Abstract
Objective
To characterize the delivery and postnatal neurodevelopmental outcomes of fetuses referred for ventriculomegaly (VM).
Methods
Under an internal review board-approved protocol, pregnant women were referred for magnetic resonance imaging (MRI) after sonographic diagnosis of VM and classified into one of four diagnostic groups: Group 1, normal central nervous system (CNS); Group 2, isolated mild VM (10–12 mm); Group 3, isolated VM > 12 mm; and Group 4, other CNS findings. Pregnancy outcome was obtained. Follow-up visits were offered with assessment of neurodevelopmental, adaptive and neurological functioning at 6 months and 1 year and/or 2 years of age. Atrial diameter and VM group differences in developmental outcomes were evaluated using repeated measures logistic regression and Fishers exact test, respectively.
Results
Of 314 fetuses, 253 (81%) were liveborn and survived the neonatal period. Fetuses in Groups 4 and 3 were less likely to progress to live delivery and to survive the neonatal period (60% and 84%, respectively) than were those in Groups 2 or 1 (93% and 100%, respectively, P < 0.001). Of the 143 fetuses followed postnatally, between 41% and 61% had a Bayley Scales of Infant Development (BSID-II) psychomotor developmental index score in the delayed range (< 85) at the follow-up visits, whereas the BSID-II mental developmental index and Vineland Adaptive Behavior composite scores were generally in line with normative expectations. Among those that were liveborn, neither VM group nor prenatal atrial diameter was related to postnatal developmental outcome.
Conclusions
Diagnostic category and degree of fetal VM based on ultrasound and MRI measurements are associated with the incidence of live births and thus abnormal outcome. Among those undergoing formal postnatal testing, VM grade is not associated with postnatal developmental outcome, but motor functioning is more delayed than is cognitive or adaptive functioning.
Keywords: adaptive behavior, Bayley Scales of Infant Development, cognitive functioning, fetus, gestational age, infant, motor functioning, MRI, neurological abnormalities, US, ventriculomegaly, Vineland Adaptive Behavior Scales
Introduction
Ventriculomegaly (VM), a non-specific dilatation of the lateral cerebral ventricles, is the most common central nervous system (CNS) abnormality identified with prenatal imaging techniques1. Counseling of patients is difficult because the cause and accurate prognosis cannot be determined with confidence2–8. Studies (often retrospective) of postnatal outcomes indicate that the clinical course and developmental sequelae of VM vary widely9–13. When a CNS anomaly is detected on ultrasound screening, fetal magnetic resonance imaging (MRI) may demonstrate additional findings that can alter patient counseling14,15. The goal of this prospective study was to characterize the delivery and postnatal neurodevelopmental outcomes of fetuses referred for MRI following suspicion on ultrasound of VM.
Patients and Methods
Study design
Analyses were based on longitudinal data collected in an ongoing prospective study performed at Beth Israel Deaconess Medical Center and Children's Hospital, Boston, as part of a National Institutes of Health-funded study investigating outcome of fetuses with VM. The study was approved by each hospital's human subjects review board and was compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Patient enrolment and imaging
Pregnant women were referred for MRI after an ultrasound examination demonstrated fetal VM, defined as ventricular size (measured at the atrium of the lateral ventricle) ≥ 10 mm. Inclusion criteria included referral for VM or an ultrasound examination at one of our institutions demonstrating VM, and agreement to participate in the study. Patients were excluded if review of medical records demonstrated that VM had never been present.
Prenatal imaging procedures as well as the prenatal diagnosis on imaging for the first 200 fetuses have been described elsewhere14,16. Atrial diameter (in mm) was obtained from the median of three measurements of the larger ventricle, one from each of three obstetric sonologists. The consensus prenatal diagnosis of the three obstetric sonologists and three pediatric neuroradiologists on MRI was utilized for categorizing patients into four groups: Group 1, normal; Group 2, isolated mild VM (measurement at level of atrium of 10–12 mm); Group 3, VM > 12 mm without other CNS anomaly; and Group 4, any other CNS anomaly. The ventricular measurement was taken from the ultrasound examination, except in two cases with unilateral VM in which the larger ventricle was not well assessed on ultrasound due to fetal position, and was better measured on MRI.
Karyotype analysis and screening for infection were performed at the discretion of the referring physician and the patient. Chart reviews were performed to obtain these results.
Postnatal follow-up
Parents were contacted regarding delivery information and invited to participate in postnatal follow-up, for which there was a separate informed consent form. Consenting caregiver–infant pairs participated in up to three follow-up visits when infants were 6 months, 1 year and 2 years old. Each visit included a neurodevelopmental and a neurological evaluation. During the neurodevelopmental assessment, one of three psychologists blinded to VM status evaluated the infant's general cognitive and motor skills using Bayley Scales of Infant Development-Second Edition (BSID-II)17 and adaptive behavior using the Vineland Adaptive Behavior Scales (VABS)18. Parents also updated demographic information and reported on their infant's receipt of early intervention services. After a rest period, one of three pediatric neurologists, with knowledge of the infant's prenatal diagnosis and medical records, evaluated their neurological status.
Instrumentation
BSID-II17 is an age-normalized assessment of infants' general cognitive and motor skills which yields two standard scores (normal mean ± SD score, 100 ± 15): the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). VABS18 is a semi-structured parent interview that generates age-referenced standard scores (normal mean ± SD score, 100 ± 15) for four subdomains of adaptive behavior (communication, daily living skills, socialization, and motor skills) and overall adaptive functioning, the Adaptive Behavior Composite (ABC). For preterm infants (< 37 gestational weeks at delivery), BSID-II and VABS scores at each visit were adjusted for gestational age at birth19. A cutoff of 85 was used to denote normal versus delayed functioning.
For the pediatric neurology examination, infants were evaluated on 37 areas of neurological functioning using a standard neurological exam (Appendix S1 online). We evaluated as a dependent variable the number of infants exhibiting a normal or abnormal finding on each of nine summary categories: head circumference, mental status, cranial nerves, hearing and vision, strength, posture and tone, deep tendon reflexes, gait and primitive reflexes. Any abnormal status with uncertain functional significance was noted for descriptive purposes.
The Hollingshead Four-Factor Index of Social Status20 was used to evaluate variations in familial socioeconomic status (SES). The Hollingshead yields a composite score based on parents' education and occupational status. Higher scores reflect higher SES.
Definition of abnormal outcome
For an overall assessment of abnormal/normal outcome, the abnormal group was defined in two manners. The first included infants with any delayed or abnormal score, postnatal exclusions due to syndromes, conditions or karyotypes associated with well-documented developmental delays, and those with in-utero/neonatal demise (Table 1). The second definition included infants with any of the aforementioned abnormal outcomes and also those pregnancies that underwent elective termination.
Table 1.
Study enrolment and follow-up of fetuses according to ventriculomegaly (VM) group
| All | Group 1 Normal | Group 2 Isolated VM, 10–12 mm | Group 3 Isolated VM, >12 mm | Group 4 Other CNS findings | |
|---|---|---|---|---|---|
| Total | 314 | 32 | 133 | 32 | 117 |
| Lost from standardized postnatal follow-up due to: | |||||
| Elective termination | 52 | 0 | 7 | 5 | 40 |
| Stillbirth/spontaneous pregnancy loss | 5 | 0 | 2 | 0 | 3 |
| Neonatal demise | 4 | 0 | 0 | 0 | 4 |
| Live birth surviving neonatal period (% of Total) | 253 (81) | 32 (100) | 124 (93) | 27 (84) | 70 (60) |
| Lost from study due to postnatal exclusion* | 17 | 1 | 6 | 1 | 9 |
| Available for postnatal enrollment | 236 | 31 | 118 | 26 | 61 |
| Lost to follow-up or declined postnatal testing | 93 | 16 | 47 | 9 | 21 |
| Postnatal neurodevelopmental testing (% of those available for postnatal enrollment) | 143 (60) | 15 (48) | 71 (61) | 17 (65) | 40 (66) |
| Known outcome† (% of Total) | 221 (70) | 16 (50) | 86 (65) | 23 (72) | 96 (82) |
Data are n or n (%).
Known abnormal outcome (syndromes, conditions or karyotypes associated with well-documented developmental delays or abnormal neurological findings) but excluded because of follow-up elsewhere.
Equals total minus those lost to follow-up. CNS, central nervous system.
Statistical analyses
The proportions of live and preterm births were compared across VM groups by Fisher's exact test. Mean BSID-II and VABS scores were compared across VM groups using one-way analysis of variance, separately for each visit. The (MDI – PDI) difference was analyzed similarly, since initial evaluation showed poorer PDI than MDI performance. Trichotomized neurodevelopmental scores (50–69, severely delayed; 70–84, mildly delayed; and ≥ 85, within normal limits) and neurological findings (normal, abnormal, abnormality with uncertain functional significance) were compared across VM groups using Fisher's exact test for each visit separately. To assess the influence of atrial diameter, we excluded Group 4 infants because ventricular size could not always be measured accurately due to other CNS abnormalities (e.g. holoprosencephaly), and because the outcome would likely be affected more by the other abnormalities than by ventricular size. Dichotomized neurodevelopmental and adaptive behavioral scores (< 85 vs. ≥ 85) and neurological findings (normal vs. abnormal, excluding abnormalities with uncertain functional significance) were modeled on atrial diameter using repeated-measures logistic regression, adjusting for: maternal age; gestational age at birth; family SES; gestational age at imaging; and the differential between nominal and actual dates of assessment.
To judge statistical significance for comparisons of each outcome among the four prenatal diagnostic groups, we employed the Bonferroni-adjusted critical P-value of 0.05 divided by the number of pre-planned comparisons among groups, which varied between one and four. Tabulated analyses are presented with exact P-values (rather than tags indicating significant vs. non-significant, or strength of significance). SAS software (version 9.1, SAS Inst. Inc., Cary, NC, USA) was used for all computations.
Results
Between 1 July 2003 and 19 October 2007 enrolled into the study were 311 consecutive pregnant women with 318 fetuses (six sets of twins and one woman with two consecutive pregnancies). The mean ± SD maternal age was 31 ± 5 (range, 17–44) years, the gestational age according to menstrual dates at the time of prenatal imaging was 26.0 ± 5.7 (range, 16.3–41.0 weeks) and the gestational age according to ultrasound was 26.1 ± 5.9 (range, 15.7–39.4) weeks. One fetus was studied twice, but only the second MRI examination was used for data analysis. Review of records showed that three fetuses never had VM, and these were excluded from the study. One patient declined postnatal follow-up and was excluded. Thus our final study sample comprised 307 women with 314 fetuses, 32 in Group 1 (normal), 133 in Group 2 (isolated VM, 10–12 mm), 32 in Group 3 (isolated VM, > 12 mm) and 117 in Group 4 (other CNS findings) (Table 1). The prenatal diagnosis of associated CNS anomalies in Group 4 is given in Table 2. The ventricular diameter, associated syndromes, karyotype abnormalities and syndromes are given in Table 3.
Table 2.
Prenatal diagnosis of additional central nervous system (CNS) anomalies in 117 fetuses with CNS findings other than ventriculomegaly (Group 4)
| Atrial diameter (mm) | |||
|---|---|---|---|
| Diagnosis (final consensus) | n | Mean ± SD | Min–Max |
| Dysgenesis of corpus callosum | 41 | 14.5 ± 5.8 | 8–42 |
| Cerebellar hypoplasia | 18 | 14.3 ± 4.2 | 10–24 |
| Spinal neural tube defect | 17 | 14.9 ± 6.7 | 9–37 |
| Hemorrhage | 17 | 17.9 ± 8.0 | 7–42 |
| Chiari malformation | 16 | 14.4 ± 6.5 | 9–37 |
| Defect of septum pellucidum | 16 | 19.4 ± 8.6 | 10–37 |
| Porencephaly | 16 | 25.5 ± 9.4 | 10–42 |
| Cyst | 13 | 12.8 ± 3.6 | 9–20 |
| Polymicrogyria, lissencephaly | 12 | 13.3 ± 3.8 | 10–23 |
| Dandy–Walker variant/ malformation | 9 | 12.6 ± 2.0 | 10–17 |
| Congenital infarction | 8 | 20.6 ± 8.9 | 12–39 |
| Heterotopias | 5 | 14.2 ± 4.0 | 10–20 |
| Holoprosencephaly | 4 | 21.3 ± 14.5 | 12–38 |
| Megacisterna magna | 3 | 12.3 ± 3.2 | 10–16 |
| Schizencephaly | 3 | 14.0 ± 1.0 | 13–15 |
| Micrencephaly | 2 | 16.5 ± 9.2 | 10–23 |
| Encephalocele | 2 | 18.0 ± 8.5 | 12–24 |
| Abnormal midbrain/thalamus | 1 | 14.0 | — |
| Craniosynostosis | 1 | 11.0 | — |
| Periventricular leukomalacia | 1 | 22.0 | — |
| Tumor | 1 | 25.0 | — |
Table 3.
Ventricular diameter, karyotype, syndromes and infection according to prenatally diagnosed ventriculomegaly (VM) group
| Group | Ventricular diameter on US (mm, mean ± SD (range)) | Abnormal karyotype, syndromes and infection | |||
|---|---|---|---|---|---|
| Chromosomal abnormalities | Proven genetic abnormalities | Syndromes | Infection | ||
| Group 1 Normal (n = 32) |
8.3 ± 1.1 (4–10)* | Trisomy 21 | |||
| Group 2 Isolated VM, 10–12 mm (n = 133) |
10.8 ± 0.9 (9–12)* | Trisomy 21 (n=5), 46,XX, add(8)(p23), 17q 12 duplication | Mutation of FGFR2 gene, Crouzon syndrome | Fanconi anemia, TTTS | |
| Group 3 Isolated VM, >12 mm (n = 32) |
13.7 ± 1.8 (9–19)† | Trisomy 21, 13q interstitial deletion | Noonan syndrome, Pierre–Robin syndrome | Parvovirus B19 | |
| Group 4 Other CNS findings (n = 117) |
15.7 ± 7.0 (7–42) | Trisomy 21 (n = 2), mosaic trisomy 8 (n = 2), inverted duplication of 8p, 47,XXY + 13 der(13) (q10;q10), 12q interstitial deletion, craniofrontal nasal syndrome, 5p15.2 microdeletion, presumed balanced translocation t(4;13)(q35;q22) | Deletion LIS 1 gene on chr 17, mutation of FGR2 gene on chr 10, Apert syndrome | Smith–Lemli–Opitz syndrome, twin reversed arterial perfusion syndrome, propionic acidemia, Zellweger syndrome, Meckel-Gruber syndrome, Schinzel-Gideon syndrome, pseudo-trisomy 13 | Cytomegalovirus |
Ranges of ventricular size overlap categories due to consensus diagnosis being used for categories, and median of largest atrial measurement being used for atrial diameters.
Two fetuses had unilateral ventriculomegaly, with the larger ventricle measured only on magnetic resonance imaging. chr, chromosome; TTTS, twin–twin transfusion syndrome; US, ultrasound.
Standardized neurological and psychological follow-up was obtained in 143 children, with 127 visits at 6 months, 122 visits at 1 year, and 79 visits at 2 years. These were performed between 14 April 2004 and 5 May 2009. There were 17 children with syndromes/findings known to be associated with neurological sequelae who did not participate in formal study follow-up due to being followed up elsewhere. These included children with trisomy 21 (n = 6), neural tube defects (n = 5) and one of each of the following syndromes: Apert syndrome with agenesis of the corpus callosum, Fanconi's anemia with closed external auditory canals and deafness, propionic acidemia with agenesis of the corpus callosum, Zellweger syndrome with polymicrogyria, chromosome 13q interstitial deletion with global developmental delay and sensorineural hearing loss, and pseudotrisomy 13. Therefore, postnatal outcome was available for 221 fetuses (143 with standardized follow-up exams, 17 with syndromes, conditions or karyotypes associated with well-documented developmental delays or abnormal neurological findings who were followed up elsewhere, four neonatal demises and 57 terminations, stillbirths or spontaneous pregnancy losses).
There was a significant decline from Group 1 to Group 4 in the percentage of live births with survival beyond the neonatal period (Groups 1–4, respectively, having 100%, 93%, 84% and 60% survival (P < 0.001)).
Sample characteristics
Regarding participants in the standardized neurodevelopmental follow-up, there were no differences between VM groups with respect to infant's gender, race, ethnicity, age at assessment, mother's marital status or parental education (Table 4). Of the infants participating in follow-up, 16% were born preterm. VM group was not associated with the proportion of preterm vs. term births (P = 0.53) or mean gestational age at delivery (mean ± SD, 38.2 ± 2.3, P = 0.77). Early intervention services were applied to 42% of infants, and infants in Group 4 were more likely to receive early intervention than were infants in the other groups (65% for Group 4 and 31–40% for the others, P = 0.02).
Table 4.
Sample characteristics of standardized neurodevelopmental follow-up, according to prenatally diagnosed ventriculomegaly (VM) group
| Characteristic | All | Group 1 Normal | Group 2 Isolated VM, 10–12 mm | Group 3 Isolated VM, >12 mm | Group 4 Other CNS findings | P |
|---|---|---|---|---|---|---|
| Infants tested | 143 | 15 | 71 | 17 | 40 | |
| Gender | ||||||
| Male | 94 (66) | 9 (60) | 47 (66) | 12 (71) | 26 (65) | 0.94* |
| Female | 49 (34) | 6 (40) | 24 (34) | 5 (29) | 14 (35) | |
| Preterm (<37 weeks at delivery) | 23 (16) | 2 (13) | 13 (18) | 4 (24) | 4 (10) | 0.53* |
| Race | ||||||
| Caucasian | 110 (77) | 13 (87) | 55 (77) | 12 (71) | 30 (75) | 0.91* |
| African-American | 10 (7) | 0 (0) | 5 (7) | 1 (6) | 4 (10) | |
| Other/unknown | 23 (16) | 2 (13) | 11 (15) | 4 (24) | 6 (15) | |
| Hispanic ethnicity | 10 (7) | 0 (0) | 5 (7) | 2 (12) | 3 (8) | 0.66* |
| Mother's marital status‡ | ||||||
| Married | 93 (65) | 11 (73) | 46 (66) | 10 (59) | 25 (63) | 0.83* |
| Other/unknown | 50 (35) | 4 (27) | 24 (34) | 7 (41) | 15 (38) | |
| Early intervention services received§ | 52 (42) | 4 (31) | 20 (33) | 6 (40) | 22 (65) | 0.02* |
| Gestational age at delivery (weeks) | 38.2 ± 2.3 | 38.5 ± 2.4 | 38.0 ± 2.6 | 38.5 ± 2.3 | 38.3 ± 1.5 | 0.77† |
| Age at assessment visit (months) | ||||||
| 6-month visit | 6.5 ± 1.1 | 6.5 ± 0.9 | 6.6 ± 1.0 | 6.1 ± 1.0 | 6.3 ± 1.3 | 0.24† |
| 1-year visit | 13.1 ± 1.9 | 13.2 ± 1.6 | 13.1 ± 2.0 | 13.1 ± 2.0 | 13.1 ± 1.9 | 0.99† |
| 2-year visit | 24.6 ± 1.8 | 24.4 ± 0.9 | 25.1 ± 2.0 | 24.4 ± 2.3 | 24.2 ± 1.4 | 0.37† |
| Maternal age at delivery (years) | 31.8 ± 5.1 | 33.2 ± 5.2 | 31.6 ± 5.1 | 34.2 ± 4.2 | 30.6 ± 5.0 | 0.06† |
| Parental education (years)‡ | ||||||
| Maternal | 15.3 ± 2.8 | 16.2 ± 2.4 | 15.2 ± 3.2 | 15.3 ± 2.5 | 15.1 ± 2.2 | 0.67† |
| Paternal | 15.2 ± 3.1 | 16.6 ± 1.9 | 15.3 ± 3.4 | 15.5 ± 2.8 | 14.6 ± 2.7 | 0.22† |
| Socioeconomic status (SES)‡ | 47 ± 13 | 55 ±8 | 47 ± 13 | 49 ± 13 | 44 ± 12 | 0.05† |
Data are n, n (%) or mean ± SD.
Tests for equal proportions by Fisher's exact test.
Tests for equal means by one-way analysis of variance.
At time of first visit.
As reported at any visit; data available for 123 subjects.
Data available on parental education and SES for 122–124 subjects; SES measured by Hollingshead Four-Factor Index of Social Status, with higher scores reflecting higher SES.
Neurodevelopmental and adaptive behavioral outcomes
Of the 143 neonates with formal postnatal follow-up, 29 were normal on all examinations and 114 were abnormal at at least one point in time. VM group was not associated with cognitive functioning as measured by BSID-II MDI at any of the three postnatal visits (Table 5). Although average MDI scores in each group were within normal limits for age (≥ 85), individual scores ranged from severely or mildly delayed to within normal limits at each visit.
Table 5.
Descriptive statistics for neurodevelopmental and adaptive behavioral standard scores according to prenatally diagnosed ventriculomegaly (VM) group
| Outcome/Visit | All | Group 1 Normal (mean ± SD) | Group 2 Isolated VM, 10–12 mm (mean ± SD) | Group 3 Isolated VM, >12 mm (mean ± SD) | P* | Group 4 Other CNS findings (mean ± SD) | P† | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min, Max | |||||||
| BSID-II MDI | ||||||||
| 6-month visit | 95.5 ± 8.5 | 71, 116 | 96.3 ± 9.2 | 96.6 ± 8.6 | 95.6 ± 6.9 | 0.93 | 93.1 ± 8.7 | 0.36 |
| 1-year visit | 94.6 ± 12.6 | 50, 124 | 93.0 ± 10.4 | 95.9 ± 12.3 | 97.8 ± 11.6) | 0.61 | 91.5 ± 14.4 | 0.36 |
| 2-year visit | 96.3 ± 15.2 | 50, 134 | 104.9 ± 15.9 | 93.9 ± 17.8 | 102.5 ± 8.2 | 0.08 | 93.1 ± 10.6 | 0.11 |
| BSID-II PDI | ||||||||
| 6-month visit | 84.9 ± 14.8 | 52, 138 | 88.2 ± 16.2 | 86.6 ± 15.1 | 83.0 ± 15.3 | 0.65 | 81.6 ± 13.2 | 0.42 |
| 1-year visit | 81.5 ± 17.2 | 50, 117 | 76.1 ± 16.6 | 86.3 ± 14.7 | 81.6 ± 13.6 | 0.11 | 74.4 ± 20.9 | 0.01 |
| 2-year visit | 84.3 ± 16.7 | 50, 113 | 90.6 ± 9.1 | 85.3 ± 17.2 | 85.9 ± 15.9 | 0.72 | 78.7 ± 18.5 | 0.34 |
| Difference (MDI - PDI) | ||||||||
| 6-month visit | 10.5 ± 11.4 | -22, 40 | 8.1 ± 10.2 | 9.9 ± 10.9 | 12.6 ± 12.7 | 0.63 | 11.5 ± 12.3 | 0.75 |
| 1-year visit | 13.2 ± 16.1 | -27, 55 | 16.9 ± 16.8 | 9.6 ± 15.0 | 16.2 ± 13.1 | 0.18 | 17.3 ± 18.2 | 0.13 |
| 2-year visit | 12.0 ± 16.3 | -35, 47 | 14.3 ± 15.3 | 8.5 ± 16.2 | 16.6 ± 15.3 | 0.31 | 14.4 ± 17.4 | 0.41 |
| VABS ABC | ||||||||
| 6-month visit | 100.9 ± 5.7 | 88, 116 | 99.6 ± 6.5 | 101.4 ± 5.6 | 101.2 ± 4.8 | 0.68 | 100.2 ± 6.1 | 0.70 |
| 1-year visit | 97.7 ± 10.0 | 62, 128 | 95.5 ± 9.6 | 99.3 ± 9.2 | 100.2 ± 7.0 | 0.39 | 94.7 ± 12.2 | 0.15 |
| 2-year visit | 88.8 ± 11.1 | 53, 111 | 93.8 ± 11.1 | 88.4 ± 9.6 | 92.7 ± 9.8 | 0.31 | 85.1 ± 3.4 | 0.16 |
One-way analysis of variance, testing for equal mean score in Groups 1–3.
Testing for equal mean score in all four groups; to account for two preplanned comparisons among diagnostic groups for each outcome at each visit, Bonferroni-adjusted criterion for statistical significance was P < 0.025. BSID-II, Bayley Scales of Infant Development, 2nd edition; Max, maximum; MDI, Mental Developmental Index; Min, minimum; PDI, Psychomotor Developmental Index; VABS ABC, Vineland Adaptive Behavior Scale, Adaptive Behavior Composite.
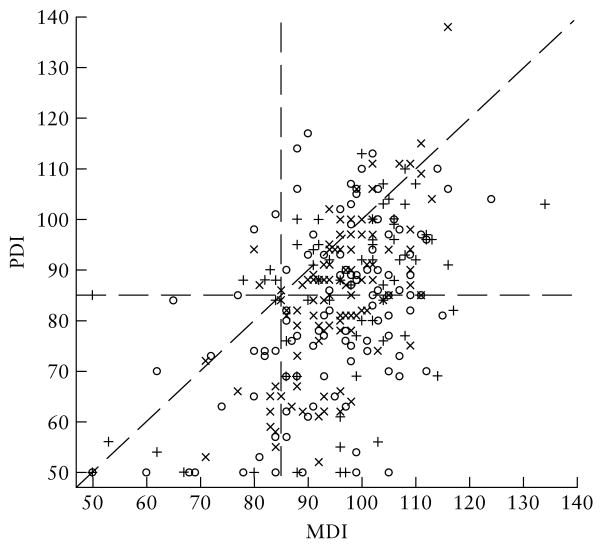
Motor functioning as measured by BSID-II PDI scores varied significantly across VM groups at the 1-year examination, with average scores being higher in cases of isolated VM (Groups 2 and 3) than in the other groups (P = 0.01), but there were no statistically significant differences among groups at the 6-month or 2-year examinations. Individual PDI scores in each VM group ranged from severely delayed to within normal limits at each visit. Infants were more delayed in motor than in mental functioning (Figure 1). The magnitude of cognitive–motor difference did not vary among VM groups (Table 5). VM group was not associated with infants' adaptive functioning as measured by the VABS ABC at any visit (Table 5).
Figure 1.
Bayley Scales of Infant Development (BSID-II): Psychomotor Developmental Index (PDI) versus Mental Developmental Index (MDI) at the 6-month (×), 1-year (○) and 2-year (+) visits.
We also examined the neurodevelopmental and adaptive behavioral outcomes as categorical variables, distinguishing three ranges of standard score: 60–69, 70–84 and ≥ 85 (Table 6). The distribution of BSID-II MDI and VABS ABC scores was generally in line with normative expectations. However, a higher percentage of infants had a PDI score in the delayed range (< 85) at each visit (45% at the 6-month visit, 53% at the 1-year visit, and 40% at the 2-year visit) than one would expect based on a normal distribution (15.8%). As for the continuous scores, only the 1-year PDI showed a significant difference across diagnostic groups, with a higher percentage in the normal range (≥ 85) for isolated VM (Groups 2 and 3) than for the normal group (Group 1) or the group with additional CNS findings (Group 4). Otherwise, VM group was not associated with the trichotomized BSID-II MDI, BSID-II PDI or VABS ABC scores.
Table 6.
Neurodevelopmental and adaptive behavior score ranges according to prenatally diagnosed ventriculomegaly (VM) group
| Outcome/Visit/Score* | All (n (%)) | Group 1 Normal (n (%)) | Group 2 Isolated VM, 10–12 mm (n (%)) | Group 3 Isolated VM, >12 mm (n (%)) | P† | Group 4 Other CNS findings (n (%)) | P‡ |
|---|---|---|---|---|---|---|---|
| BSID-II MDI | |||||||
| 6-month visit | |||||||
| ≥85 | 100 (90) | 7 (78) | 56 (95) | 12 (86) | 0.10 | 25 (86) | 0.17 |
| 70–84 | 11 (10) | 2 (22) | 3 (5) | 2 (14) | 4 (14) | ||
| 50–69 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 1-year visit | |||||||
| ≥85 | 90 (82) | 10 (71) | 47 (85) | 10 (77) | 0.24 | 23 (82) | 0.24 |
| 70–84 | 14 (13) | 4 (29) | 5 (9) | 3 (23) | 2 (7) | ||
| 50–69 | 6 (5) | 0 (0) | 3 (5) | 0 (0) | 3 (11) | ||
| 2-year visit | |||||||
| ≥85 | 58 (83) | 7 (88) | 26 (79) | 11 (100) | 0.57 | 14 (78) | 0.70 |
| 70–84 | 7 (10) | 1 (13) | 3 (9) | 0 (0) | 3 (17) | ||
| 50–69 | 5 (7) | 0 (0) | 4 (12) | 0 (0) | 1 (6) | ||
| BSID-II PDI | |||||||
| 6-month visit | |||||||
| ≥85 | 60 (55) | 6 (67) | 33 (57) | 8 (57) | 0.92 | 13 (45) | 0.78 |
| 70–84 | 30 (27) | 1 (11) | 15 (26) | 3 (21) | 11 (38) | ||
| 50–69 | 20 (18) | 2 (22) | 10 (17) | 3 (21) | 5 (17) | ||
| 1-year visit | |||||||
| ≥85 | 51 (47) | 5 (36) | 30 (55) | 7 (54) | 0.53 | 9 (33) | 0.02 |
| 70–84 | 33 (30) | 5 (36) | 19 (35) | 4 (31) | 5 (19) | ||
| 50–69 | 25 (23) | 4 (29) | 6 (11) | 2 (15) | 13 (48) | ||
| 2-year visit | |||||||
| ≥85 | 42 (60) | 6 (75) | 21 (64) | 7 (64) | 0.75 | 8 (44) | 0.59 |
| 70–84 | 13 (19) | 2 (25) | 5 (15) | 2 (18) | 4 (22) | ||
| 50–69 | 15 (21) | 0 (0) | 7 (21) | 2 (18) | 6 (33) | ||
| VABS ABC | |||||||
| 6-month visit | |||||||
| ≥85 | 112 (100) | 9 (100) | 59 (100) | 14 (100) | — | 30 (100) | — |
| 70–84 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 50–69 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 1-year visit | |||||||
| ≥85 | 103 (92) | 13 (93) | 52 (93) | 13 (100) | 0.99 | 25 (86) | 0.51 |
| 70–84 | 7 (6) | 1 (7) | 4 (7) | 0 (0) | 2 (7) | ||
| 50–69 | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | ||
| 2-year visit | |||||||
| ≥85 | 50 (71) | 6 (75) | 25 (76) | 10 (91) | 0.80 | 9 (50) | 0.28 |
| 70–84 | 18 (26) | 2 (25) | 7 (21) | 1 (9) | 8 (44) | ||
| 50–69 | 2 (3) | 0 (0) | 1 (3) | 0 (0) | 1 (6) |
Score of ≥ 85, within normal limits; 70–84, mildly delayed performance; 50–69, significantly delayed performance.
Fisher's exact test for equal proportions in Groups 1–3.
Testing for equal proportions in all four diagnostic groups; to account for two preplanned comparisons among diagnostic groups for each outcome at each visit, Bonferroni-adjusted criterion for statistical significance was P < 0.025. BSID-II, Bayley Scales of Infant Development, 2nd edition; MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index; VABS ABC, Vineland Adaptive Behavior Scale, Adaptive Behavior Composite.
Postnatal neurological status
Head circumference was abnormal in 22%, 24% and 26% of infants across groups at 6-month, 1-year and 2-year follow-up visits, respectively (Table 7), while at these same time points mental status was abnormal in 6–8%, cranial nerve testing in 4–8% and strength in 1–2% of infants across groups. At 6-month, 1-year and 2-year follow-up visits there were abnormalities of uncertain functional importance of the cranial nerves in 6–10% of infants, of hearing and vision in 0–1% of infants and of strength in 8–12% of infants. VM diagnostic group was not significantly associated with head circumference, mental status, cranial nerve testing, hearing and vision or strength testing (Table 7). However, abnormalities of uncertain functional importance were more common in Group 4 strength testing at each visit.
Table 7.
Neurological status compared among prenatally diagnosed ventriculomegaly (VM) groups
| Parameter/Visit/Neurologcial status | All (n (%)) | Group 1 Normal (n (%)) | Group 2 Isolated VM, 10 – 12 mm (n (%)) | Group 3 Isolated VM, >12 mm (n (%)) | P* | Group 4 Other CNS, findings (n (%)) | P† | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head circumference | |||||||||||
| 6-month visit | |||||||||||
| Normal | 93 (78) | 9 (82) | 50 (81) | 10 (71) | 0.71 | 24 (75) | 0.82 | ||||
| Abnormal | 26 (22) | 2 (18) | 12 (19) | 4 (29) | 8 (25) | ||||||
| 1-year visit | |||||||||||
| Normal | 85 (76) | 11 (73) | 41 (75) | 10 (77) | ‡ | 23 (79) | 0.96 | ||||
| Abnormal | 27 (24) | 4 (27) | 14 (25) | 3 (23) | 6 (21) | ||||||
| 2-year visit | |||||||||||
| Normal | 56 (74) | 8 (89) | 24 (69) | 10 (83) | 0.45 | 14 (70) | 0.61 | ||||
| Abnormal | 20 (26) | 1 (11) | 11 (31) | 2 (17) | 6 (30) | ||||||
| Mental status | |||||||||||
| 6-month visit | |||||||||||
| Normal | 115 (94) | 11 (100) | 59 (94) | 15 (94) | ‡ | 30 (91) | 0.89 | ||||
| Abnormal | 8 (7) | 0 (0) | 4 (6) | 1 (6) | 3 (9) | ||||||
| 1-year visit | |||||||||||
| Normal | 110 (94) | 14 (93) | 52 (93) | 14 (93) | ‡ | 30 (97) | 0.94 | ||||
| Abnormal | 7 (6) | 1 (7) | 4 (7) | 1 (7) | 1 (3) | ||||||
| 2-year visit | |||||||||||
| Normal | 72 (92) | 7 (78) | 33 (92) | 12 (100) | 0.19 | 20 (95) | 0.34 | ||||
| Abnormal | 6 (8) | 2 (22) | 3 (8) | 0 (0) | 1 (5) | ||||||
| Cranial nerves | |||||||||||
| 6-month visit | |||||||||||
| Normal | 106 (86) | 11 (100) | 56 (89) | 15 (94) | ‡ | 24 (73) | 0.52 | ||||
| Abnormal | 6 (5) | 0 (0) | 3 (5) | 0 (0) | 3 (9) | ||||||
| Ab/UFS | 11 (9) | 0 (0) | 4 (6) | 1 (6) | ‡ | 6 (18) | 0.32 | ||||
| 1-year visit | |||||||||||
| Normal | 100 (86) | 14 (93) | 50 (89) | 13 (93) | 0.82 | 23 (74) | 0.80 | ||||
| Abnormal | 9 (8) | 1 (7) | 5 (9) | 0 (0) | 3 (10) | ||||||
| Ab/UFS | 7 (6) | 0 (0) | 1 (2) | 1 (7) | 0.59 | 5 (16) | 0.15 | ||||
| 2-year visit | |||||||||||
| Normal | 67 (86) | 9 (100) | 33 (92) | 11 (92) | 0.62 | 14 (67) | 0.72 | ||||
| Abnormal | 3 (4) | 0 (0) | 1 (3) | 1 (8) | 1 (5) | ||||||
| Ab/UFS | 8 (10) | 0 (0) | 2 (6) | 0 (0) | 0.86 | 6 (29) | 0.06 | ||||
| Hearing and vision | |||||||||||
| 6-month visit | |||||||||||
| Normal | 118 (98) | 11 (100) | 63 (100) | 16 (100) | ‡ | 28 (90) | 0.06 | ||||
| Abnormal | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 3 (10) | ||||||
| Ab/UFS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ‡ | 0 (0) | 0.06 | ||||
| 1-year visit | |||||||||||
| Normal | 114 (97) | 14 (93) | 56 (100) | 15 (100) | ‡ | 29 (94) | 0.26 | ||||
| Abnormal | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (6) | ||||||
| Ab/UFS | 1 (1) | 1 (7) | 0 (0) | 0 (0) | 0.35 | 0 (0) | 0.10 | ||||
| 2-year visit | |||||||||||
| Normal | 77 (99) | 9 (100) | 36 (100) | 12 (100) | ‡ | 20 (95) | 0.54 | ||||
| Abnormal | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | ||||||
| Ab/UFS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ‡ | 0 (0) | 0.54 | ||||
| Strength | |||||||||||
| 6-month visit | |||||||||||
| Normal | 108 (88) | 11 (100) | 59 (94) | 15 (94) | ‡ | 23 (70) | ‡ | ||||
| Abnormal | 1 (1) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | ||||||
| Ab/UFS | 14 (11) | 0 (0) | 3 (5) | 1 (6) | ‡ | 10 (30) | 0.005 | ||||
| 1-year visit | |||||||||||
| Normal | 101 (86) | 14 (93) | 53 (95) | 14 (93) | ‡ | 20 (65) | 0.12 | ||||
| Abnormal | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (6) | ||||||
| Ab/UFS | 14 (12) | 1 (7) | 3 (5) | 1 (7) | ‡ | 9 (29) | 0.006 | ||||
| 2-year visit | |||||||||||
| Normal | 71 (91) | 9 (100) | 35 (97) | 12 (100) | ‡ | 15 (71) | 0.52 | ||||
| Abnormal | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | ||||||
| Ab/UFS | 6 (8) | 0 (0) | 1 (3) | 0 (0) | ‡ | 5 (24) | 0.025 | ||||
| Posture and tone | |||||||||||
| 6-month visit | |||||||||||
| Normal | 92 (75) | 10 (91) | 51 (81) | 14 (88) | 0.70 | 17 (52) | 0.003 | ||||
| Abnormal | 18 (15) | 1 (9) | 6 (10) | 0 (0) | 11 (33) | ||||||
| Ab/UFS | 13 (11) | 0 (0) | 6 (10) | 2 (13) | 0.70 | 5 (15) | 0.011 | ||||
| 1-year visit | |||||||||||
| Normal | 80 (68) | 13 (81) | 44 (80) | 9 (60) | ‡ | 14 (45) | < 0.001 | ||||
| Abnormal | 13 (11) | 0 (0) | 3 (5) | 0 (0) | 10 (32) | ||||||
| Ab/UFS | 24 (21) | 3 (20) | 8 (14) | 6 (40) | 0.23 | 7 (23) | < 0.001 | ||||
| 2-year visit | |||||||||||
| Normal | 56 (72) | 7 (80) | 29 (80) | 9 (75) | ‡ | 11 (52) | 0.018 | ||||
| Abnormal | 8 (10) | 0 (0) | 2 (6) | 0 (0) | 6 (29) | ||||||
| Ab/UFS | 14 (18) | 2 (20) | 5 (14) | 3 (25) | 0.82 | 4 (19) | 0.10 | ||||
| Deep tendon reflexes | |||||||||||
| 6-month visit | |||||||||||
| Normal | 113 (92) | 11 (100) | 61 (97) | 16 (100) | ‡ | 25 (76) | 0.003 | ||||
| Ab/UFS | 10 (8) | 0 (0) | 2 (3) | 0 (0) | 8 (24) | ||||||
| 1-year visit | |||||||||||
| Normal | 108 (92) | 15 (100) | 55 (98) | 15 (100) | ‡ | 23 (74) | < 0.001 | ||||
| Ab/UFS | 9 (8) | 0 (0) | 1 (2) | 0 (0) | 8 (26) | ||||||
| 2-year visit | |||||||||||
| Normal | 70 (90) | 9 (100) | 33 (91) | 12 (100) | 0.74 | 16 (76) | 0.15 | ||||
| Ab/UFS | 8 (10) | 0 (0) | 3 (9) | 0 (0) | 5 (24) | ||||||
| Gait§ | |||||||||||
| 1-year visit | |||||||||||
| Normal | 98 (84) | 14 (93) | 53 (96) | 12 (80) | 0.07 | 19 (61) | < 0.001 | ||||
| Abnormal | 18 (16) | 1 (7) | 2 (4) | 3 (20) | 12 (39) | ||||||
| 2-year visit | |||||||||||
| Normal | 70 (95) | 9 (100) | 34 (97) | 11 (100) | ‡ | 16 (84) | 0.20 | ||||
| Abnormal | 4 (5) | 0 (0) | 1 (3) | 0 (0) | 3 (16) | ||||||
| Primitive reflexes | |||||||||||
| 6-month visit | |||||||||||
| Normal | 99 (84) | 8 (80) | 50 (83) | 14 (88) | 0.06 | 27 (82) | 0.03 | ||||
| Abnormal | 7 (6) | 2 (20) | 1 (2) | 0 (0) | 4 (12) | ||||||
| Ab/UFS | 13 (11) | 0 (0) | 9 (15) | 2 (13) | 0.11 | 2(6) | 0.09 | ||||
| 1-year visit | |||||||||||
| Normal | 106 (91) | 14 (93) | 53 (95) | 15 (100) | ‡ | 24 (77) | 0.003 | ||||
| Abnormal | 5 (4) | 0 (0) | 0 (0) | 0 (0) | 5 (16) | ||||||
| Ab/UFS | 6 (5) | 1 (7) | 3 (5) | 0 (0) | ‡ | 2(6) | 0.03 | ||||
| 2-year visit | |||||||||||
| Normal | 75 (97) | 9 (100) | 36 (100) | 12 (100) | ‡ | 18 (90) | 0.20 | ||||
| Abnormal | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | ||||||
| Ab/UFS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ‡ | 0(0) | 0.20 | ||||
Denominator in some cases is smaller than total tested because of missing observations.
Fisher's exact test, comparing percentages of neurological status (two or three categories as indicated by square brackets) across Groups 1–3.
Comparing all four diagnostic groups; to account for multiple preplanned comparisons among diagnostic groups for each outcome at each visit, Bonferroni-adjusted criterion for statistical significance was P < 0.025 in the case of two neurological status categories (two comparisons) and P < 0.0125 in the case of three categories (four comparisons).
Negligible or no variation in percentages of neurological status across diagnostic groups; P = 1 or undefined, indicating maximal non-significance.
Assessment of gait not applicable at 6 months of age. Ab/UFS, abnormality with uncertain functional significance.
Posture and tone were abnormal in 10–15% of infants at the different time points, with infants in Group 4 exhibiting more abnormal posture and tone at each visit (33% at 6 months, P = 0.003; 32% at 1 year, P < 0.001; 29% at 2 years, P = 0.018). There was a higher percentage of infants at the 1-year visit in Group 4 (39%) and Group 3 (20%) with abnormal gait compared with those in Group 1 (7%) or Group 2 (4%, P < 0.001). This difference between groups had resolved by the 2-year visit. There was also a higher percentage of infants at the 1-year visit in Group 4 with abnormal primitive reflexes (16%, P = 0.004) than in the other groups.
Atrial diameter
There was a mild and non-significant association (P = 0.12) between larger atrial diameter and abnormal outcome as shown by the odds ratio of 1.14 per 1-mm increment (95% CI, 0.97–1.34, Table 8). When elective terminations were excluded from the abnormal outcome group, the association was attenuated (odds ratio 1.04 per 1-mm increment; 95% CI, 0.87–1.24, P = 0.66). Among fetuses that were liveborn and enrolled in postnatal follow-up, atrial diameter was not associated with BSID-II MDI, PDI, VABS ABC scores, neurological findings or composite abnormal/normal status (Table 8).
Table 8.
Outcome in infants with normal diagnosis or with isolated ventriculomegaly (VM)
| Outcome | Comparison of visits* | OR (95% CI) per increment in covariate‡ | |||||
|---|---|---|---|---|---|---|---|
| 6-month (n (%)) | 1-year (n (%)) | 2-year (n (%)) | P† | Atrial diameter, 1-mm increment | Gestational age at birth, 1-week increment | Hollingshead SES scale, 10-point increment | |
| Low developmental score (<85) | |||||||
| BSID-II MDI | 6 (8) | 13 (18) | 8 (17) | 0.25 | 0.76 (0.58–1.01) | 1.03 (0.87–1.23) | 1.03 (0.69–1.54) |
| BSID-II PDI | 28 (39) | 34 (47) | 17 (35) | 0.38 | 1.03 (0.87–1.24) | 0.99 (0.87–1.12) | 0.80 (0.60–1.08) |
| VABS ABC | 0 (0) | 3 (4) | 11 (23) | 0.006 | 0.75 (0.50–1.13) | 0.96 (0.75–1.22) | 0.75 (0.39–1.43) |
| Any | 29 (40) | 38 (52) | 23 (48) | 0.38 | 0.98 (0.82–1.16) | 0.99 (0.87–1.11) | 0.89 (0.66–1.19) |
| Abnormal neurological exam§ | |||||||
| Head circumference | 16 (23) | 18 (24) | 11 (23) | 0.85 | 1.09 (0.86–1.39) | 1.06 (0.89–1.28) | — |
| Mental status | 5 (7) | 6 (8) | 4 (9) | 0.98 | 0.84 (0.61–1.15) | 0.90 (0.76–1.08) | — |
| Cranial nerves | 3 (4) | 6 (8) | 2 (4) | —¶ | — | — | — |
| Hearing and vision | 0 (0) | 0 (0) | 0 (0) | — | — | — | — |
| Strength | 1 (1) | 0 (0) | 0 (0) | — | — | — | — |
| Posture and tone | 6 (9) | 3 (5) | 2 (5) | 0.51 | 0.90 (0.47–1.71) | 0.68 (0.51–0.90) | — |
| Deep tendon reflexes | 0 (0) | 0 (0) | 0 (0) | — | — | — | — |
| Gait | —** | 5 (7) | 1 (2) | 0.14 | 1.25 (0.87–1.79) | 1.73 (0.93–3.21) | — |
| Primitive reflexes | 3 (4) | 0 (0) | 0 (0) | — | — | — | — |
| Any | 24 (36) | 30 (45) | 17 (41) | 0.49 | 1.01 (0.82–1.25) | 0.95 (0.83–1.09) | — |
| Abnormal postnatal exam§,†† | 48 (55) | 54 (65) | 32 (59) | 0.37 | 1.04 (0.88–1.23) | — | — |
| Abnormal outcome | |||||||
| Incl. elective termination§,‡‡ | 70 (64) | 76 (72) | 54 (71) | 0.42 | 1.14 (0.97–1.34) | — | — |
| Excl. elective termination§,# | 58 (60) | 64 (69) | 42 (66) | 0.41 | 1.04 (0.87–1.24) | — | — |
Denominators vary between 46 and 113 depending on missing values. Sample restricted to diagnosis of normal (Group 1) or isolated VM (Groups 2 and 3), with all covariates recorded. Ambiguous findings and abnormalities with uncertain functional significance excluded from analysis of neurological outcomes.
Repeated-measures multiple logistic regression analysis, comparing frequency of outcome across visits, adjusted for listed covariates, mother's age, biparietal diameter at time of imaging and offset between nominal and actual visit date.
Odds ratio (OR) from regression analysis, indicating multiplicative change in odds of outcome attributable to indicated increment of covariate, adjusted for other covariates.
Hollingshead SES scale omitted as covariate for neurological outcomes; gestational age and testing date offset additionally omitted for combined outcomes.
Sparse outcome; regression analysis failed to converge.
Assessment of gait not applicable at 6 months of age.
Low developmental score or abnormal neurological exam.
Elective termination (n = 12), stillbirth and spontaneous pregnancy loss (n = 2) and severe postnatal anomalies (n = 8) included as abnormal outcome at every visit, in addition to those with low developmental score or abnormal neurological exam at each particular visit.
Stillbirth and spontaneous pregnancy loss (n = 2) and severe postnatal anomalies (n = 8) included as abnormal outcome at every visit, in addition to those with low developmental score or abnormal neurological exam at each particular visit. BSID-II, Bayley Scales of Infant Development, 2nd edition; Excl., excluding; Incl., including; MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index; VABS ABC, Vineland Adaptive Behavior Scale, Adaptive Behavior Composite.
Discussion
It is generally agreed that infants with mild isolated VM have more favorable outcomes than do those with more severe ventricular dilatation or VM with additional CNS anomalies5,8,13,21,22. Some have suggested that mild isolated VM (10–12 mm) that resolves spontaneously may represent a normal variant6,21,23. Yet, overall, the literature suggests that the degree of VM in fetal or neonatal imaging is not associated with outcome in a simple or consistent way9–13,24. Our findings are in accord with this concept. Fetuses with larger degrees of VM and those with associated abnormalities were less likely to proceed to a live birth with survival beyond the neonatal period. However, once born and participating in follow-up, infants in each group had a similar range of outcomes on measures of mental and adaptive function. Associated CNS anomalies were correlated at 6 months and 1 year with abnormal findings on neurological exam, including abnormal posture and tone, abnormal deep tendon and primitive reflexes and abnormal gait (at 1 year); however, only the differences in posture and tone persisted until 2 years of age.
Of interest were our contrasting findings between the infants' MDI and PDI scores across groups. Whereas MDI scores were typically in the normal range, only 47–60% of PDI scores were. Corroborating retrospective findings by Ouahba et al.11, these results further suggest that infants referred for fetal VM are at increased risk for neuromotor delays, regardless of the severity of isolated VM or the presence of associated CNS abnormalities.
The regression results corroborate the general lack of significant findings for VM grade and postnatal neurodevelopmental outcome, once the infant has survived the neonatal period. Adjusting for covariates, atrial diameter was not associated with low BSID-II mental or motor scores, low VABS adaptive behavior or the presence of neurological abnormalities at any visit. Prenatal measures of atrial size may thus be of limited prognostic utility for predicting the postnatal neurodevelopmental outcome of infants referred for VM prenatally, at least until the age of 2 years.
Prenatal imaging diagnosis is the basis for counseling parents whose fetuses have been referred for VM, and when postnatal imaging or autopsy is not available, it is also used for counseling regarding the risk of recurrence in future pregnancies. Yet it is well recognized that measurement variability can be a factor in the accuracy of prenatal diagnosis25,26. It is likely that some fetuses in our ‘normal’ group actually had VM that resolved or had borderline VM but, by consensus, were not diagnosed16. Because anomalies associated with VM are not always detected during routine prenatal ultrasound screening, Lee et al.9 urge physicians to exercise caution in giving a prognosis for fetal VM, even when ultrasound indicates that the VM is mild and isolated. Part of the difficulty in giving an accurate prognosis of outcome in this population may also stem from methodological problems in cohort studies. For instance, the use of retrospective studies, small heterogeneous samples and inconsistent imaging and follow-up methods have likely contributed to variable results. We attempted to overcome some of these difficulties by using a prospective design, state-of-the-art prenatal ultrasound and MRI techniques, and longitudinal evaluation of multiple neurodevelopmental outcomes.
Our study has several limitations. The first is attrition. Among our fetal population, 81% were liveborn surviving the neonatal period, with lower survival among fetuses with isolated VM (> 12 mm) and those with other CNS anomalies. Postnatal attrition also occurred in the normal and mild isolated VM groups. Several parents felt their child was normal and thus did not enroll for postnatal follow-up. This attrition may have skewed our follow-up towards preferential assessment of those neonates from the normal/mild VM groups in whom the parents had perceived that a developmental issue might be present. There may also have been insufficient statistical power to detect subtle VM-related effects.
Another limitation is that there is currently no reference standard for prenatal imaging diagnosis, and our imaging was performed at a mean of 26 weeks' gestation (with some studies as early as 16 weeks). We used the opinion of the ultrasound/MRI consensus conference as our final diagnosis. This reference standard of imaging concordance has been used by others27–29. While more information from imaging would be available at later gestational ages, we felt it reasonable to use diagnosis at the earliest ultrasound/MRI assessment since the purpose of this study was to obtain data to better counsel patients at the time of presentation with VM.
A third potential limitation is referral bias. Mild VM cases that have normal-looking brains on careful ultrasound examination may not be referred. Another limitation in this cohort is the complexity of diagnoses outside the CNS; we did not, for example, assess the impact of cardiac abnormalities.
A final limitation is the lack of assessment of the caregiving environment. Parenting style and quality of the home environment may exert a stronger impact on children's functioning than do environmental measures such as SES30,31. Moreover, most participants in our sample were working to upper middle class, which may limit generalizability to low-income samples. Moreover, early intervention was skewed towards infants with greater degrees of VM and other CNS problems, which could have attenuated VM effects on our outcomes32, since early intervention has been shown to maximize developmental potential in infants at risk for developmental problems33,34.
In conclusion, our results suggest that when based on ultrasound and MRI measurements made at a single prenatal assessment, VM diagnostic category and degree of VM are associated with the incidence of live birth and survival beyond the neonatal period. Among liveborns who participated in neurodevelopmental and neurological testing, motor outcomes were more severely affected than were cognitive or adaptive outcomes; however, neither VM group nor prenatal atrial diameter was associated consistently with postnatal developmental outcome. Prenatal counseling should indicate that fetal VM is associated with heterogeneous developmental outcomes, with increased risk for mild neuromotor delays, even if the sonogram and MRI study appear normal at the time of referral for prenatal MRI. Further follow-up of this cohort to older ages is warranted, since many functional domains potentially linked to fetal VM cannot be assessed reliably during infancy.
Supplementary Material
Acknowledgments
Patient studies were funded by NIH NIBIB 01998. Additional support was given by Advanced Fetal Care Center, Children's Hospital Boston and General Clinical Research Center, Children's Hospital Boston.
Footnotes
Supporting Information on The Internet: The following supporting information may be found in the online version of this article: Appendix S1 Scoring criteria for pediatric neurological examination at 6-month, 1-year and 2-year follow-up visits.
References
- 1.Garel C, Luton RJ. Ventricular dilatations. Childs Nerv Syst. 2003;19:301–308. doi: 10.1007/s00381-003-0795-0. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides KH, Berry S, Snijders RJM, Thorpe-Beeston JG, Gosden C. Fetal lateral cerebral ventriculomegaly: Associated malformations and chromosomal defects. Fetal Diagn Ther. 1990;5:5–14. doi: 10.1159/000263529. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein I, Reece EA, Pilu GL. Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles. IV. The posterior horn. Am J Perinatol. 1990;7:79–83. doi: 10.1055/s-2007-999452. [DOI] [PubMed] [Google Scholar]
- 4.Bromley B, Frigoletto FD, Jr, Benacerraf BR. Mild fetal lateral cerebral ventriculomegaly: clinical course and outcome. Am J Obstet Gynecol. 1991;164:863–867. doi: 10.1016/0002-9378(91)90530-5. [DOI] [PubMed] [Google Scholar]
- 5.Bloom SL, Bloom DD, DellaNebbia C, Martin LB, Lucas MJ, Twickler DM. The developmental outcome of children with antenatal mild isolated ventriculomegaly. Obstet Gynecol. 1997;90:93–97. doi: 10.1016/S0029-7844(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm C, Keck C, Hess S, Korinthenberg R, Breckwoldt M. Ventriculomegaly diagnosed by prenatal ultrasound and mental development of the children. Fetal Diagn Ther. 1998;13:162–166. doi: 10.1159/000020830. [DOI] [PubMed] [Google Scholar]
- 7.Rankin J, Robson S, Wariyar U. Outcome of prenatally detected mild/moderate cerebral ventriculomegaly. Eur J Pediatr Surg. 1998;8(Suppl 1):72. [PubMed] [Google Scholar]
- 8.Vergani P, Locatelli A, Strobelt N, Cavallone MD, Ceruti P, Paterlini G. Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol. 1998;178:218–222. doi: 10.1016/s0002-9378(98)80003-3. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Hong SH, Wang KC, Kim SK, Park JS, Jun JK, Yoon BH, Lee YH, Shin SM, Lee YK, Cho BK. Fetal ventriculomegaly: Prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg. 2006;105(Suppl 4):265–270. doi: 10.3171/ped.2006.105.4.265. [DOI] [PubMed] [Google Scholar]
- 10.Wyldes M, Watkinson M. Isolated mild fetal ventriculomegaly. Arch Dis Child Fetal Neonatal Ed. 2004;89:F9–F13. doi: 10.1136/fn.89.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouahba J, Luton D, Vuillard E, Garel C, Gressens P, Blanc N, Yoon BH, Lee YH, Shin SM, Lee YK, Cho BK. Prenatal isolated mild ventriculomegaly: outcome in 167 cases. BJOG. 2006;113:1072–1079. doi: 10.1111/j.1471-0528.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- 12.Falip C, Blanc N, Maes E, Zaccaria I, Oury JF, Sebag G, Garel C. Postnatal clinical and imaging follow-up of infants with prenatal isolated mild ventriculomegaly: a series of 101 cases. Pediatr Radiol. 2007;37:981–989. doi: 10.1007/s00247-007-0582-2. [DOI] [PubMed] [Google Scholar]
- 13.Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropolis S, Todros T. Fetal cerebral ventriculomegaly: Outcome in 176 cases. Ultrasound Obstet Gynecol. 2005;25:372–377. doi: 10.1002/uog.1857. [DOI] [PubMed] [Google Scholar]
- 14.Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS. Fast MR imaging of fetal central nervous system abnormalities. Radiology. 2003;229:51–61. doi: 10.1148/radiol.2291020770. [DOI] [PubMed] [Google Scholar]
- 15.Morris JE, Rickard S, Paley MN, Griffiths PD, Rigby PD, Whitby EH. The value of in-utero magnetic resonance imaging in ultrasound diagnosed foetal isolated cerebral ventriculomegaly. Clin Radiol. 2007;62:140–144. doi: 10.1016/j.crad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Levine D, Feldman HA, Kazan Tannus JF, Estroff JA, Magnino M, Robson C, Poussaint TY, Barnewolt CE, Mehta TS, Robertson RL. Frequency and etiology of disagreements in diagnoses for fetuses referred for VM. Radiology. 2008;247:516–527. doi: 10.1148/radiol.2472071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development. 2nd. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 18.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales – Interview Edition. American Guidance Service, Inc.; Circle Pines, MN: 1984. [Google Scholar]
- 19.Aylward GP. Methodological issues in outcome studies of at-risk infants. J Pediatr Psy. 2002;27:37–45. doi: 10.1093/jpepsy/27.1.37. [DOI] [PubMed] [Google Scholar]
- 20.Hollingshead A. Four-factor Index of Social Status. Yale University; New Haven, CT: 1979. [Google Scholar]
- 21.Patel MD, Filly AL, Hersh DR, Goldstein RB. Isolated mild cerebral ventriculomegaly: Clinical course and outcome. Radiology. 1994;192:759–764. doi: 10.1148/radiology.192.3.7520183. [DOI] [PubMed] [Google Scholar]
- 22.Tercanli S, Danzer E, Batukan C, Kupesic S, Holzgreve W. The fetus with ventriculomegaly: Prenatal diagnosis, associated malformations, pathophysiology, and prognostic factors. Ultrasound Rev Obstet Gynecol. 2001;1:6–13. [Google Scholar]
- 23.Lipitz S, Yagel S, Malinger G, Meizner I, Zalel Y, Achiron R. Outcome of fetuses with isolated borderline unilateral ventriculomegaly diagnosed at mid-gestation. Ultrasound Obstet Gynecol. 1998;12:23–26. doi: 10.1046/j.1469-0705.1998.12010023.x. [DOI] [PubMed] [Google Scholar]
- 24.Melchiorre K, Bhide A, Gika AD, Pilu G, Papageorghiou AT. Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol. 2009;34:212–224. doi: 10.1002/uog.7307. [DOI] [PubMed] [Google Scholar]
- 25.Borrell A, Costa D, Delgado RD, Martinez JM, Borrell C, Fortuny A. Interobserver variability of midtrimester fetal nuchal thickness. Eur J Obstet Gynecol Reprod Biol. 1997;72:27–29. doi: 10.1016/s0301-2115(96)02659-0. [DOI] [PubMed] [Google Scholar]
- 26.Rovas L, Sladkevicius P, Strobel E, Valentin L. Intraobserver and interobserver reproducibility of three-dimensional grayscale and power Doppler ultrasound examinations of the cervix in pregnant women. Ultrasound Obstet Gynecol. 2005;26:132–137. doi: 10.1002/uog.1884. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Bindman R, Hosmer WD, Caponigro M, Cunningham G. The variability in the interpretation of prenatal diagnostic ultrasound. Ultrasound Obstet Gynecol. 2001;17:326–332. doi: 10.1046/j.1469-0705.2001.00346.x. [DOI] [PubMed] [Google Scholar]
- 28.Birkelo CC, Chamberlain WE, Phelps PS. Tuberculosis case finding. A comparison of the effectiveness of various roentgeno-graphic and photofluorographic methods. JAMA. 1947;133:359–366. doi: 10.1001/jama.1947.02880060001001. [DOI] [PubMed] [Google Scholar]
- 29.Borgstede JP, Lewis RS, Bhargavan M, Sunshine JH. RADPEER quality assurance program: a multifacility study of interpretive disagreement rates. J Am Coll Radiol. 2004;1:59–65. doi: 10.1016/S1546-1440(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 30.Landry SH, Smith KE, Swank PR, Miller-Loncar CL. Early maternal and child influences on children's later independent cognitive and social functioning. Child Dev. 2002;71:358–375. doi: 10.1111/1467-8624.00150. [DOI] [PubMed] [Google Scholar]
- 31.Bornstein MH, Bradley RH, editors. SES, Parenting, and Child Development. Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- 32.Telzrow CF. Impact of perinatal complications on education. In: Gray JW, Dean RS, editors. Neuropsychology of Perinatal Complications. Springer; New York: 1991. pp. 161–185. [Google Scholar]
- 33.Berlin LJ, Brooks-Gunn J, McCarton C, McCormick MC. The effectiveness of early intervention: Examining risk factors and pathways to enhanced development. Prev Med. 1998;27:238–245. doi: 10.1006/pmed.1998.0282. [DOI] [PubMed] [Google Scholar]
- 34.Ramey CT, Ramey SL. Early intervention and early experience. Am Psychol. 1998;53:109–120. doi: 10.1037//0003-066x.53.2.109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.