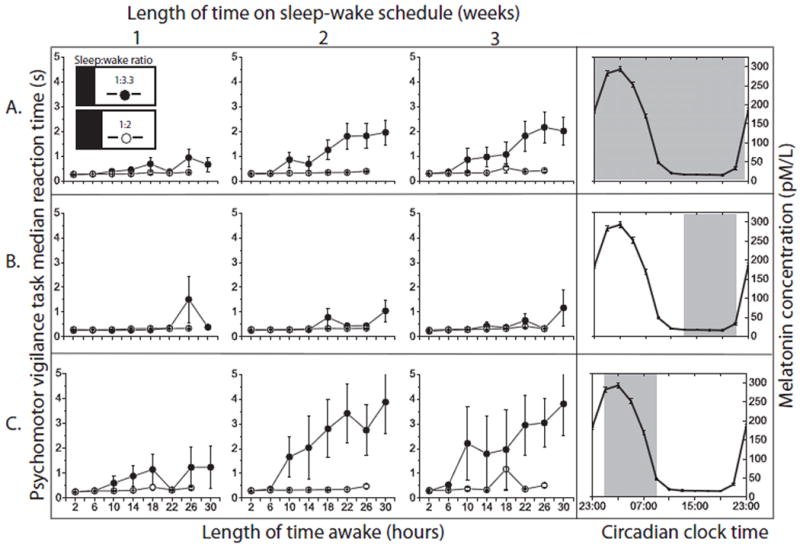
Effect of consecutive hours awake on observed PVT median RT (mean and standard error of the mean). Weeks on the experimental schedule are shown from left-to-right. The graphs on the far right side show the range of circadian phases for which data are included in each row using melatonin as a phase marker of the circadian clock. During entrained baseline conditions, the melatonin maximum occurred at approximately 3am. (A) The homeostatic response across all circadian phases. (B) The homeostatic response during the circadian afternoon/early evening. (C) The homeostatic response during the late circadian night. Independent of circadian phase and across all weeks of the protocol, performance returns to near-baseline levels for at least the first six hours after waking. Chronic sleep loss (closed circles) accelerates the decline in performance over consecutive hours awake, predominantly during the late circadian night with remarkably preserved performance during the circadian afternoon/early evening. Variability is greatest with longer consecutive hours awake, weeks of chronic sleep loss, and during the late circadian night. Note that the graphs do not indicate the trajectory of an individual with increasing time awake (accompanied by change in circadian phase), but rather the theoretical homeostatic effect of increasing time awake at a fixed circadian phase. In the control group, there was a slowing of RT by approximately 30% between 2 and 26 hours awake, but this deterioration within wake episodes (at an average circadian phase) is not apparent at this scale.
In contrast to previous chronic sleep loss protocols, the data also can detail performance within a wake episode as a function of circadian timing. Plasma melatonin was the primary marker of the phase of the circadian clock, with the melatonin maximum assigned a phase of 0 degrees. Fig. 3A demonstrates that PVT performance was worst at circadian phases 0-60 degrees (corresponding to approximately 3am-7am during entrained conditions) and it was best at 180-240 degrees (corresponding to approximately 3pm-7pm during entrained conditions), phases at which the circadian pacemaker strongly promotes wakefulness (14, 15). Across both experimental protocols, the amplitude of the circadian oscillation in performance increased with the number of consecutive hours awake, such that after only a few hours awake the variation in performance across circadian phase was small, whereas with longer time awake, the variation in performance across circadian phase was much larger. This is consistent with prior reports (5, 8). In addition, chronic sleep loss, assessed both as a function of week within the chronic sleep loss protocol and differences between experimental groups (with different sleep:wake ratios), increased the amplitude of the circadian performance rhythm. This was evident as a significant four-way interaction between circadian phase, length of time awake, week on the FD protocol, and group, on PVT median RT (p<0.0001). Note that the apparent increase in circadian amplitude with chronic sleep restriction was primarily associated with worsening of the performance nadir in the late circadian night during extended wakefulness (Fig. 3B and C, Fig. 4). Other PVT measures also showed this significant four-way interaction, including the mean RT, PVT lapses (trials with RT>0.5 sec), 5th percentile RT, and 95th percentile RT (p<0.0001). The most robust differences were seen between the first and second week of chronic sleep restriction (Fig 2-4). Acute and chronic sleep loss and adverse circadian phases generally increased the probability of slow RTs within this 10-minute PVT, but the fastest possible reaction times were relatively preserved (see Supplemental Materials). This shift in the rightward tail of the response distribution has been interpreted to reflect an impairment of attention rather than primary visual or motor processes (16).
Figure 3. Circadian rhythm of performance.
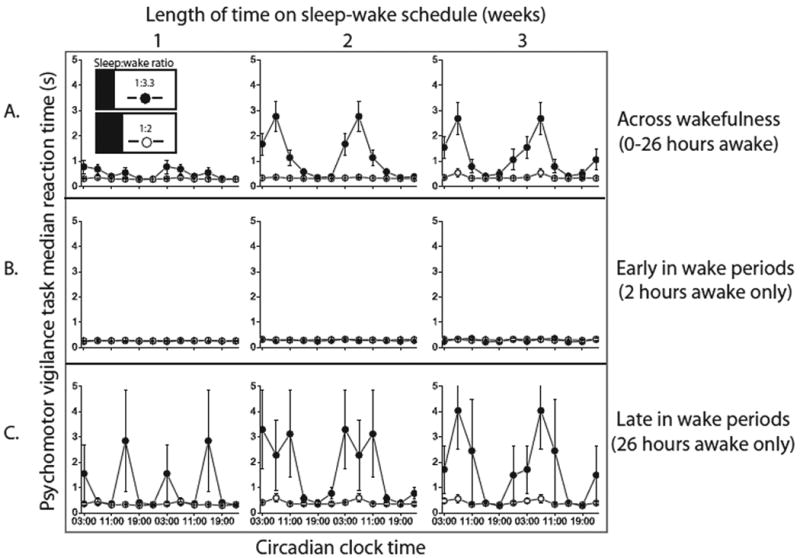
Effect of circadian phase on observed PVT median RT (mean and standard error of the mean). Weeks on the experimental schedule are shown from left-to-right. (A) The circadian oscillation in performance averaged across 26 hours of wakefulness per wake episode (B) The circadian oscillation early in each wake episode at 2 hours awake (C) The circadian oscillation late in each wake episode at 26 hours awake. As can be seen in row B, across all weeks of the protocol, the amplitude of the circadian oscillation is small shortly after waking. However, chronic sleep loss (closed circles) increases the amplitude of the circadian oscillation, predominantly after extended wakefulness (row C). Chronic sleep loss alone therefore is not sufficient to appreciably increase the circadian amplitude; rather it intensifies the interaction between acute sleep loss and circadian phase. In the control group, the 116msec average peak-to-trough difference in the circadian performance rhythm (averaged across 26 hours awake) is not apparent at this scale.
Figure 4. Circadian and homeostatic regulation of performance.
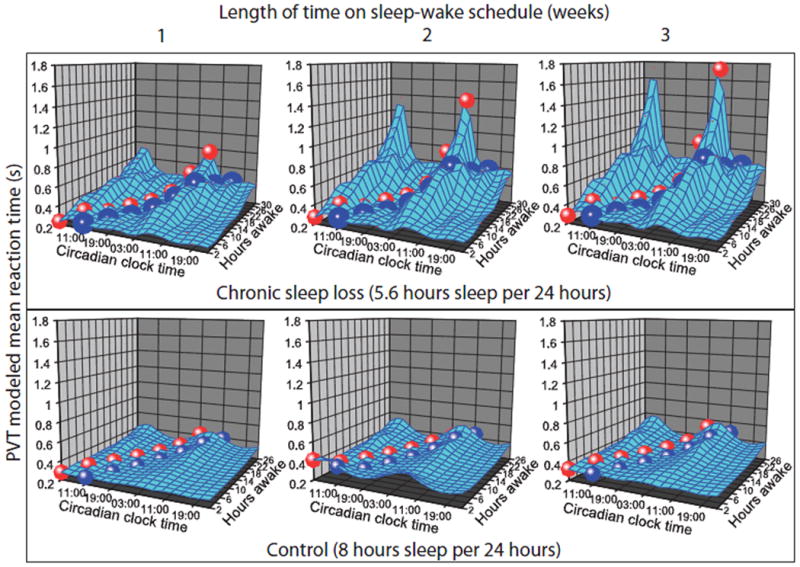
Mixed-effect statistical model predictions of PVT mean RT data at each combination of circadian phase and length of time awake within each week on the FD protocol are shown. Within each week of the chronic sleep loss protocol (top row), circadian amplitude increased with longer consecutive hours awake. Across weeks (left-to-right within each row), there was a disproportionate deterioration of performance during the late circadian night across three weeks of chronic sleep loss. Projected trajectories demonstrate the combination of consecutive hours awake and corresponding circadian time when individuals awaken at their normal entrained circadian time (blue path) and when they awaken two hours prior to the melatonin peak (red path) as may occur during jet leg or night shift schedules. The cumulative cost of chronic sleep loss is most pronounced during these conditions of circadian misalignment. The 85% average increase in predicted mean RT in the control condition between 2 hours awake during the circadian “day” and 26 hours awake during the circadian “night” is not apparent at this scale.
Generalization of our results to predict the average level of impairment that can be expected at a population level requires accounting for inter-individual differences. Statistical models can take into account such differences in order to make predictions about performance. For example, performance at all the different combinations of time awake, circadian phase, and week of sleep restriction can be visualized as potential points in a state space of circadian and homeostatic conditions (Fig. 4). However, as a wake episode unfolds, there is a simultaneous change in the number of hours awake and circadian phase, so individuals can only experience limited combinations of homeostatic pressure and circadian phase within a wake episode. Based on the circadian phase at awakening, the expected trajectory of how performance will change throughout the wake episode can be determined. These models are therefore useful for predicting the effects of acute and chronic sleep loss combined with various levels of circadian misalignment, such as occurs during shift work conditions. Note that the statistical model does not take into account differences in the sex distribution between the chronic sleep restriction group (4 female, 5 male) and the control group (8 male). Although a recent study demonstrated slightly slower reaction times in women compared to men, sex differences did not influence the deterioration of performance during acute sleep loss in that study (17). Nevertheless, sex differences in response to chronic sleep loss may exist.
In this chronic sleep loss protocol, sleep efficiency [(total sleep time/time in bed) × 100%] was ∼90% (mean +/- s.e.m. of 90.14+/- 0.08%) for all 3 weeks of the protocol. This sleep efficiency corresponds to approximately 9 hours of sleep per sleep episode or 5.0 hours of sleep per 24 hours. For the FD in the control group with a 1:2 sleep:wake ratio, sleep efficiency was 84.0 +/- 0.1%, corresponding to 12 hours of sleep per sleep episode or 6.7 hours of sleep per 24 hours.
Discussion
Our findings demonstrate that homeostatic regulation of alertness and performance in humans is composed of at least two dissociable components that act on different time scales. Recent animal data support our findings: the short-term homeostatic process acts on the order of hours and may be mediated by accumulating levels of extracellular adenosine in the basal forebrain and other cerebral sites as a product of energy metabolism (18-22), though this hypothesis has been challenged (23). The characteristics of the short-term homeostatic process are similar to the homeostatic regulation of slow wave sleep (24). On longer time scales, the reduced sleep:wake ratio of chronic sleep loss (10) may cause an up-regulation of adenosine receptor density, sensitizing the system and increasing sleep drive for a given number of consecutive hours awake (25, 26).
Data from this experiment reveal that individuals can develop a chronic sleep debt in the face of apparent full recovery from acute sleep loss. It is common for individuals to have relatively long sleep bouts on weekends or holidays but short sleep episodes on work or school days. Under such conditions, a chronically sleep-restricted individual may have a false sense of recovery from their prior sleep debt as a result of performing well for the first several hours of a usual waking day. In addition, during circadian phases typically corresponding to afternoon/early evening, the circadian system has the remarkable capacity to override the deleterious effects of prior sleep loss (Fig. 2B). Thus, the short-term restorative properties of recent extended sleep combined with the alerting properties of the normally timed circadian rhythm can mask the effects of chronic sleep loss during a typical waking day. However, during subsequent bouts of extended wakefulness, the cumulative effect of chronic sleep loss may cause performance to deteriorate much more rapidly, particularly during the late circadian night. In addition, these data demonstrate that chronic sleep loss reduces an individual's ability to cope with circadian misalignment from rotating shift schedules or jet lag, since the alerting phase of the circadian rhythm may be inappropriately timed to counter the deterioration in performance experienced after several hours awake.
A practical application of this work relates to individuals who work extended hours, such as health care workers, military personnel, emergency response teams, and transportation workers. After such workers remain awake all day, their performance may deteriorate overnight, when increasing consecutive hours of wakefulness combine with the circadian performance nadir to make them increasingly vulnerable to accidents and errors. This degraded performance may be somewhat mitigated if the individual were to have adequate sleep for weeks prior to this challenge (27), as demonstrated by smaller impairments seen in the control group during extended wakefulness. It is important to bear in mind that acute sleep loss alone is hazardous: 19 hours of sustained wakefulness from 8am to 3am is associated with performance deficits equivalent to a blood alcohol concentration of 0.05%, and after 24 hours of sustained wakefulness the performance deficit is equivalent to an alcohol concentration of 0.10% (28). Yet, using the metric we have chosen to evaluate the additional impact of chronic sleep loss, these significant performance deficits from acute sleep loss experienced in the control condition appear indistinguishable from baseline when presented at the same scale as the chronic sleep loss condition. We found that when participants were pre-loaded with fatigue due to chronic sleep loss, the deterioration in performance during the biological night induced by acute sleep loss dwarfed the impairment induced by acute sleep loss alone in people who were not chronically sleep restricted. This has important safety implications for the 16% of Americans who routinely sleep six hours per night or less (29), particularly those in safety sensitive industries like long haul trucking (30). Mathematical models that reflect the three-way interaction between chronic sleep loss, long consecutive wake hours, and circadian phase as well as the time course of the decline in performance documented above can aid in the development of work schedules that reduce the probability of occupational errors, injuries, motor vehicle crashes, and other potential adverse health outcomes. In addition, public health education campaigns should emphasize the potentially covert consequences of chronic sleep loss.
Materials and Methods
Participants
Nine healthy volunteers were studied (4 female, 5 male) in the chronic sleep loss protocol. Participants were 21-34 years old (mean 27), were medically healthy by history, physical examination, and laboratory testing of hematological or metabolic measures. There was no evidence of psychological disturbance as judged by questionnaires and a clinical interview with a psychologist. They did not have clinical sleep disorders as assessed by questionnaires and one night of screening clinical polysomnography. Inclusion criteria included self-reported habitual sleep duration of 6.5-9 hours averaged across the entire week, with no history of night shift work or transmeridian travel for at least three months prior to enrollment. To minimize self-imposed sleep loss prior to entering the Brigham & Women's Hospital General Clinical Research Center (GCRC) research facilities, participants were maintained on regular 10-hour per night nocturnal sleep schedule at times of their choosing at home for at least three weeks prior to entering the research facility, which was verified by wrist actigraphy, sleep logs, and time-stamped voicemail messages left immediately prior to going to bed and upon waking. Only deviations of less than 30 minutes from this schedule were allowed.
The eight control participants studied in the FD with 1:2 sleep:wake ratio were male (ages 18-30 years). Their physical exam, laboratory, psychological and questionnaire screening and eligibility criteria were the same as for the chronic sleep loss protocol. They had 8-hours time in bed at home prior to entering the GCRC. Data from this group have been previously reported (8).
For both protocols, participants refrained from alcohol, caffeine, and nicotine, for three weeks at home and while in the GCRC; this was verified by toxicology screening. All participants gave written informed consent. The protocols were approved by the Partners Healthcare Institutional Review Board.
Chronic Sleep Loss Protocol
The chronic sleep loss experiment included a 38-day protocol (Fig. 1) within the GCRC. The protocol began with a 12-hour overnight sleep opportunity and a 4-hour daytime nap opportunity for the first three days, to minimize any residual sleep loss. This was followed by two baseline days with a 10-hour overnight sleep opportunity at the same clock time as their home schedule. The participants then entered the forced desynchrony (FD) segment of the protocol, which lasted 21 calendar days, and contained 12 cycles of the 42.85 hour sleep-wake schedule. Participants then had 10 recovery days, consisting of 14 hours of scheduled wakefulness and 10-hour scheduled sleep episodes, which is the same pattern as the two baseline days. Circadian phase estimates using temperature data collected during FD were used to realign the sleep-wake schedule so that recovery sleep episodes occurred at the same circadian phase as during the baseline entrained conditions. During the last baseline day and during the FD segment of the protocol, a technician remained in the room during scheduled wakefulness to minimize inadvertent sleep.
Within each week of the FD protocol, the four scheduled sleep episodes (occurring 42.85 hours apart) and other periodic events in the sleep-wake schedule were relatively evenly distributed across all circadian phases (9). The experimental environment is free of time cues to minimize the potential synchronizing influences on the circadian system. Light levels were at 4 lux during wakefulness and 0 lux during sleep to minimize any circadian phase-shifting properties of light (31). Circadian phase was assessed with hourly serum melatonin samples as the primary phase marker and continuous core body temperature measurements via a rectal sensor as a secondary measure. Non-orthogonal spectral analysis (9, 32) of melatonin and temperature data was used to estimate the intrinsic period of each participants' endogenous circadian pacemaker (mean 24.17 ± 0.21 hours; range 23.73-24.43 hours across all nine participants) and the timing of protocol events relative to the melatonin rhythm.
Psychomotor Vigilance Task (PVT)
Participants were tested using 25-minute test batteries every two hours, starting two hours after waking to allow the dissipation of sleep inertia (33), which is the cognitive impairment observed immediately upon waking. Data from the other performance and mood testing during these batteries are not reported here. Alternating test batteries were used such that the PVT was administered every four hours in the chronic sleep loss group and every two hours in the control group starting two hours after waking. In the PVT, participants are instructed to maintain the fastest possible RTs to a simple visual stimulus. The inter-stimulus interval involves a high signal rate, randomly varying between 2 and 10 seconds. Participants sat 57cm from a monitor screen, measured prior to each test administration, to ensure a consistent visual angle for all testing. Responses were made with the dominant thumb on a response button, and visual feedback of each RT was provided. Anticipatory responses prior to the appearance of the target (i.e., false-alarms) were discouraged and visual feedback of anticipatory responses was immediately presented on the screen when they occurred. Each administration of the PVT lasted 10 minutes. The PVT does not display appreciable practice effects (34), making it an ideal test to compare performance across protocols that have different frequencies of exposure to the task.
Control Group
The protocol for the eight control participants with a 1:2 sleep:wake ratio within a 42.85-hr FD protocol (8) was conducted in the same facility as the current chronic sleep loss protocol. Following three baseline days, consisting of 16 hours scheduled wakefulness and 8 hours scheduled sleep, participants underwent 14 cycles of FD with a 42.85 hour sleep-wake cycle but with a “normal” sleep:wake ratio of 1:2. Only data from the first 12 cycles of FD were used, to match number of FD cycles in the chronic sleep loss protocol. Performance tests were given every 2 hours starting 1.5 hours after scheduled wake time. The data were binned into four-hour time-awake bins so that all data could be included and analyzed identically between both the chronic sleep loss protocol and control groups.
Sleep Data
Sleep was assessed by polysomnography (Vitaport digital sleep recorder, TEMEC Instruments B.V. Kerkrade, The Netherlands). The sampling rate was 256 Hz. The EEG montage consisted of the electrodes: C3, C4, O1, and O2 referenced to contralateral mastoid A1, A2). Sleep data were visually scored according to standard criteria (35). Sleep efficiency was defined as the total sleep time/time in bed *100%).
Statistical Analysis
The duration of one complete circadian cycle, designated as the estimated intrinsic period of the melatonin rhythm of each participant, was divided into 360 degrees, with the fitted melatonin maximum designated at 0 degrees (approximately 3am for an individual with a habitual sleep schedule of 11pm to 7am). The phase of awakening was calculated for each subject using 90 degree phase bins to determine the relative alignment between the circadian cycle and the sleep-wake cycle at baseline and throughout the FD protocol. On average, the phase relationship between the circadian rhythm and scheduled events approximated baseline conditions after each calendar week and 4 sleep-wake cycles of the FD protocol. However, for the purpose of analysis, the exact number of sleep-wake cycles included in each week was determined individually based on the circadian phase at awakening for each participant. Performance tests were analyzed with a resolution of 60 degree circadian phase bins to match the every-four-hour sampling rate of the performance tests across wakefulness.
All administrations of the PVT were identified with length of time awake, week within the FD protocol, and circadian phase in degrees. Mixed-effects statistical modeling was used. GROUP, LENGTH OF TIME AWAKE, CIRCADIAN PHASE, and WEEK on the protocol were fixed effects; PARTICIPANT was a random effect to model inter-individual differences. This model also takes into account correlation of performance measurements within an individual. We assessed for significant two-way interactions between LENGTH OF TIME AWAKE and CIRCADIAN PHASE within each week; three-way interactions were determined by the change in the time awake x phase interactions by WEEK, and four-way interactions were determined by differences in the change in the time awake x phase interactions across weeks as a function of GROUP. All analyses were done using SAS PROC MIXED with unstructured correlation structure. From the mixed-effect model, a predicted value for mean RT was determined for each combination of group, time awake, and circadian phase within each week (Fig. 4); the model accounts for the observed inter-individual variation in performance. Model predicted RTs tended to be lower than the observed group means during the late circadian night and long consecutive hours awake, physiological conditions in which variability was highest. Therefore, the model predictions are a conservative estimate of the degree of impairment that can be expected at a population level from sleep loss and circadian misalignment.
Supplementary Material
Acknowledgments
Research Supported by: NIH T32-HLO701-10 (DC), NIH K02-HD045459 (EBK), AFOSR FA9550-06-0080/ O5NL132 (CAC,EBK, REK, WW), AFOSR F49620-95-1-0388 (CAC, DJD, JKW), NSBRI HFP01604 (EBK, REK, WW), NIH M01-RR-02635
Competing interests
DC
Funding: none
Financial relationships: None
Patents: None
WW
Funding: None
Financial relationships: None
Patents: None
JKW
Funding: none related to or supporting the work in this manuscript: Investigator initiated (but no salary support) from Respironics Sleep and Respiratory Research Foundation (ongoing); Site PI for clinical trial (but no salary support) from Respironics Inc. (ended in 2007); Co-PI (with salary support) on NIH R01 MH067057 (ended 5/2009)
Financial relationships Speakers bureau for Vital Issues in Medicine (4 paid lectures given in 2007 on shift work sleep disorder and sleep in medical residents)
Patents: none
REK
Funding: none
Financial relationships: none
Patents: Patent related to the use of light to shift the timing of circadian rhythms
DJD
Funding: AFOSR, BBSRC, H Lundbeck A/S (Investigator Initiated), Philips Lighting (Investigator Initiated) Wellcome Trust, Organon, Takeda, Merck&Co Inc, Glaxo-smith Kline
Financial Relationships; Consulting for: Sanofi Aventis, Ono-pharmaceuticals, Actelion, Cephalon, Glaxo-Smith Kline, Lilly, H Lundbeck A/S, Merck&Co Inc., Pfizer Inc, Philips Lighting, Takeda, Lilly
Patents: Beneficiary on a patent related to the effects of light on circadian rhythms.
CAC
Funding: NASA, NIH, NIOSH/CDC, NSBRI, DHS FEMA
Financial relationships: Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd.; Bombardier, Inc.; Cephalon, Inc.; Delta Airlines; Eli Lilly and Co.; Fedex Kinko's; Federal Motor Carrier Safety Administration (FMCSA), U.S. Department of Transportation; Fusion Medical Education, LLC; Garda Síoch á na Inspectorate (Dublin, Ireland); Hypnion, Inc. (acquired by Eli Lilly and Co. in April 2007); Global Ground Support; Johnson & Johnson; Koninklijke Philips Electronics, N.V.; Morgan Stanley; Sanofi-Aventis Groupe; Portland Trail Blazers; Respironics, Inc.; Sepracor, Inc.; Sleep Multimedia, Inc.; Sleep Research Society (for which Dr.Czeisler served as president); Somnus Therapeutics, Inc.; Takeda Pharmaceuticals; Vanda Pharmaceuticals, Inc.; Vital Issues in Medicine; Warburg-Pincus and Zeo Inc.
Dr. Czeisler owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc., and Zeo Inc., and received royalties from McGraw Hill, the New York Times and Penguin Press.
Dr. Czeisler has received lecture fees from the Accreditation Council of Graduate Medical Education; Alfresa; the American Academy of Allergy, Asthma and Immunology Program Directors; American Physiological Society; Association of University Anesthesiologists; Baylor College of Medicine; Beth-Israel Deaconess Medical Center; Brown Medical School/Rhode Island Hospital; Cephalon, Inc.; Clinical Excellence Commission (Australia); Dalhousie University; Duke University Medical Center; Harvard School of Public Health, Harvard University; Institute of Sleep Health Promotion (NPO); London Deanery; Morehouse School of Medicine; Mount Sinai School of Medicine; National Emergency Training Center; National Institutes of Health; North East Sleep Society; Osaka University School of Medicine; Partners HealthCare, Inc.; Sanofi-Aventis, Inc.; St. Lukes Roosevelt Hospital; Takeda; Tanabe Seiyaku Co., Ltd.; Tokyo Electric Power Company (TEPCO); University of Michigan; University of Pennsylvania; University of Pittsburgh; University of Tsukuba; University of Virginia Medical School; University of Washington Medical Center; University of Wisconsin Medical School; World Federation of Sleep Research and Sleep Medicine Societies.
Dr. Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine; American Clinical and Climatological Association; Association for Patient-Oriented Research; National Institute for Occupational Safety and Health; National Sleep Foundation; and Sleep Research Society; clinical trial research contracts from Cephalon, Inc., Merck & Co., Inc., and Pfizer, Inc.; an investigator-initiated research grant from Cephalon, Inc.; and his research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc., Koninklijke Philips Electronics, N.V., ResMed, and the Brigham and Women's Hospital.
The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from: Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck & Co., Inc., Peter C. Farrell, Ph.D., Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals and Tempur-Pedic.
The HMS/DSM has received gifts from many outside organizations and individuals including: Axon Sleep Research Laboratories, Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Catalyst Group, Cephalon, Inc., Clarus Ventures, Eli Lilly and Co., Farrell Family Foundation, Fisher & Paykel Healthcare Corporation, George H. Kidder, Esq., GlaxoSmithKline, Hypnion, Inc., Jordan's Furniture, Merck Research Laboratories, Park Place Corporation, Respironics, Inc., Sanofi-Aventis, Inc., Select Comfort Corporation, Sepracor, Inc., Sleep Health Centers LLC, Takeda Pharmaceuticals, Tempur-Pedic Medical Division, Total Sleep Holdings, Vanda Pharmaceuticals, Inc.
The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc.
Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
Patents: beneficiary or inventor on several patents related: assessment and modification of the phase and amplitude of the endogenous circadian rhythm, apparatus for delivering high intensity light to modify circadian rhythms, a method to modify circadian rhythms with short wavelength light, and a test for evaluating visual function in visually impaired people.
EBK
Funding: NSBRI, NIH, Respironics (investigator initiated), DARPA/Air Force (2004), Army (2005), Vanda Pharmaceuticals (investigator initiated, no salary support, 2004-2006), Takeda Pharmaceuticals NA (investigator initiated, 2007-2009).
Financial relationships:
Grant reviews: (all <$10,000): NIH (2004,2006,2007,2009);NASA (check from Diversified Global resources) (2009); Ministry of Health, Singapore (2007);USAMRMC (check from American Institute of Biological Sciences) (2006)
Speaker Honoraria (all <$10,000): India Science and Engineering Research Council (2008); USAMRMC (check from MTS Systems) (2008);American Academy of Sleep Medicine (2007);Morehouse School of Medicine (2007); University of Pittsburgh (2004).
Consulting (all <$10,000): Vanda Pharmaceuticals (2004); Sanofi –Aventis (2009)
Patents: None
Footnotes
List of Supplemental Material: Supplemental Figure
Authors contributions: DC – data collection, analysis, manuscript preparation; WW – analysis, manuscript preparation; JKW – data collection, analysis, manuscript preparation; REK – study design, analysis, manuscript preparation; DJD – study design, analysis, manuscript preparation; CAC – study design, analysis, manuscript preparation; EBK – study design, data collection, analysis, manuscript preparation
References and Notes
- 1.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 2.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: The mind's clock. Oxford University Press; New York: 1991. [Google Scholar]
- 3.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 4.Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007;30:257–262. doi: 10.1093/sleep/30.3.257. [DOI] [PubMed] [Google Scholar]
- 5.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 7.Jewett ME, Kronauer RE. Interactive mathematical models of subjective alertness and cognitive throughput in humans. J Biol Rhythms. 1999;14:588–597. doi: 10.1177/074873099129000920. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 10.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HP. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–1033. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 13.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 14.Lavie P. Ultrashort sleep-waking schedule III. “Gates” and “forbidden zones” for sleep. Electroenceph Clin Neurophysiol. 1986;63:414–425. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 15.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The Impact of Sleep Timing and Bright Light Exposure on Attentional Impairment during Night Work. J Biol Rhythms. 2008;23:341–352. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–317. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 19.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 20.Christie MA, Bolortuya Y, Chen LC, McKenna JT, McCarley RW, Strecker RE. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep. 2008;31:1393–1398. [PMC free article] [PubMed] [Google Scholar]
- 21.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Porkka-Heiskanen T. Adenosine in sleep and wakefulness. Ann Med. 1999;31:125–129. doi: 10.3109/07853899908998788. [DOI] [PubMed] [Google Scholar]
- 23.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borbely AA. From slow waves to sleep homeostasis: new perspectives. Arch Ital Biol. 2001;139:53–61. [PubMed] [Google Scholar]
- 25.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 26.Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2008 doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking Sleep: Realization of Benefits During Subsequent Sleep Restriction and Recovery. Sleep. 2009;32:311–321. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 29.Executive summary of the 2005 “Sleep in America” poll. National Sleep Foundation; 2005. [Google Scholar]
- 30.Mitler MM, Miller JC, Lipsitz JJ, Walsh JK, Wylie CD. The sleep of long-haul truck drivers. N Engl J Med. 1997;337:755–761. doi: 10.1056/NEJM199709113371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM, Knauer RS. In: Disorders of Sleeping and Waking: Indications and Techniques. Guilleminault C, editor. Addison-Wesley; Menlo Park, CA: 1982. pp. 297–329. [Google Scholar]
- 32.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 33.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 34.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human Subjects. U.S Government Printing Office; Washington, D.C: 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


